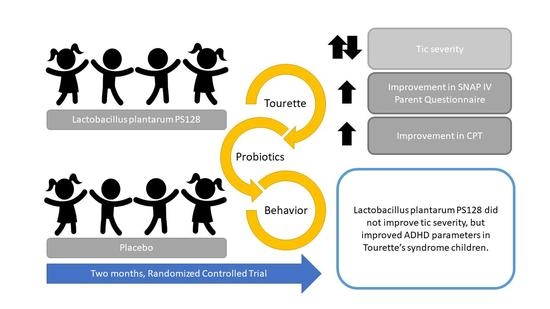
Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Randomization and Blinding
2.4. Intervention
2.5. Outcomes
2.5.1. Tic Severity—Yale Global Tic Severity Scale (YGTSS)
2.5.2. ADHD—SNAP-IV Parent and Teacher Evaluation
2.5.3. ADHD—Conners’ Continuous Performance Test II (CPT-2)
2.5.4. OCD—Obsessive-Compulsive Inventory-Revised (OCI-R)
2.5.5. Migraine—Migraine Disability Assessment (MIDAS)
2.5.6. Depression—Children’s Depression Inventory, Taiwan Version (CDI-TW)
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. Participant Flow and Recruitment
3.2. Baseline Data
3.3. Outcomes
3.3.1. YGTSS
3.3.2. SNAP-IV
3.3.3. CPT
3.3.4. CDI
3.3.5. OCI-R
3.3.6. MIDAS
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirschtritt, M.E.; Lee, P.C.; Pauls, D.L.; Dion, Y.; Grados, M.A.; Illmann, C.; King, R.A.; Sandor, P.; McMahon, W.M.; Lyon, G.J.; et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry 2015, 72, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Maia, T.V.; Marsh, R.; Colibazzi, T.; Gerber, A.; Peterson, B.S. The neural circuits that generate tics in Tourette’s syndrome. Am. J. Psychiatry 2011, 168, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Plessen, K.J.; Bansal, R.; Peterson, B.S. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J. Psychosom. Res. 2009, 67, 559–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, H.S.; Szymanski, S.; Giuliano, J.; Yokoi, F.; Dogan, A.S.; Brasic, J.R.; Zhou, Y.; Grace, A.A.; Wong, D.F. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am. J. Psychiatry 2002, 159, 1329–1336. [Google Scholar] [CrossRef]
- Leckman, J.F.; Goodman, W.K.; Anderson, G.M.; Riddle, M.A.; Chappell, P.B.; McSwiggan-Hardin, M.T.; McDougle, C.J.; Scahill, L.D.; Ort, S.I.; Pauls, D.L.; et al. Cerebrospinal fluid biogenic amines in obsessive compulsive disorder, Tourette’s syndrome, and healthy controls. Neuropsychopharmacology 1995, 12, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Chou, I.C.; Tsai, C.H.; Wang, T.R.; Li, T.C.; Tsai, F.J. Dopamine receptor D2 gene polymorphisms are associated in Taiwanese children with Tourette syndrome. Pediatr. Neurol. 2005, 33, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Paschou, P.; Fernandez, T.V.; Sharp, F.; Heiman, G.A.; Hoekstra, P.J. Genetic susceptibility and neurotransmitters in Tourette syndrome. Int. Rev. Neurobiol. 2013, 112, 155–177. [Google Scholar]
- Jankovic, J. Therapeutic Developments for Tics and Myoclonus. Mov. Disord. 2015, 30, 1566–1573. [Google Scholar] [CrossRef]
- Robertson, M.M. The Gilles de la Tourette syndrome: The current status. Arch. Dis. Child. Educ. Pract. Ed. 2012, 97, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liong, M.T.; Chung, Y.E.; Huang, H.Y.; Peng, W.S.; Cheng, Y.F.; Lin, Y.S.; Wu, Y.Y.; Tsai, Y.C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, D.J.; Doerr, H.M.; Grzelak, A.K.; Busi, S.B.; Jasarevic, E.; Ericsson, A.C.; Bryda, E.C. Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Sci. Rep. 2016, 6, 33726. [Google Scholar] [CrossRef] [PubMed]
- Cohen Kadosh, K.; Basso, M.; Knytl, P.; Johnstone, N.; Lau, J.Y.F.; Gibson, G.R. Psychobiotic interventions for anxiety in young people: A systematic review and meta-analysis, with youth consultation. Transl. Psychiatry 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Quero, C.D.; Manonelles, P.; Fernández, M.; Abellán-Aynés, O.; López-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Gálvez, I.; Ortega, E. Differential Health Effects on Inflammatory, Immunological and Stress Parameters in Professional Soccer Players and Sedentary Individuals after Consuming a Synbiotic. A Triple-Blinded, Randomized, Placebo-Controlled Pilot Study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef] [PubMed]
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.-F.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in Multiple Sclerosis; Where Are We, What We Know and Do Not Know. Brain Sci. 2020, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinson’s Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Ligezka, A.N.; Sonmez, A.I.; Corral-Frias, M.P.; Golebiowski, R.; Lynch, B.; Croarkin, P.E.; Romanowicz, M. A systematic review of microbiome changes and impact of probiotic supplementation in children and adolescents with neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110187. [Google Scholar] [CrossRef]
- Warner, B.B. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr. Res. 2019, 85, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.H.; Chuang, H.L.; Huang, Y.T.; Wu, C.C.; Chou, G.T.; Wang, S.; Tsai, Y.C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298 Pt B, 202–209. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), t.e. In Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Chao, S.H.; Wu, R.J.; Watanabe, K.; Tsai, Y.C. Diversity of lactic acid bacteria in suan-tsai and fu-tsai, traditional fermented mustard products of Taiwan. Int. J. Food Microbiol. 2009, 135, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Yang, C.H.; Lin, C.T.; Li, S.W.; Cheng, W.S.; Jiang, Y.P.; Wu, C.C.; Chang, C.H.; Tsai, Y.C. Genome architecture of Lactobacillus plantarum PS128, a probiotic strain with potential immunomodulatory activity. Gut Pathog. 2015, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Walkup, J.T.; Woods, D.W.; Peterson, A.; Piacentini, J.; Wilhelm, S.; Katsovich, L.; McGuire, J.F.; Dziura, J.; Scahill, L. Detecting a clinically meaningful change in tic severity in Tourette syndrome: A comparison of three methods. Contemp. Clin. Trials 2013, 36, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child. Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Gau, S.S.; Shang, C.Y.; Liu, S.K.; Lin, C.H.; Swanson, J.M.; Liu, Y.C.; Tu, C.L. Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, version IV scale—parent form. Int. J. Methods Psychiatr. Res. 2008, 17, 35–44. [Google Scholar] [CrossRef]
- Gau, S.S.-F.; Lin, C.-H.; Hu, F.-C.; Shang, C.-Y.; Swanson, J.M.; Liu, Y.-C.; Liu, S.-K. Psychometric Properties of the Chinese Version of the Swanson, Nolan, and Pelham, Version IV Scale-Teacher Form. J. Pediatr. Psychol. 2008, 34, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Bennett, D.A. How can I deal with missing data in my study? Aust. N. Z. J. Public Health 2001, 25, 464–469. [Google Scholar] [CrossRef]
- Conners, C.K.; Sitarenios, G. Conners’ Continuous Performance Test (CPT). In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 681–683. [Google Scholar]
- Foa, E.B.; Huppert, J.D.; Leiberg, S.; Langner, R.; Kichic, R.; Hajcak, G.; Salkovskis, P.M. The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychol. Assess. 2002, 14, 485–496. [Google Scholar] [CrossRef]
- Hon, S.K.H.; Siu, B.W.M.; Cheng, C.W.; Wong, W.C.W.; Foa, E.B. Validation of the Chinese Version of Obsessive-Compulsive Inventory-Revised. East Asian Arch. Psychiatry 2019, 29, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Hung, P.H.; Fuh, J.L.; Wang, S.J. Validity, reliability and application of the taiwan version of the migraine disability assessment questionnaire. J. Formos. Med. Assoc. 2006, 105, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.U. Children’s Depression Inventory—Taiwan Version Manual; Psychological Publishing Co., Ltd.: Taipei, Taiwan, 2008. (In Chinese) [Google Scholar]
- Roessner, V.; Schoenefeld, K.; Buse, J.; Bender, S.; Ehrlich, S.; Munchau, A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology 2013, 68, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.S.; Hahn, I.H.; Moran, T.H. Abnormal dopamine uptake sites in postmortem striatum from patients with Tourette’s syndrome. Ann. Neurol. 1991, 30, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Noudoost, B.; Moore, T. The role of neuromodulators in selective attention. Trends Cogn. Sci. 2011, 15, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Oades, R.D. The Roles of Norepinephrine and Serotonin in Attention Deficit Hyperactivity Disorder. In Attention Deficit Hyperactivity Disorder: From Genes to Patients; Gozal, D., Molfese, D.L., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 97–130. [Google Scholar]
- Arnsten, A.F. The use of α-2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev. Neurother. 2010, 10, 1595–1605. [Google Scholar] [CrossRef] [Green Version]
- Lissemore, J.I.; Sookman, D.; Gravel, P.; Berney, A.; Barsoum, A.; Diksic, M.; Nordahl, T.E.; Pinard, G.; Sibon, I.; Cottraux, J.; et al. Brain serotonin synthesis capacity in obsessive-compulsive disorder: Effects of cognitive behavioral therapy and sertraline. Transl. Psychiatry 2018, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloch, M.H.; Peterson, B.S.; Scahill, L.; Otka, J.; Katsovich, L.; Zhang, H.; Leckman, J.F. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch. Pediatr. Adolesc. Med. 2006, 160, 65–69. [Google Scholar] [CrossRef] [Green Version]

| Variable | PS128 (N = 28) | Placebo (N = 29) | |||
|---|---|---|---|---|---|
| N | Median (Q1, Q3) or n (%) | N | Median (Q1, Q3) or n (%) | p-Value | |
| Age | 28 | 9.3 (8.5, 10.3) | 29 | 10.4 (8.7, 12.2) | 0.139 |
| Gender | |||||
| Male | 28 | 24 (85.71) | 29 | 24 (82.76) | 0.999 a |
| Female | 4 (14.29) | 5 (17.24) | |||
| IQ | 28 | 102.0 (95.0, 113.0) | 29 | 103.0 (96.0, 108.0) | 0.546 |
| Medication use | 0.346 a | ||||
| No | 28 | 11 (39.30) | 29 | 15 (51.70) | |
| Yes | 17 (60.70) | 14 (48.30) | |||
| Medication type | |||||
| Aripiprazole | 28 | 16 (57.14) | 29 | 12 (41.38) | 0.234 a |
| Biperiden | 28 | 1 (3.57) | 29 | 0 (0) | 0.491 a |
| Clonazepam | 28 | 4 (14.29) | 29 | 1 (3.45) | 0.194 a |
| Clonidine | 28 | 2 (7.14) | 29 | 2 (6.90) | 0.999 a |
| Methylphenidate | 28 | 1 (3.57) | 29 | 0 (0) | 0.491 a |
| Risperidone | 28 | 1 (3.57) | 29 | 0 (0) | 0.491 a |
| Sulpride | 28 | 0 (0) | 29 | 1 (3.45) | 0.999 a |
| Other | 28 | 1 (3.57) | 29 | 1 (3.45) | 0.999 a |
| YGTSS | |||||
| YGTSS Total | 28 | 18.0 (13.0, 22.0) | 29 | 19.0 (13.0, 26.0) | 0.460 |
| YGTSS Global | 28 | 27.0 (18.5, 39.5) | 29 | 30.0 (19.0, 46.0) | 0.634 |
| SNAPIV | |||||
| Teacher | |||||
| Total | 23 | 11.0 (6.0, 20.0) | 28 | 14.5 (7.7, 26.5) | 0.281 |
| Inattention | 23 | 8.0 (2.0, 12.0) | 28 | 7.0 (5.0, 13.5) | 0.540 |
| Hyperactivity/Impulsivity | 23 | 3.0 (1.0, 5.0) | 28 | 3.0 (1.5, 7.0) | 0.299 |
| Oppositional behavior | 23 | 1.0 (0.0, 3.0) | 28 | 3.0 (0.5, 4.5) | 0.160 |
| Parent | |||||
| Total | 25 | 24.0 (22.0, 32.0) | 28 | 26.5 (18.0, 38.5) | 0.187 b |
| Inattention | 25 | 10.0 (7.0, 14.0) | 28 | 10.0 (6.0, 15.5) | 0.845 |
| Hyperactivity/Impulsivity | 25 | 6.0 (4.5, 11.0) | 28 | 9.0 (4.5, 11.5) | 0.333 b |
| Oppositional behavior | 25 | 6.0 (4.0, 8.0) | 28 | 9.5 (5.0, 12.0) | 0.039 * |
| CPT | |||||
| Omissions T-score | 28 | 45.8 (43.3, 51.1) | 24 | 45.5 (43.6, 50.9) | 0.862 |
| Commissions | 28 | 48.4 (36.8, 53.3) | 24 | 49.3 (41.3, 59.0) | 0.326 |
| Hit RT | 28 | 49.4 (41.5, 60.7) | 24 | 48.5 (38.6, 53.3) | 0.159 b |
| Hit RT Std. Error | 28 | 49.1 (42.6, 54.5) | 24 | 45.7 (37.2, 55.5) | 0.344 |
| Variability | 28 | 48.0 (41.7, 53.7) | 24 | 45.5 (39.2, 54.7) | 0.611 b |
| Detectability (d’) | 28 | 49.9 (43.8, 55.4) | 24 | 49.4 (44.2, 58.4) | 0.452 b |
| Response Style (B) | 28 | 50.5 (45.2, 55.8) | 24 | 46.4 (45.4, 52.5) | 0.235 |
| Perseverations | 28 | 46.3 (43.8, 51.4) | 24 | 48.0 (45.6, 55.7) | 0.198 |
| OCI-R | |||||
| score | 28 | 9.0 (5.5, 12.0) | 29 | 14.0 (5.0, 19.0) | 0.307 |
| ≥21 patient number | 28 | 4 (14.29) | 29 | 6 (20.69) | 0.730 a |
| CDI | 28 | 7.5 (4.5, 13.5) | 29 | 12.0 (5.0, 17.0) | 0.179 |
| MIDAS | 28 | 0.0 (0.0, 0.0) | 29 | 0.0 (0.0, 0.0) | 0.813 |
| N | PS128 (N = 28) | p-Value | N | Placebo (N = 29) | p-Value | |
|---|---|---|---|---|---|---|
| 1 month and baseline | ||||||
| YGTSS Total | 28 | −2.21 ± 4.71 | 0.019 * | 29 | −3.10 ± 7.12 | 0.026 * |
| YGTSS Global | 28 | −7.43 ± 16.77 | 0.027 * | 29 | −5.17 ± 13.91 | 0.055 |
| 2 months and 1 month | ||||||
| YGTSS Total | 27 | 0.11 ± 5.25 | 0.915 | 27 | −0.14 ± 6.06 | 0.903 |
| YGTSS Global | 26 | 2.82 ± 12.24 | 0.233 | 27 | −2.38 ± 13.09 | 0.336 |
| 2 months and baseline | ||||||
| YGTSS Total | 28 | −2.11 ± 5.32 | 0.046 * | 29 | −3.24 ± 5.79 | 0.005 * |
| YGTSS Global | 28 | −4.61 ± 16.68 | 0.156 | 29 | −7.55 ± 16.37 | 0.019 * |
| N | PS128 (N = 28) | p-Value | N | Placebo (N = 29) | p-Value | |
|---|---|---|---|---|---|---|
| 1 month and baseline | ||||||
| %change of YGTSS Total | 28 | −16.31 ± 29.36 | 0.007 * | 29 | −14.93 ± 39.67 | 0.052 * |
| %change of YGTSS Global | 28 | −19.43 ± 46.74 | 0.037 * | 29 | −14.02 ± 41.40 | 0.079 |
| 2 months and 1 month | ||||||
| %change of YGTSS Total | 27 | 7.97 ± 59.65 | 0.494 | 27 | 7.79 ± 69.69 | 0.566 |
| %change of YGTSS Global | 26 | 22.58 ± 100.50 | 0.263 | 27 | −13.47 ± 33.26 | 0.045 * |
| 2 months and baseline | ||||||
| %change of YGTSS Total | 28 | −15.02 ± 37.49 | 0.043 * | 29 | −13.66 ± 28.52 | 0.015 * |
| %change of YGTSS Global | 28 | −14.20 ± 59.21 | 0.215 | 29 | −20.57 ± 35.78 | 0.004 * |
| Variable | PS128 (N = 21) | p-Value | Placebo (N = 20) | p-Value |
|---|---|---|---|---|
| Teacher | ||||
| Total | 1.00 ± 7.62 | 0.554 | −2.75 ± 9.79 | 0.224 |
| Inattention | 0.86 ± 4.05 | 0.344 | −1.45 ± 4.49 | 0.165 |
| Hyperactivity/Impulsivity | 0.48 ± 2.91 | 0.462 | −0.20 ± 3.86 | 0.819 |
| Oppositional behavior | 0.33 ± 2.18 | 0.491 | −1.10 ± 3.28 | 0.150 |
| PS128 (N = 25) | p-Value | Placebo (N = 26) | p-Value | |
| Parent | ||||
| Total | −3.88 ± 7.88 | 0.021 * | −3.69 ± 10.12 | 0.075 |
| Inattention | −2.04 ± 3.27 | 0.005 * | −1.27 ± 3.21 | 0.054 |
| Hyperactivity/Impulsivity | −1.76 ± 3.74 | 0.027 * | −1.12 ± 4.09 | 0.177 |
| Oppositional behavior | −0.08 ± 3.30 | 0.905 | −1.31 ± 4.90 | 0.186 |
| Variable | PS128 (N = 28) | p-Value | Placebo (N = 24) | p-Value |
|---|---|---|---|---|
| Omissions T-score | 0.91 ± 11.05 | 0.667 | 0.91 ± 10.69 | 0.681 |
| Commissions | −4.25 ± 9.22 | 0.022 * | −3.02 ± 8.12 | 0.082 |
| Hit RT | 2.09 ± 6.99 | 0.126 | 2.61 ± 11.62 | 0.282 |
| Hit RT Std. Error | 2.10 ± 6.56 | 0.103 | 1.28 ± 8.50 | 0.468 |
| Variability | 1.84 ± 8.39 | 0.257 | 1.73 ± 9.62 | 0.389 |
| Detectability (d’) | −4.71 ± 10.02 | 0.019 * | −4.73 ± 13.29 | 0.094 |
| Response Style (B) | 1.60 ± 13.93 | 0.548 | 2.75 ± 13.50 | 0.329 |
| Perseverations | 0.56 ± 19.10 | 0.879 | −1.38 ± 24.00 | 0.781 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Wong, L.-C.; Hsu, C.-J.; Yang, C.-W.; Tsai, Y.-C.; Cheng, F.-S.; Hu, H.-Y.; Lee, W.-T. Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome. Nutrients 2021, 13, 3698. https://doi.org/10.3390/nu13113698
Wu C-C, Wong L-C, Hsu C-J, Yang C-W, Tsai Y-C, Cheng F-S, Hu H-Y, Lee W-T. Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome. Nutrients. 2021; 13(11):3698. https://doi.org/10.3390/nu13113698
Chicago/Turabian StyleWu, Chang-Chun, Lee-Chin Wong, Chia-Jui Hsu, Chianne-Wen Yang, Ying-Chieh Tsai, Feng-Shiang Cheng, Hsiao-Yun Hu, and Wang-Tso Lee. 2021. "Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome" Nutrients 13, no. 11: 3698. https://doi.org/10.3390/nu13113698
APA StyleWu, C.-C., Wong, L.-C., Hsu, C.-J., Yang, C.-W., Tsai, Y.-C., Cheng, F.-S., Hu, H.-Y., & Lee, W.-T. (2021). Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome. Nutrients, 13(11), 3698. https://doi.org/10.3390/nu13113698