Multivariate Analysis Reveals That Unsubstituted β-Ring and C8-Keto Structures Are Important Factors for Anti-Inflammatory Activity of Carotenoids
Abstract
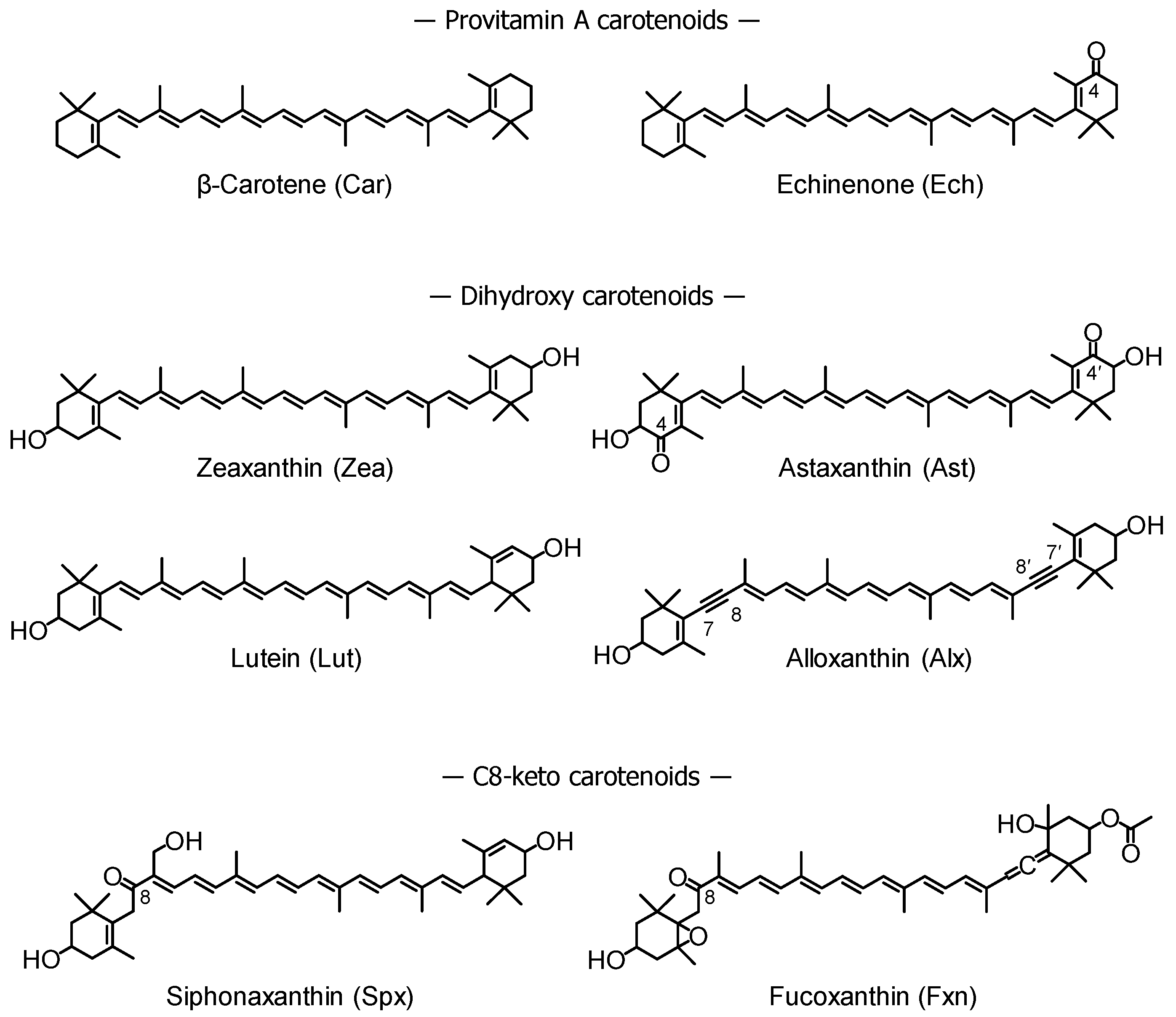
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Reagents
2.3. Carotenoid Treatment
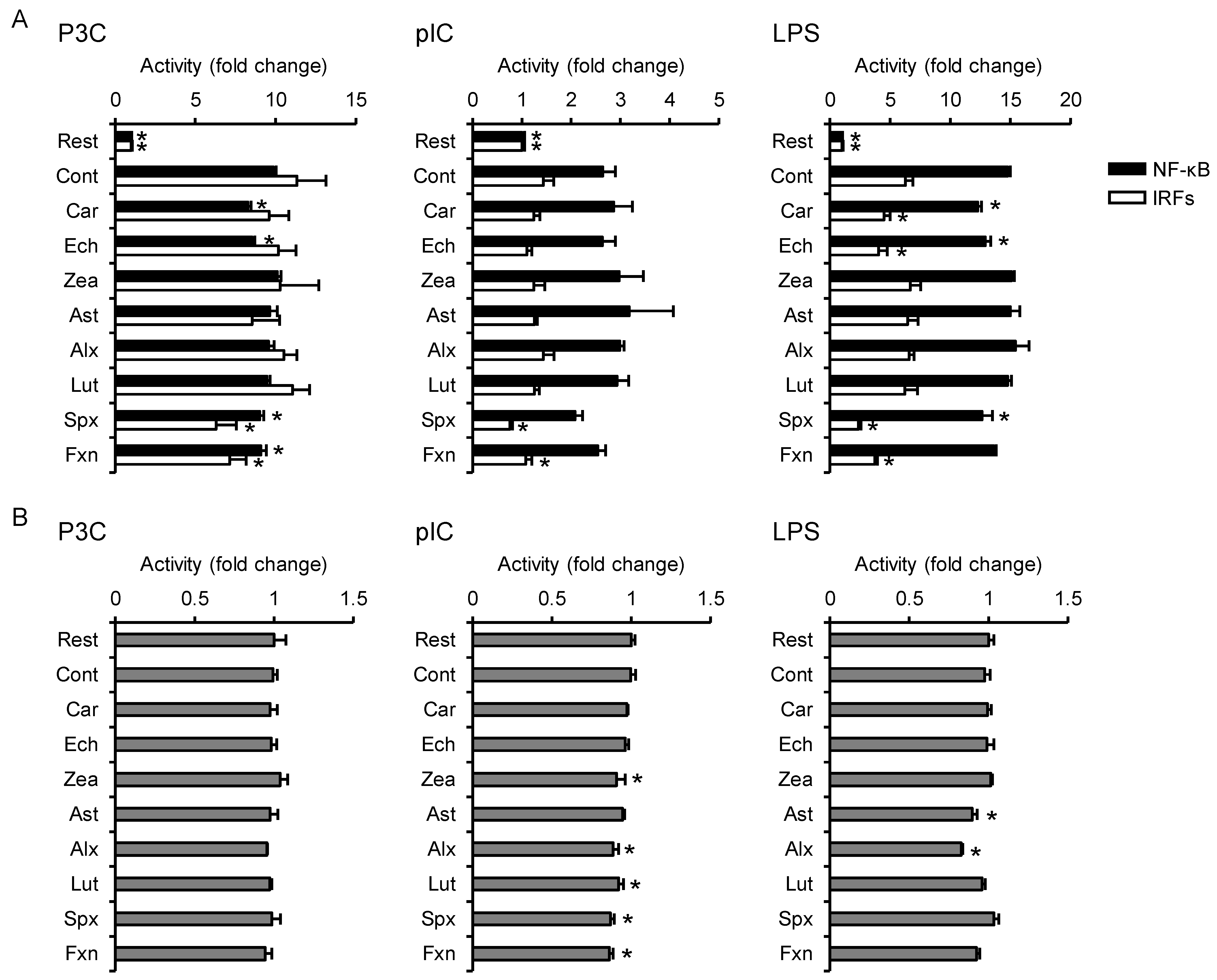
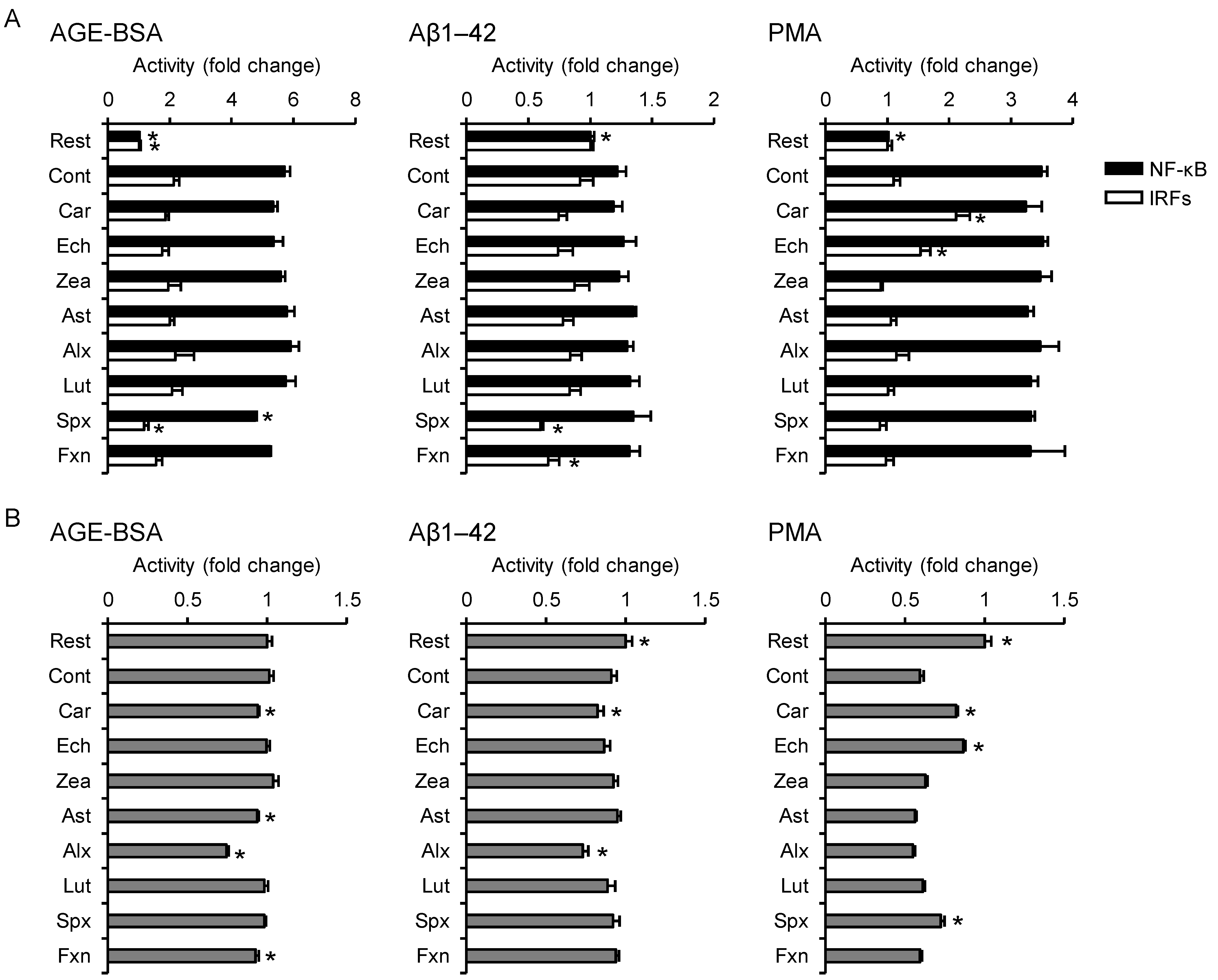
2.4. Reporter Gene Assay
2.5. MTT Assay
2.6. LC-MS/MS Analysis
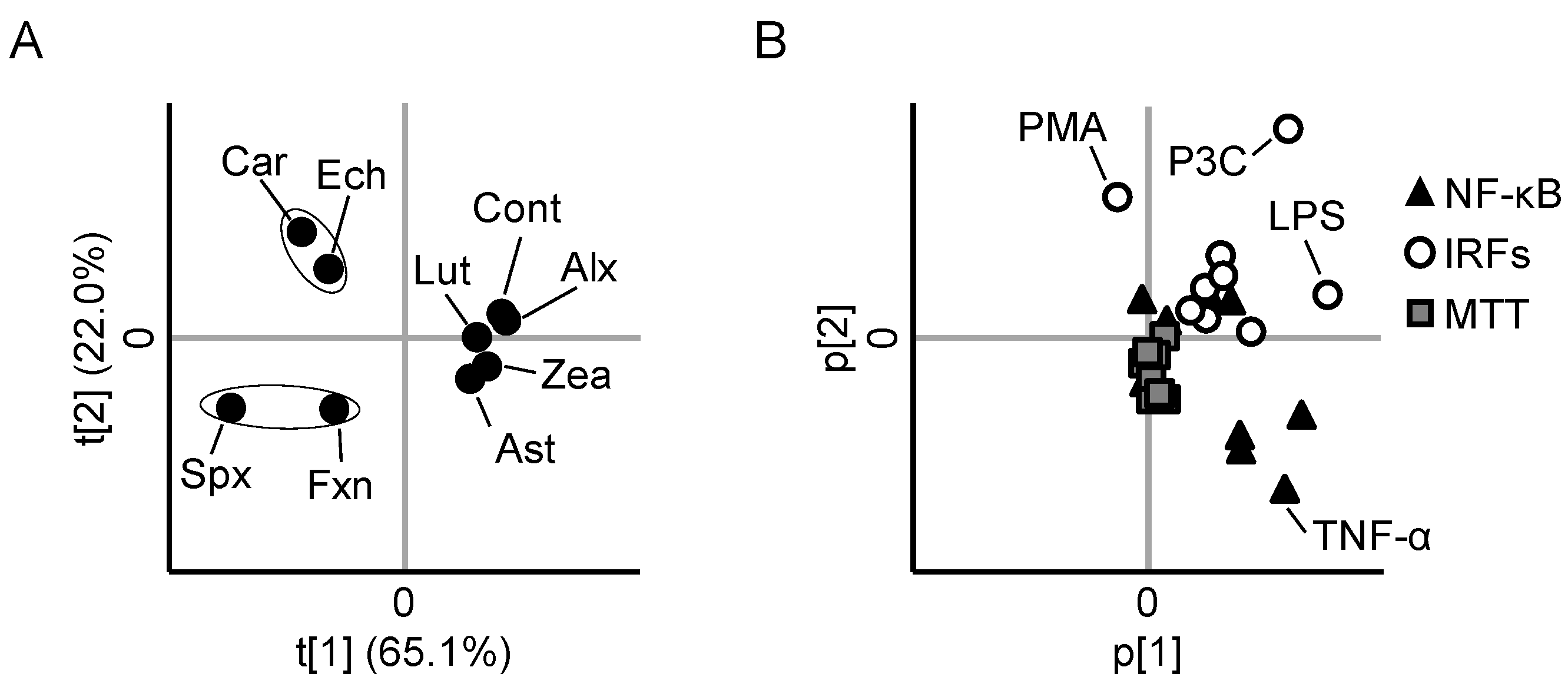
2.7. Multivariate Analysis
2.8. Statistical Analysis
3. Results
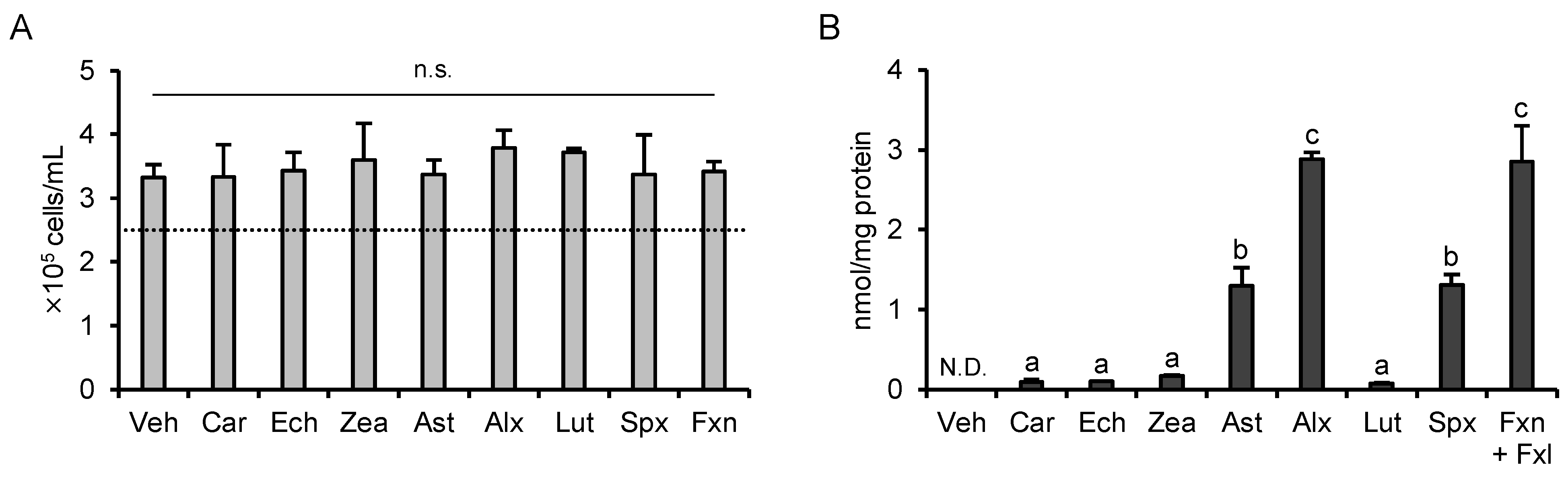
3.1. Determination of the Treatment Concentration
3.2. Effects on TLR-Mediated Inflammatory Responses
3.3. Effects on Proinflammatory Cytokine-Induced Inflammatory Responses
3.4. Effects on Non-Infectious Inflammatory Responses
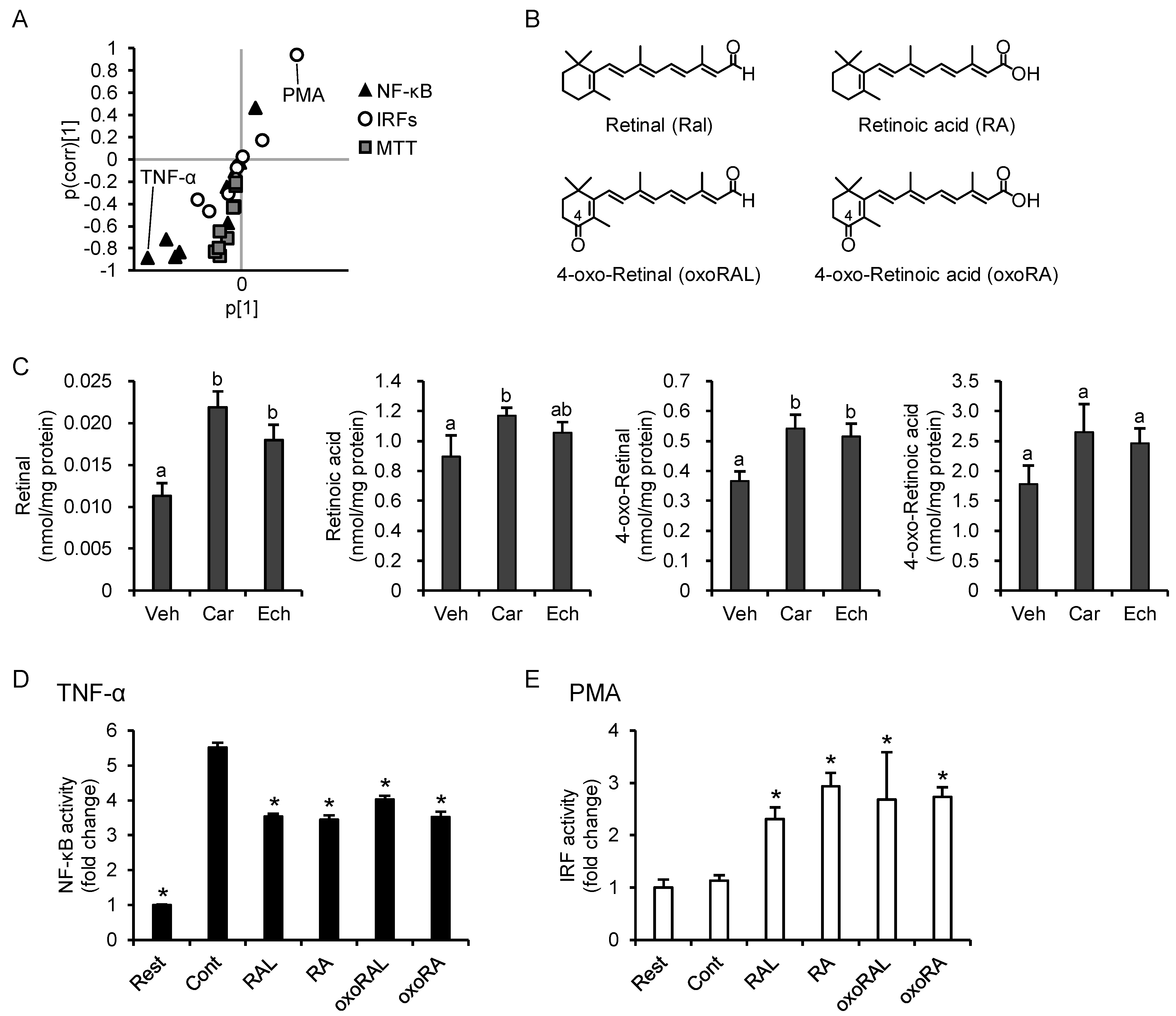
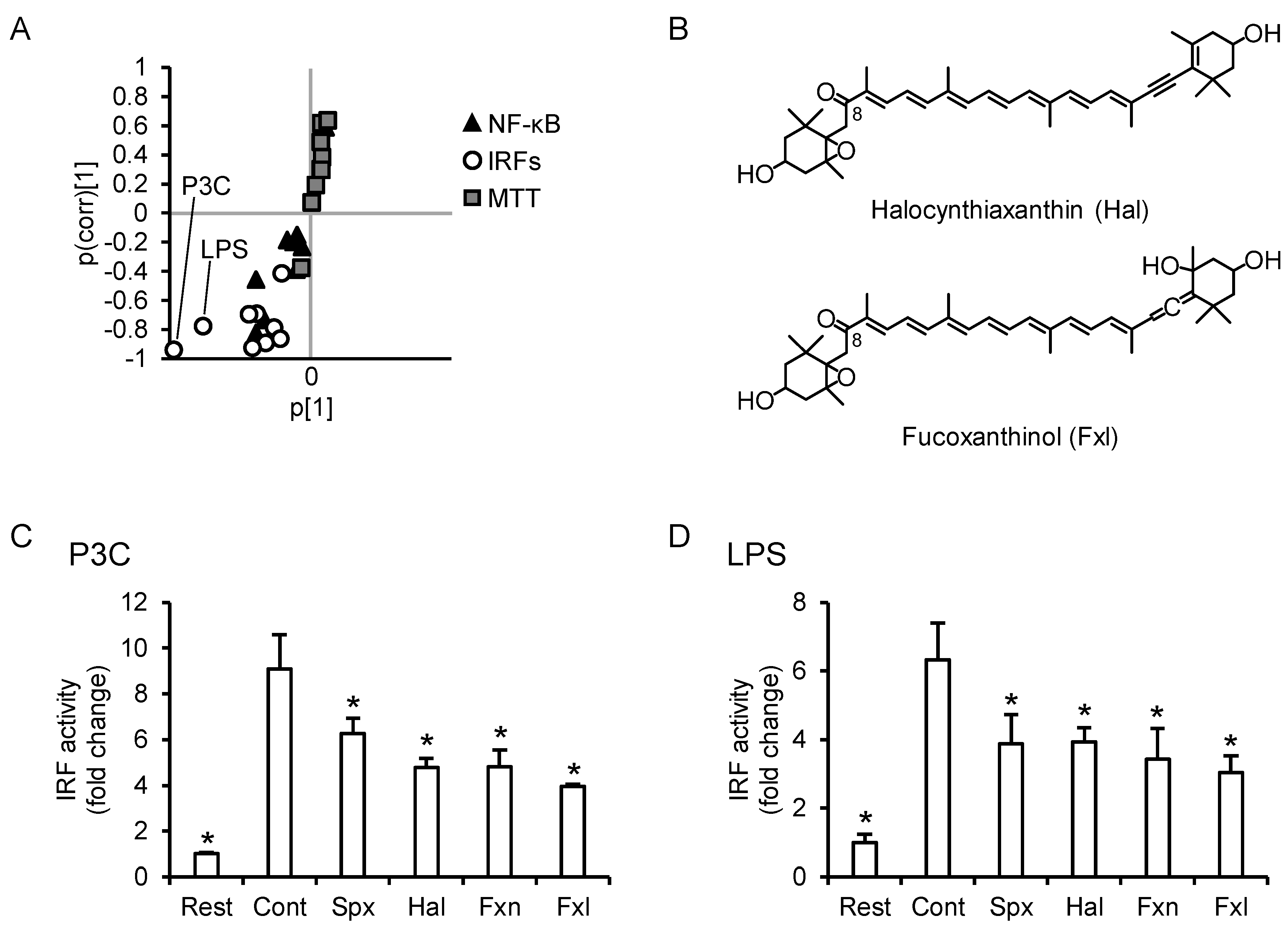
3.5. PCA Reveals Differences between the Anti-Inflammatory Profiles of the Carotenoids
3.6. Cytotoxicity and Cellular Accumulation Cannot Explain the Results of PCA
3.7. Increase in Intracellular Retinoid Levels Is Important for the Effects of β-Carotene and Echinenone
3.8. C8-Keto Carotenoids Inhibit P3C- and LPS-Induced IRF Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamagishi, S.; Matsui, T. Role of hyperglycemia-induced advanced glycation end product (AGE) accumulation in atherosclerosis. Ann. Vasc. Dis. 2018, 11, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khachik, F.; Spangler, C.J.; Smith, J.C.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, Quantification, and Relative Concentrations of Carotenoids and Their Metabolites in Human Milk and Serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids supplementation and inflammation: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.H.; Paul-Labrador, M.J.; Fan, J.; Shircore, A.M.; Bairey Merz, C.N.; Dwyer, K.M. Progression of carotid intima-media thickness and plasma antioxidants: The Los Angeles Atherosclerosis Study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sugiura, M.; Aoki, N. High β-carotene and β-cryptoxanthin are associated with low pulse wave velocity. Atherosclerosis 2006, 184, 363–369. [Google Scholar] [CrossRef]
- Zou, Z.; Xu, X.; Huang, Y.; Xiao, X.; Ma, L.; Sun, T.; Dong, P.; Wang, X.; Lin, X. High serum level of lutein may be protective against early atherosclerosis: The Beijing atherosclerosis study. Atherosclerosis 2011, 219, 789–793. [Google Scholar] [CrossRef]
- Manabe, Y.; Takii, Y.; Sugawara, T. Siphonaxanthin, a carotenoid from green algae, suppresses advanced glycation end product-induced inflammatory responses. J. Nat. Med. 2020, 74, 127–134. [Google Scholar] [CrossRef]
- Manabe, Y.; Hirata, T.; Sugawara, T. Inhibitory effect of carotenoids on ligand-induced lipid raft translocation of immunoreceptors. J. Oleo Sci. 2019, 68, 149–158. [Google Scholar] [CrossRef]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Maoka, T.; Miyashita, K. Suppressive effects of alloxanthin and diatoxanthin from Halocynthia roretzi on LPS-induced expression of pro-inflammatory genes in RAW264.7 cells. J. Oleo Sci. 2008, 57, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Murakami, A.; Nakashima, M.; Koshiba, T.; Maoka, T.; Nishino, H.; Yano, M.; Sumida, T.; Kyung Kim, O.; Koshimizu, K.; Ohigashi, H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leukocytes. Cancer Lett. 2000, 149, 115–123. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Goldstein, R.S.; Gallowitsch-Puerta, M.; Yang, L.; Valdés-Ferrer, S.I.; Patel, N.B.; Chavan, S.; Al-Abed, Y.; Yang, H.; Tracey, K.J. The selective α7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol. Med. 2009, 15, 195–202. [Google Scholar] [CrossRef]
- Sugawara, T.; Kushiro, M.; Zhang, H.; Nara, E.; Ono, H.; Nagao, A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001, 131, 2921–2927. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Manabe, Y.; Hirata, T.; Sugawara, T. Suppressive effects of carotenoids on the antigen-induced degranulation in RBL-2H3 rat basophilic leukemia cells. J. Oleo Sci. 2014, 63, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef] [Green Version]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Volume 1B: Spectroscopy. In Carotenoids; Birkhäuser Verlag: Basel, Switzerland, 1995. [Google Scholar]
- Manabe, Y.; Ichihara, M.; Fukuda, K.; Tomonaga, N.; Li, Z.S.; Yamanashi, Y.; Suzuki, H.; Takada, T.; Matsuo, M.; Sugawara, T. Niemann-Pick C1-like 1 promotes intestinal absorption of siphonaxanthin. Lipids 2019, 54, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The green algal carotenoid siphonaxanthin inhibits adipogenesis in 3T3-L1 preadipocytes and the accumulation of lipids in white adipose tissue of KK-Aymice. J. Nutr. 2015, 145, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Li, Z.; Manabe, Y.; Kim, M.; Goto, T.; Kawada, T.; Sugawara, T. Siphonaxanthin, a Carotenoid from Green Algae, Inhibits Lipogenesis in Hepatocytes via the Suppression of Liver X Receptor α Activity. Lipids 2018, 53, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Yates, S.L.; Burgess, L.H.; Kocsis-Angle, J.; Antal, J.M.; Dority, M.D.; Embury, P.B.; Piotrkowski, A.M.; Brunden, K.R. Amyloid β and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J. Neurochem. 2000, 74, 1017–1025. [Google Scholar] [CrossRef]
- Udan, M.L.D.; Ajit, D.; Crouse, N.R.; Nichols, M.R. Toll-like receptors 2 and 4 mediate Aβ(1-42) activation of the innate immune response in a human monocytic cell line. J. Neurochem. 2008, 104, 524–533. [Google Scholar] [CrossRef]
- Thomas, L.D.; Bandara, S.; Parmar, V.M.; Srinivasagan, R.; Khadka, N.; Golczak, M.; Kiser, P.D.; von Lintig, J. The human mitochondrial enzyme BCO2 exhibits catalytic activity toward carotenoids and apocarotenoids. J. Biol. Chem. 2020, 295, 15553–15565. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Nitta, T.; Ichihara, M.; Maoka, T.; Sugawara, T. Siphonaxanthin, a carotenoid of green algae, is metabolically converted to three different dehydro-forms. unpublished.
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bationo, J.F.; Zeba, A.N.; Abbeddou, S.; Coulibaly, N.D.; Sombier, O.O.; Sheftel, J.; Bassole, I.H.N.; Barro, N.; Ouedraogo, J.B.; Tanumihardjo, S.A. Serum carotenoids reveal poor fruit and vegetable intake among schoolchildren in burkina faso. Nutrients 2018, 10, 1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Kiko, T.; Hatade, K.; Asai, A.; Kimura, F.; Sookwong, P.; Tsuduki, T.; Arai, H.; Miyazawa, T. Development of a high-performance liquid chromatography-based assay for carotenoids in human red blood cells: Application to clinical studies. Anal. Biochem. 2008, 381, 129–134. [Google Scholar] [CrossRef]
- Hartmann, D.; Thürmann, P.A.; Spitzer, V.; Schalch, W.; Manner, B.; Cohn, W. Plasma kinetics of zeaxanthin and 3′-dehydro-lutein after multiple oral doses of synthetic zeaxanthin. Am. J. Clin. Nutr. 2004, 79, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Landrum, J.T.; Bone, R.A.; Joa, H.; Kilburn, M.D.; Moore, L.L.; Sprague, K.E. A one year study of the macular pigment: The effect of 140 days of a lutein supplement. Exp. Eye Res. 1997, 65, 57–62. [Google Scholar] [CrossRef]
- Østerlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Harrison, E.H.; Kopec, R.E. Enzymology of vertebrate carotenoid oxygenases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158653. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Andersson, S. Biochemical properties of purified recombinant human β-carotene 15,15′-monooxygenase. J. Biol. Chem. 2002, 277, 23942–23948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.F.; Lee, C.Y.; Lai, L.C.; Tsai, M.H.; Lu, T.P.; Chuang, E.Y. CellExpress: A comprehensive microarray-based cancer cell line and clinical sample gene expression analysis online system. Database 2018, 2018, bax101. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.N.T.; Leclerc, D.; Lévesque, N.; Deng, L.; Rozen, R. β,β-Carotene 15,15′-monooxygenase and its substrate β-carotene modulate migration and invasion in colorectal carcinoma cells. Am. J. Clin. Nutr. 2013, 98, 413–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Ma, Y.; Ross, A.C. Opposing cytokine-specific effects of all trans-retinoic acid on the activation and expression of signal transducer and activator of transcription (STAT)-1 in THP-1 cells. Immunology 2002, 107, 199–208. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [Green Version]
- Oosenbrug, T.; van de Graaff, M.J.; Haks, M.C.; van Kasteren, S.; Ressing, M.E. An alternative model for type I interferon induction downstream of human TLR2. J. Biol. Chem. 2020, 295, 14325–14342. [Google Scholar] [CrossRef]
- Triantafilou, M.; Gamper, F.G.J.; Haston, R.M.; Mouratis, M.A.; Morath, S.; Hartung, T.; Triantafilou, K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 2006, 281, 31002–31011. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, Y.; Taguchi, C.; Kishimoto, Y.; Kondo, K. Japanese carotenoid database with α- And β-carotene, β-cryptoxanthin, lutein, zeaxanthin, lycopene, and fucoxanthin and intake in adult women. Int. J. Vitam. Nutr. Res. 2021, 7, 1–12. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manabe, Y.; Tomonaga, N.; Maoka, T.; Sugawara, T. Multivariate Analysis Reveals That Unsubstituted β-Ring and C8-Keto Structures Are Important Factors for Anti-Inflammatory Activity of Carotenoids. Nutrients 2021, 13, 3699. https://doi.org/10.3390/nu13113699
Manabe Y, Tomonaga N, Maoka T, Sugawara T. Multivariate Analysis Reveals That Unsubstituted β-Ring and C8-Keto Structures Are Important Factors for Anti-Inflammatory Activity of Carotenoids. Nutrients. 2021; 13(11):3699. https://doi.org/10.3390/nu13113699
Chicago/Turabian StyleManabe, Yuki, Nami Tomonaga, Takashi Maoka, and Tatsuya Sugawara. 2021. "Multivariate Analysis Reveals That Unsubstituted β-Ring and C8-Keto Structures Are Important Factors for Anti-Inflammatory Activity of Carotenoids" Nutrients 13, no. 11: 3699. https://doi.org/10.3390/nu13113699
APA StyleManabe, Y., Tomonaga, N., Maoka, T., & Sugawara, T. (2021). Multivariate Analysis Reveals That Unsubstituted β-Ring and C8-Keto Structures Are Important Factors for Anti-Inflammatory Activity of Carotenoids. Nutrients, 13(11), 3699. https://doi.org/10.3390/nu13113699





