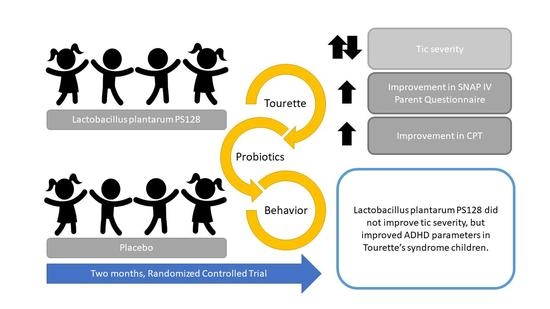
Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Randomization and Blinding
2.4. Intervention
2.5. Outcomes
2.5.1. Tic Severity—Yale Global Tic Severity Scale (YGTSS)
2.5.2. ADHD—SNAP-IV Parent and Teacher Evaluation
2.5.3. ADHD—Conners’ Continuous Performance Test II (CPT-2)
2.5.4. OCD—Obsessive-Compulsive Inventory-Revised (OCI-R)
2.5.5. Migraine—Migraine Disability Assessment (MIDAS)
2.5.6. Depression—Children’s Depression Inventory, Taiwan Version (CDI-TW)
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. Participant Flow and Recruitment
3.2. Baseline Data
3.3. Outcomes
3.3.1. YGTSS
3.3.2. SNAP-IV
3.3.3. CPT
3.3.4. CDI
3.3.5. OCI-R
3.3.6. MIDAS
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirschtritt, M.E.; Lee, P.C.; Pauls, D.L.; Dion, Y.; Grados, M.A.; Illmann, C.; King, R.A.; Sandor, P.; McMahon, W.M.; Lyon, G.J.; et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry 2015, 72, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Maia, T.V.; Marsh, R.; Colibazzi, T.; Gerber, A.; Peterson, B.S. The neural circuits that generate tics in Tourette’s syndrome. Am. J. Psychiatry 2011, 168, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Plessen, K.J.; Bansal, R.; Peterson, B.S. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J. Psychosom. Res. 2009, 67, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.S.; Szymanski, S.; Giuliano, J.; Yokoi, F.; Dogan, A.S.; Brasic, J.R.; Zhou, Y.; Grace, A.A.; Wong, D.F. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am. J. Psychiatry 2002, 159, 1329–1336. [Google Scholar] [CrossRef]
- Leckman, J.F.; Goodman, W.K.; Anderson, G.M.; Riddle, M.A.; Chappell, P.B.; McSwiggan-Hardin, M.T.; McDougle, C.J.; Scahill, L.D.; Ort, S.I.; Pauls, D.L.; et al. Cerebrospinal fluid biogenic amines in obsessive compulsive disorder, Tourette’s syndrome, and healthy controls. Neuropsychopharmacology 1995, 12, 73–86. [Google Scholar] [CrossRef][Green Version]
- Lee, C.C.; Chou, I.C.; Tsai, C.H.; Wang, T.R.; Li, T.C.; Tsai, F.J. Dopamine receptor D2 gene polymorphisms are associated in Taiwanese children with Tourette syndrome. Pediatr. Neurol. 2005, 33, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Paschou, P.; Fernandez, T.V.; Sharp, F.; Heiman, G.A.; Hoekstra, P.J. Genetic susceptibility and neurotransmitters in Tourette syndrome. Int. Rev. Neurobiol. 2013, 112, 155–177. [Google Scholar]
- Jankovic, J. Therapeutic Developments for Tics and Myoclonus. Mov. Disord. 2015, 30, 1566–1573. [Google Scholar] [CrossRef]
- Robertson, M.M. The Gilles de la Tourette syndrome: The current status. Arch. Dis. Child. Educ. Pract. Ed. 2012, 97, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liong, M.T.; Chung, Y.E.; Huang, H.Y.; Peng, W.S.; Cheng, Y.F.; Lin, Y.S.; Wu, Y.Y.; Tsai, Y.C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.J.; Doerr, H.M.; Grzelak, A.K.; Busi, S.B.; Jasarevic, E.; Ericsson, A.C.; Bryda, E.C. Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Sci. Rep. 2016, 6, 33726. [Google Scholar] [CrossRef] [PubMed]
- Cohen Kadosh, K.; Basso, M.; Knytl, P.; Johnstone, N.; Lau, J.Y.F.; Gibson, G.R. Psychobiotic interventions for anxiety in young people: A systematic review and meta-analysis, with youth consultation. Transl. Psychiatry 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Quero, C.D.; Manonelles, P.; Fernández, M.; Abellán-Aynés, O.; López-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Gálvez, I.; Ortega, E. Differential Health Effects on Inflammatory, Immunological and Stress Parameters in Professional Soccer Players and Sedentary Individuals after Consuming a Synbiotic. A Triple-Blinded, Randomized, Placebo-Controlled Pilot Study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef] [PubMed]
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.-F.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in Multiple Sclerosis; Where Are We, What We Know and Do Not Know. Brain Sci. 2020, 10, 234. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinson’s Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Ligezka, A.N.; Sonmez, A.I.; Corral-Frias, M.P.; Golebiowski, R.; Lynch, B.; Croarkin, P.E.; Romanowicz, M. A systematic review of microbiome changes and impact of probiotic supplementation in children and adolescents with neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110187. [Google Scholar] [CrossRef]
- Warner, B.B. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr. Res. 2019, 85, 216–224. [Google Scholar] [CrossRef]
- Liu, W.H.; Chuang, H.L.; Huang, Y.T.; Wu, C.C.; Chou, G.T.; Wang, S.; Tsai, Y.C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298 Pt B, 202–209. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), t.e. In Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Chao, S.H.; Wu, R.J.; Watanabe, K.; Tsai, Y.C. Diversity of lactic acid bacteria in suan-tsai and fu-tsai, traditional fermented mustard products of Taiwan. Int. J. Food Microbiol. 2009, 135, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Yang, C.H.; Lin, C.T.; Li, S.W.; Cheng, W.S.; Jiang, Y.P.; Wu, C.C.; Chang, C.H.; Tsai, Y.C. Genome architecture of Lactobacillus plantarum PS128, a probiotic strain with potential immunomodulatory activity. Gut Pathog. 2015, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Walkup, J.T.; Woods, D.W.; Peterson, A.; Piacentini, J.; Wilhelm, S.; Katsovich, L.; McGuire, J.F.; Dziura, J.; Scahill, L. Detecting a clinically meaningful change in tic severity in Tourette syndrome: A comparison of three methods. Contemp. Clin. Trials 2013, 36, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child. Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Gau, S.S.; Shang, C.Y.; Liu, S.K.; Lin, C.H.; Swanson, J.M.; Liu, Y.C.; Tu, C.L. Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, version IV scale—parent form. Int. J. Methods Psychiatr. Res. 2008, 17, 35–44. [Google Scholar] [CrossRef]
- Gau, S.S.-F.; Lin, C.-H.; Hu, F.-C.; Shang, C.-Y.; Swanson, J.M.; Liu, Y.-C.; Liu, S.-K. Psychometric Properties of the Chinese Version of the Swanson, Nolan, and Pelham, Version IV Scale-Teacher Form. J. Pediatr. Psychol. 2008, 34, 850–861. [Google Scholar] [CrossRef]
- Bennett, D.A. How can I deal with missing data in my study? Aust. N. Z. J. Public Health 2001, 25, 464–469. [Google Scholar] [CrossRef]
- Conners, C.K.; Sitarenios, G. Conners’ Continuous Performance Test (CPT). In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 681–683. [Google Scholar]
- Foa, E.B.; Huppert, J.D.; Leiberg, S.; Langner, R.; Kichic, R.; Hajcak, G.; Salkovskis, P.M. The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychol. Assess. 2002, 14, 485–496. [Google Scholar] [CrossRef]
- Hon, S.K.H.; Siu, B.W.M.; Cheng, C.W.; Wong, W.C.W.; Foa, E.B. Validation of the Chinese Version of Obsessive-Compulsive Inventory-Revised. East Asian Arch. Psychiatry 2019, 29, 103–111. [Google Scholar] [CrossRef]
- Hung, P.H.; Fuh, J.L.; Wang, S.J. Validity, reliability and application of the taiwan version of the migraine disability assessment questionnaire. J. Formos. Med. Assoc. 2006, 105, 563–568. [Google Scholar] [CrossRef]
- Chen, S.U. Children’s Depression Inventory—Taiwan Version Manual; Psychological Publishing Co., Ltd.: Taipei, Taiwan, 2008. (In Chinese) [Google Scholar]
- Roessner, V.; Schoenefeld, K.; Buse, J.; Bender, S.; Ehrlich, S.; Munchau, A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology 2013, 68, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.S.; Hahn, I.H.; Moran, T.H. Abnormal dopamine uptake sites in postmortem striatum from patients with Tourette’s syndrome. Ann. Neurol. 1991, 30, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Noudoost, B.; Moore, T. The role of neuromodulators in selective attention. Trends Cogn. Sci. 2011, 15, 585–591. [Google Scholar] [CrossRef]
- Oades, R.D. The Roles of Norepinephrine and Serotonin in Attention Deficit Hyperactivity Disorder. In Attention Deficit Hyperactivity Disorder: From Genes to Patients; Gozal, D., Molfese, D.L., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 97–130. [Google Scholar]
- Arnsten, A.F. The use of α-2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev. Neurother. 2010, 10, 1595–1605. [Google Scholar] [CrossRef]
- Lissemore, J.I.; Sookman, D.; Gravel, P.; Berney, A.; Barsoum, A.; Diksic, M.; Nordahl, T.E.; Pinard, G.; Sibon, I.; Cottraux, J.; et al. Brain serotonin synthesis capacity in obsessive-compulsive disorder: Effects of cognitive behavioral therapy and sertraline. Transl. Psychiatry 2018, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.H.; Peterson, B.S.; Scahill, L.; Otka, J.; Katsovich, L.; Zhang, H.; Leckman, J.F. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch. Pediatr. Adolesc. Med. 2006, 160, 65–69. [Google Scholar] [CrossRef]

| Variable | PS128 (N = 28) | Placebo (N = 29) | |||
|---|---|---|---|---|---|
| N | Median (Q1, Q3) or n (%) | N | Median (Q1, Q3) or n (%) | p-Value | |
| Age | 28 | 9.3 (8.5, 10.3) | 29 | 10.4 (8.7, 12.2) | 0.139 |
| Gender | |||||
| Male | 28 | 24 (85.71) | 29 | 24 (82.76) | 0.999 a |
| Female | 4 (14.29) | 5 (17.24) | |||
| IQ | 28 | 102.0 (95.0, 113.0) | 29 | 103.0 (96.0, 108.0) | 0.546 |
| Medication use | 0.346 a | ||||
| No | 28 | 11 (39.30) | 29 | 15 (51.70) | |
| Yes | 17 (60.70) | 14 (48.30) | |||
| Medication type | |||||
| Aripiprazole | 28 | 16 (57.14) | 29 | 12 (41.38) | 0.234 a |
| Biperiden | 28 | 1 (3.57) | 29 | 0 (0) | 0.491 a |
| Clonazepam | 28 | 4 (14.29) | 29 | 1 (3.45) | 0.194 a |
| Clonidine | 28 | 2 (7.14) | 29 | 2 (6.90) | 0.999 a |
| Methylphenidate | 28 | 1 (3.57) | 29 | 0 (0) | 0.491 a |
| Risperidone | 28 | 1 (3.57) | 29 | 0 (0) | 0.491 a |
| Sulpride | 28 | 0 (0) | 29 | 1 (3.45) | 0.999 a |
| Other | 28 | 1 (3.57) | 29 | 1 (3.45) | 0.999 a |
| YGTSS | |||||
| YGTSS Total | 28 | 18.0 (13.0, 22.0) | 29 | 19.0 (13.0, 26.0) | 0.460 |
| YGTSS Global | 28 | 27.0 (18.5, 39.5) | 29 | 30.0 (19.0, 46.0) | 0.634 |
| SNAPIV | |||||
| Teacher | |||||
| Total | 23 | 11.0 (6.0, 20.0) | 28 | 14.5 (7.7, 26.5) | 0.281 |
| Inattention | 23 | 8.0 (2.0, 12.0) | 28 | 7.0 (5.0, 13.5) | 0.540 |
| Hyperactivity/Impulsivity | 23 | 3.0 (1.0, 5.0) | 28 | 3.0 (1.5, 7.0) | 0.299 |
| Oppositional behavior | 23 | 1.0 (0.0, 3.0) | 28 | 3.0 (0.5, 4.5) | 0.160 |
| Parent | |||||
| Total | 25 | 24.0 (22.0, 32.0) | 28 | 26.5 (18.0, 38.5) | 0.187 b |
| Inattention | 25 | 10.0 (7.0, 14.0) | 28 | 10.0 (6.0, 15.5) | 0.845 |
| Hyperactivity/Impulsivity | 25 | 6.0 (4.5, 11.0) | 28 | 9.0 (4.5, 11.5) | 0.333 b |
| Oppositional behavior | 25 | 6.0 (4.0, 8.0) | 28 | 9.5 (5.0, 12.0) | 0.039 * |
| CPT | |||||
| Omissions T-score | 28 | 45.8 (43.3, 51.1) | 24 | 45.5 (43.6, 50.9) | 0.862 |
| Commissions | 28 | 48.4 (36.8, 53.3) | 24 | 49.3 (41.3, 59.0) | 0.326 |
| Hit RT | 28 | 49.4 (41.5, 60.7) | 24 | 48.5 (38.6, 53.3) | 0.159 b |
| Hit RT Std. Error | 28 | 49.1 (42.6, 54.5) | 24 | 45.7 (37.2, 55.5) | 0.344 |
| Variability | 28 | 48.0 (41.7, 53.7) | 24 | 45.5 (39.2, 54.7) | 0.611 b |
| Detectability (d’) | 28 | 49.9 (43.8, 55.4) | 24 | 49.4 (44.2, 58.4) | 0.452 b |
| Response Style (B) | 28 | 50.5 (45.2, 55.8) | 24 | 46.4 (45.4, 52.5) | 0.235 |
| Perseverations | 28 | 46.3 (43.8, 51.4) | 24 | 48.0 (45.6, 55.7) | 0.198 |
| OCI-R | |||||
| score | 28 | 9.0 (5.5, 12.0) | 29 | 14.0 (5.0, 19.0) | 0.307 |
| ≥21 patient number | 28 | 4 (14.29) | 29 | 6 (20.69) | 0.730 a |
| CDI | 28 | 7.5 (4.5, 13.5) | 29 | 12.0 (5.0, 17.0) | 0.179 |
| MIDAS | 28 | 0.0 (0.0, 0.0) | 29 | 0.0 (0.0, 0.0) | 0.813 |
| N | PS128 (N = 28) | p-Value | N | Placebo (N = 29) | p-Value | |
|---|---|---|---|---|---|---|
| 1 month and baseline | ||||||
| YGTSS Total | 28 | −2.21 ± 4.71 | 0.019 * | 29 | −3.10 ± 7.12 | 0.026 * |
| YGTSS Global | 28 | −7.43 ± 16.77 | 0.027 * | 29 | −5.17 ± 13.91 | 0.055 |
| 2 months and 1 month | ||||||
| YGTSS Total | 27 | 0.11 ± 5.25 | 0.915 | 27 | −0.14 ± 6.06 | 0.903 |
| YGTSS Global | 26 | 2.82 ± 12.24 | 0.233 | 27 | −2.38 ± 13.09 | 0.336 |
| 2 months and baseline | ||||||
| YGTSS Total | 28 | −2.11 ± 5.32 | 0.046 * | 29 | −3.24 ± 5.79 | 0.005 * |
| YGTSS Global | 28 | −4.61 ± 16.68 | 0.156 | 29 | −7.55 ± 16.37 | 0.019 * |
| N | PS128 (N = 28) | p-Value | N | Placebo (N = 29) | p-Value | |
|---|---|---|---|---|---|---|
| 1 month and baseline | ||||||
| %change of YGTSS Total | 28 | −16.31 ± 29.36 | 0.007 * | 29 | −14.93 ± 39.67 | 0.052 * |
| %change of YGTSS Global | 28 | −19.43 ± 46.74 | 0.037 * | 29 | −14.02 ± 41.40 | 0.079 |
| 2 months and 1 month | ||||||
| %change of YGTSS Total | 27 | 7.97 ± 59.65 | 0.494 | 27 | 7.79 ± 69.69 | 0.566 |
| %change of YGTSS Global | 26 | 22.58 ± 100.50 | 0.263 | 27 | −13.47 ± 33.26 | 0.045 * |
| 2 months and baseline | ||||||
| %change of YGTSS Total | 28 | −15.02 ± 37.49 | 0.043 * | 29 | −13.66 ± 28.52 | 0.015 * |
| %change of YGTSS Global | 28 | −14.20 ± 59.21 | 0.215 | 29 | −20.57 ± 35.78 | 0.004 * |
| Variable | PS128 (N = 21) | p-Value | Placebo (N = 20) | p-Value |
|---|---|---|---|---|
| Teacher | ||||
| Total | 1.00 ± 7.62 | 0.554 | −2.75 ± 9.79 | 0.224 |
| Inattention | 0.86 ± 4.05 | 0.344 | −1.45 ± 4.49 | 0.165 |
| Hyperactivity/Impulsivity | 0.48 ± 2.91 | 0.462 | −0.20 ± 3.86 | 0.819 |
| Oppositional behavior | 0.33 ± 2.18 | 0.491 | −1.10 ± 3.28 | 0.150 |
| PS128 (N = 25) | p-Value | Placebo (N = 26) | p-Value | |
| Parent | ||||
| Total | −3.88 ± 7.88 | 0.021 * | −3.69 ± 10.12 | 0.075 |
| Inattention | −2.04 ± 3.27 | 0.005 * | −1.27 ± 3.21 | 0.054 |
| Hyperactivity/Impulsivity | −1.76 ± 3.74 | 0.027 * | −1.12 ± 4.09 | 0.177 |
| Oppositional behavior | −0.08 ± 3.30 | 0.905 | −1.31 ± 4.90 | 0.186 |
| Variable | PS128 (N = 28) | p-Value | Placebo (N = 24) | p-Value |
|---|---|---|---|---|
| Omissions T-score | 0.91 ± 11.05 | 0.667 | 0.91 ± 10.69 | 0.681 |
| Commissions | −4.25 ± 9.22 | 0.022 * | −3.02 ± 8.12 | 0.082 |
| Hit RT | 2.09 ± 6.99 | 0.126 | 2.61 ± 11.62 | 0.282 |
| Hit RT Std. Error | 2.10 ± 6.56 | 0.103 | 1.28 ± 8.50 | 0.468 |
| Variability | 1.84 ± 8.39 | 0.257 | 1.73 ± 9.62 | 0.389 |
| Detectability (d’) | −4.71 ± 10.02 | 0.019 * | −4.73 ± 13.29 | 0.094 |
| Response Style (B) | 1.60 ± 13.93 | 0.548 | 2.75 ± 13.50 | 0.329 |
| Perseverations | 0.56 ± 19.10 | 0.879 | −1.38 ± 24.00 | 0.781 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Wong, L.-C.; Hsu, C.-J.; Yang, C.-W.; Tsai, Y.-C.; Cheng, F.-S.; Hu, H.-Y.; Lee, W.-T. Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome. Nutrients 2021, 13, 3698. https://doi.org/10.3390/nu13113698
Wu C-C, Wong L-C, Hsu C-J, Yang C-W, Tsai Y-C, Cheng F-S, Hu H-Y, Lee W-T. Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome. Nutrients. 2021; 13(11):3698. https://doi.org/10.3390/nu13113698
Chicago/Turabian StyleWu, Chang-Chun, Lee-Chin Wong, Chia-Jui Hsu, Chianne-Wen Yang, Ying-Chieh Tsai, Feng-Shiang Cheng, Hsiao-Yun Hu, and Wang-Tso Lee. 2021. "Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome" Nutrients 13, no. 11: 3698. https://doi.org/10.3390/nu13113698
APA StyleWu, C.-C., Wong, L.-C., Hsu, C.-J., Yang, C.-W., Tsai, Y.-C., Cheng, F.-S., Hu, H.-Y., & Lee, W.-T. (2021). Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome. Nutrients, 13(11), 3698. https://doi.org/10.3390/nu13113698