Feasibility Study to Assess the Impact of a Lifestyle Intervention during Colorectal Cancer Screening in France
Abstract
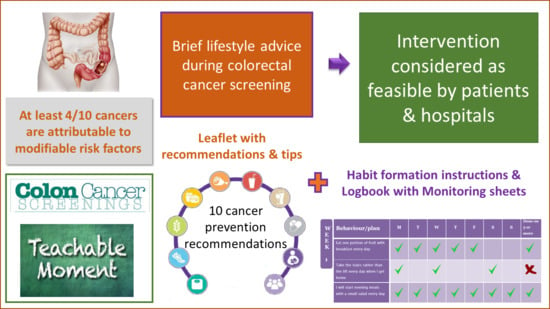
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Lifestyle Intervention
2.4. Measurements
2.5. Data Analysis
3. Results
4. Discussion
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
IARC Disclaimer
References
- International Agency for Research on Cancer. Global Cancer Observatory (GCO). Available online: https://gco.iarc.fr/ (accessed on 2 September 2021).
- Ferlay, J.; Soerjomataram, I.; Ervik, M. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef]
- World Cancer Research Fund-American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet., Nutrition, Physical ACTIVITY and Colorectal Cancer. Available online: dietandcancerreport.org (accessed on 2 September 2021).
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of Leisure-Time Physical Activity with Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Morris, J.S.; Bradbury, K.E.; Cross, A.J.; Gunter, M.J.; Murphy, N. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br. J. Cancer 2018, 118, 920–929. [Google Scholar] [CrossRef]
- WCRF/AICR. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2018. [Google Scholar]
- Marant-Micallef, C.; Shield, K.D.; Vignat, J.; Hill, C.; Menvielle, G.; Dossus, L.; Ormsby, J.N.; Rehm, J.; Rushton, L.; Vineis, P.; et al. Nombre et fractions de cancers attribuables au mode de vie et a l’environnement en France metropolitaine en 2015: Resultats principaux. Bull. Epidemiol. Hebd. 2018, 21, 442–448. [Google Scholar]
- WCRF/AICR. World Cancer Research Fund’s (WCRF) Continuous Update Project. Available online: https://www.wcrf.org/diet-and-cancer/continuous-update-project/ (accessed on 2 September 2021).
- Romaguera, D.; Vergnaud, A.C.; Peeters, P.H.; van Gils, C.H.; Chan, D.S.; Ferrari, P.; Romieu, I.; Jenab, M.; Slimani, N.; Clavel-Chapelon, F.; et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am. J. Clin. Nutr. 2012, 96, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Ward, H.; Wark, P.A.; Vergnaud, A.C.; Peeters, P.H.; van Gils, C.H.; Ferrari, P.; Fedirko, V.; Jenab, M.; Boutron-Ruault, M.C.; et al. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: A cohort study. BMC Med. 2015, 13, 107. [Google Scholar] [CrossRef]
- Vergnaud, A.C.; Romaguera, D.; Peeters, P.H.; van Gils, C.H.; Chan, D.S.; Romieu, I.; Freisling, H.; Ferrari, P.; Clavel-Chapelon, F.; Fagherazzi, G.; et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: Results from the European Prospective Investigation into Nutrition and Cancer cohort study1,4. Am. J. Clin. Nutr. 2013, 97, 1107–1120. [Google Scholar] [CrossRef]
- Jankovic, N.; Geelen, A.; Winkels, R.M.; Mwungura, B.; Fedirko, V.; Jenab, M.; Illner, A.K.; Brenner, H.; Ordonez-Mena, J.M.; Kiefte de Jong, J.C.; et al. Adherence to the WCRF/AICR Dietary Recommendations for Cancer Prevention and Risk of Cancer in Elderly from Europe and the United States: A Meta-Analysis within the CHANCES Project. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. 2017, 26, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Bravi, F.; Di Maso, M.; Bosetti, C.; Polesel, J.; Serraino, D.; Dalmartello, M.; Giacosa, A.; Montella, M.; Tavani, A.; et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and colorectal cancer risk. Eur. J. Cancer 2017, 85, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Rabin, C. Promoting Lifestyle Change Among Cancer Survivors: When Is the Teachable Moment? Am. J. Lyfestyle Med. 2009, 5, 142. [Google Scholar] [CrossRef]
- Berstad, P.; Loberg, M.; Larsen, I.K.; Kalager, M.; Holme, O.; Botteri, E.; Bretthauer, M.; Hoff, G. Long-term lifestyle changes after colorectal cancer screening: Randomised controlled trial. Gut 2015, 64, 1268–1276. [Google Scholar] [CrossRef]
- Stevens, C.; Vrinten, C.; Smith, S.G.; Waller, J.; Beeken, R.J. Determinants of willingness to receive healthy lifestyle advice in the context of cancer screening. Br. J. Cancer 2018, 119, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, C.R.; Brett, J.; Bukach, C.; Webster, R.; Stewart-Brown, S.; Munafo, M.; Austoker, J. Impact of screening on future health-promoting behaviors and health beliefs: A systematic review. Int. J. Technol. Assess. 2005, 21, 147. [Google Scholar] [CrossRef]
- Anderson, A.S.; Craigie, A.M.; Caswell, S.; Treweek, S.; Stead, M.; Macleod, M.; Daly, F.; Belch, J.; Rodger, J.; Kirk, A.; et al. The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: Randomised controlled trial. BMJ 2014, 348, g1823. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Macleod, M.; Mutrie, N.; Sugden, J.; Dobson, H.; Treweek, S.; O’Carroll, R.E.; Thompson, A.; Kirk, A.; Brennan, G.; et al. Breast cancer risk reduction--is it feasible to initiate a randomised controlled trial of a lifestyle intervention programme (ActWell) within a national breast screening programme? Int. J. Behav. Nutr. Phys. Act. 2014, 11, 156. [Google Scholar] [CrossRef][Green Version]
- Beeken, R.J.; Leurent, B.; Vickerstaff, V.; Wilson, R.; Croker, H.; Morris, S.; Omar, R.Z.; Nazareth, I.; Wardle, J. A brief intervention for weight control based on habit-formation theory delivered through primary care: Results from a randomised controlled trial. Int. J. Obes. 2017, 41, 246–254. [Google Scholar] [CrossRef]
- Gardner, B.; Lally, P.; Wardle, J. Making health habitual: The psychology of ‘habit formation’ and general practice. Br. J. Gen. Pract. 2012, 62, 664–666. [Google Scholar] [CrossRef]
- Vart, G.; Banzi, R.; Minozzi, S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: A systematic review and meta-analysis. Prev. Med. 2012, 55, 87–92. [Google Scholar] [CrossRef]
- van Rossum, L.G.; van Rijn, A.F.; Laheij, R.J.; van Oijen, M.G.; Fockens, P.; van Krieken, H.H.; Verbeek, A.L.; Jansen, J.B.; Dekker, E. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008, 135, 82–90. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Polkowski, M.; Kraszewska, E.; Rupinski, M.; Butruk, E.; Regula, J. A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut 2014, 63, 1112–1119. [Google Scholar] [CrossRef]
- Gunter, M.J.; Alhomoud, S.; Arnold, M.; Brenner, H.; Burn, J.; Casey, J.; Chan, A.T.; Cross, A.J.; Giovannucci, E.; Hoover, R.; et al. International cancer seminars: A focus on colorectal cancer. Ann. Oncol. 2018. Under review. [Google Scholar]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef]
- Lally, P.; Gardner, B. Promoting habit formation. Health Psychol. Rev. 2013, 7, S137–S158. [Google Scholar] [CrossRef]
- Lally, P.; Van Jaarsveld, C.H.M.; Potts, H.W.W.; Wardle, J. How are habits formed: Modelling habit formation in the real world. Eur. J. Soc. Psychol. 2010, 40, 998–1009. [Google Scholar] [CrossRef]
- Gardner, B.; Sheals, K.; Wardle, J.; McGowan, L. Putting habit into practice, and practice into habit: A process evaluation and exploration of the acceptability of a habit-based dietary behaviour change intervention. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 135. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Houben, K.; Hofmann, W.; Roefs, A.; Jansen, A. Control Yourself or Just Eat What You Like? Weight Gain Over a Year Is Predicted by an Interactive Effect of Response Inhibition and Implicit Preference for Snack Foods. Health Psychol. 2010, 29, 389–393. [Google Scholar] [CrossRef]
- Morin, H. En France, quatre cancers sur dix pourraient être évités. Le Monde 2018, 11, 142. [Google Scholar]
- Elster, N. Modern myths about cancer from ‘chemicals’ in food to wifi. Guardian 2018, 11, 452. [Google Scholar]
- Robb, K.A.; Power, E.; Kralj-Hans, I.; Atkin, W.S.; Wardle, J. The impact of individually-tailored lifestyle advice in the colorectal cancer screening context: A randomised pilot study in North-West London. Prev. Med. 2010, 51, 505–508. [Google Scholar] [CrossRef] [PubMed]
- LoConte, N.K.; Gershenwald, J.E.; Thomson, C.A.; Crane, T.E.; Harmon, G.E.; Rechis, R. Lifestyle Modifications and Policy Implications for Primary and Secondary Cancer Prevention: Diet, Exercise, Sun Safety, and Alcohol Reduction. Am. Soc. Clin. Oncol. Educ. Book 2018, 93, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, M.D.; Hjartåker, A.; Robb, K.A.; de Lange, T.; Hoff, G.; Berstad, P. Improving Cancer Preventive Behaviors: A Randomized Trial of Tailored Lifestyle Feedback in Colorectal Cancer Screening. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. 2018, 27, 1442–1449. [Google Scholar] [CrossRef]
- Conway, E.; Wyke, S.; Sugden, J.; Mutrie, N.; Anderson, A.S. Can a lifestyle intervention be offered through NHS breast cancer screening? Challenges and opportunities identified in a qualitative study of women attending screening. BMC Public Health 2016, 16, 758. [Google Scholar] [CrossRef] [PubMed]
- Caswell, S.; Anderson, A.S.; Steele, R.J. Bowel health to better health: A minimal contact lifestyle intervention for people at increased risk of colorectal cancer. Br. J. Nutr. 2009, 102, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Smith, S.G.; Vrinten, C.; Waller, J.; Beeken, R.J. Lifestyle changes associated with participation in colorectal cancer screening: Prospective data from the English Longitudinal Study of Ageing. J. Med. Screen. 2019, 26, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, C.N.; Legler, J.M.; Warren, J.L.; Laura-Mae, B.; Schrag, D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann. Epidemiol. 2007, 17, 584–590. [Google Scholar] [CrossRef]
- de Bruijn, K.M.J.; Arends, L.R.; Hansen, B.E.; Leeflang, S.; Ruiter, R.; van Eijck, C.H.J. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br. J. Surg. 2013, 100, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
| n (%) or Mean (Min-Max) | |
|---|---|
| Sex | |
| Men | 20 (41%) |
| Women | 29 (59%) |
| Age | 56.6 (23–75) * |
| Hospital visit at which the intervention was performed | |
| Pre-colonoscopy | 4 (8%) |
| Post-colonoscopy | 41 (84%) |
| Hospitalization for colonoscopy | 4 (8%) |
| Reason for the colonoscopy | |
| Family history of Colorectal cancer (CRC) | 7 (14%) |
| CRC symptoms | 13 (27%) |
| CRC screening (positive FIT test) | 7 (14%) |
| Medical follow-up (e.g., Lynch) | 22 (45%) |
| Interested in reducing CRC risk | |
| Yes | 49 (100) |
| No | 0 |
| Knowledge on how to reduce CRC risk | |
| Yes | 19 (39%) |
| No | 30 (61%) |
| Do healthy lifestyle behaviours help reduce CRC risk? | |
| Yes | 41 (84%) |
| No | 2 (4%) |
| Don’t know | 6 (12%) |
| Ready to change lifestyle behaviours | |
| Yes | 43 (88%) |
| No | 1 (2%) |
| Don’t know | 5 (10%) |
| Interested in taking part in LIFE-SCREEN | |
| Yes | 45 (92%) |
| No | 2 (4%) |
| Do not know | 2 (4%) |
| Compliance to the Intervention | n (%) |
|---|---|
| Achieved at least one healthy behaviour using the tick sheets | |
| Yes | 13 (50%) |
| No | 10 (38%) |
| Missing | 3 (12%) |
| Made plan(s) to achieve at least one healthy behaviour | |
| Yes | 15 (58%) |
| No | 9 (34%) |
| Missing | 2 (8%) |
| Made adjustment to plan(s) to achieve at least one healthy behaviour | |
| Yes | 14 (58%) |
| No | 9 (34%) |
| Missing | 3 (8%) |
| Intends to continue following the intervention? | |
| Yes | 19 (74%) |
| No | 7 (26%) |
| Missing | - |
| Feedback on the Intervention Delivery and Content | n (%) |
|---|---|
| Interested in taking part in this intervention if attending cancer screening again in the future | |
| Not very interested | 6 (23%) |
| Somewhat interested | 11 (42%) |
| Very interested | 5 (20%) |
| Not applicable or missing | 4 (15%) |
| Interested in taking part in this intervention even if randomied to intervention or control condition | |
| Not very interested | 5 (20%) |
| Somewhat interested | 12 (46%) |
| Very interested | 5 (19%) |
| Not applicable or missing | 4 (15%) |
| Interested in receiving text-messages and emails reminding about the healthy behaviours | |
| Not very interested | 9 (35%) |
| Somewhat interested | 9 (35%) |
| Very interested | 6 (23%) |
| Not applicable or missing | 2 (7%) |
| The educational material was easy to understand | |
| Yes | 26 (100%) |
| No | 0 |
| The educational material covered the kind of information you would expect | |
| Yes | 24 (92%) |
| No | 0 |
| Missing | 2 (8%) |
| The educational materials are attractive and eye-catching | |
| Yes | 23 (88%) |
| No | 1 (4%) |
| Missing | 2 (8%) |
| Is there any other information or tips that you would like to see in the leaflet or logbook? | |
| Yes | 7 (27%) |
| No | 18 (69%) |
| Missing | 1 (4%) |
| Was the timing of the delivery adequate? | |
| Yes | 24 (92%) |
| No | 1 (4%) |
| Missing | 1 (4%) |
| Would it be helpful to have access to a mobile or web-based app to monitor your behaviours? | |
| Yes | 11 (42%) |
| No | 14 (54%) |
| Missing | 1 (4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huybrechts, I.; Kliemann, N.; Perol, O.; Cattey-Javouhey, A.; Benech, N.; Maire, A.; Lignini, T.; Carretier, J.; Saurin, J.-C.; Fervers, B.; et al. Feasibility Study to Assess the Impact of a Lifestyle Intervention during Colorectal Cancer Screening in France. Nutrients 2021, 13, 3685. https://doi.org/10.3390/nu13113685
Huybrechts I, Kliemann N, Perol O, Cattey-Javouhey A, Benech N, Maire A, Lignini T, Carretier J, Saurin J-C, Fervers B, et al. Feasibility Study to Assess the Impact of a Lifestyle Intervention during Colorectal Cancer Screening in France. Nutrients. 2021; 13(11):3685. https://doi.org/10.3390/nu13113685
Chicago/Turabian StyleHuybrechts, Inge, Nathalie Kliemann, Olivia Perol, Anne Cattey-Javouhey, Nicolas Benech, Aurelia Maire, Tracy Lignini, Julien Carretier, Jean-Christophe Saurin, Beatrice Fervers, and et al. 2021. "Feasibility Study to Assess the Impact of a Lifestyle Intervention during Colorectal Cancer Screening in France" Nutrients 13, no. 11: 3685. https://doi.org/10.3390/nu13113685
APA StyleHuybrechts, I., Kliemann, N., Perol, O., Cattey-Javouhey, A., Benech, N., Maire, A., Lignini, T., Carretier, J., Saurin, J.-C., Fervers, B., & Gunter, M. J. (2021). Feasibility Study to Assess the Impact of a Lifestyle Intervention during Colorectal Cancer Screening in France. Nutrients, 13(11), 3685. https://doi.org/10.3390/nu13113685