Three-Generation Study of Male Rats Gestationally Exposed to High Butterfat and Bisphenol A: Impaired Spermatogenesis, Penetrance with Reduced Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Hormone Treatment SD Rat Model
2.3. Natural Aging Model
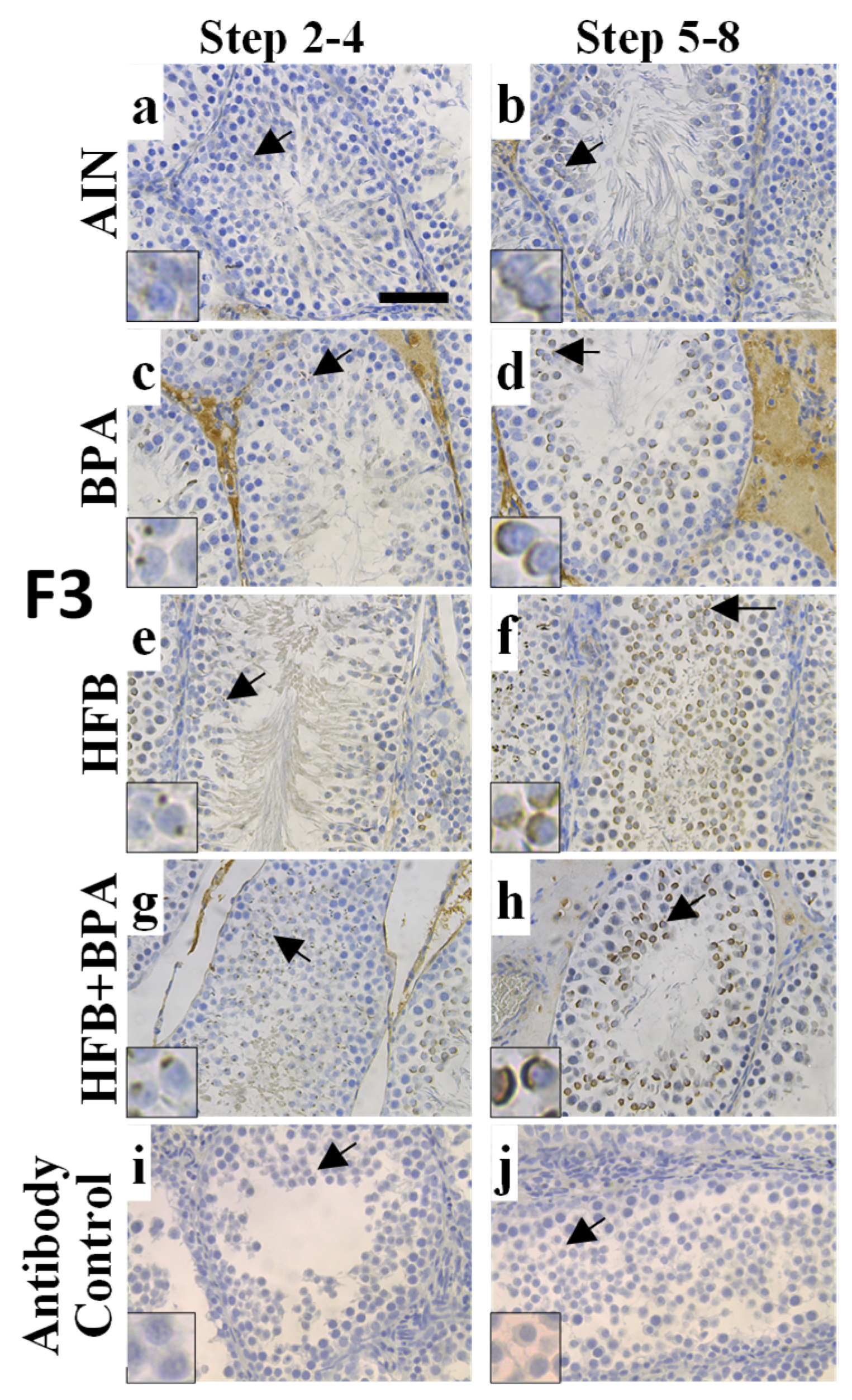
2.4. Tissue Collection and Immunohistochemistry
2.5. Quantitative Real-Time PCR (qPCR)
2.6. Statistical Analysis
3. Results
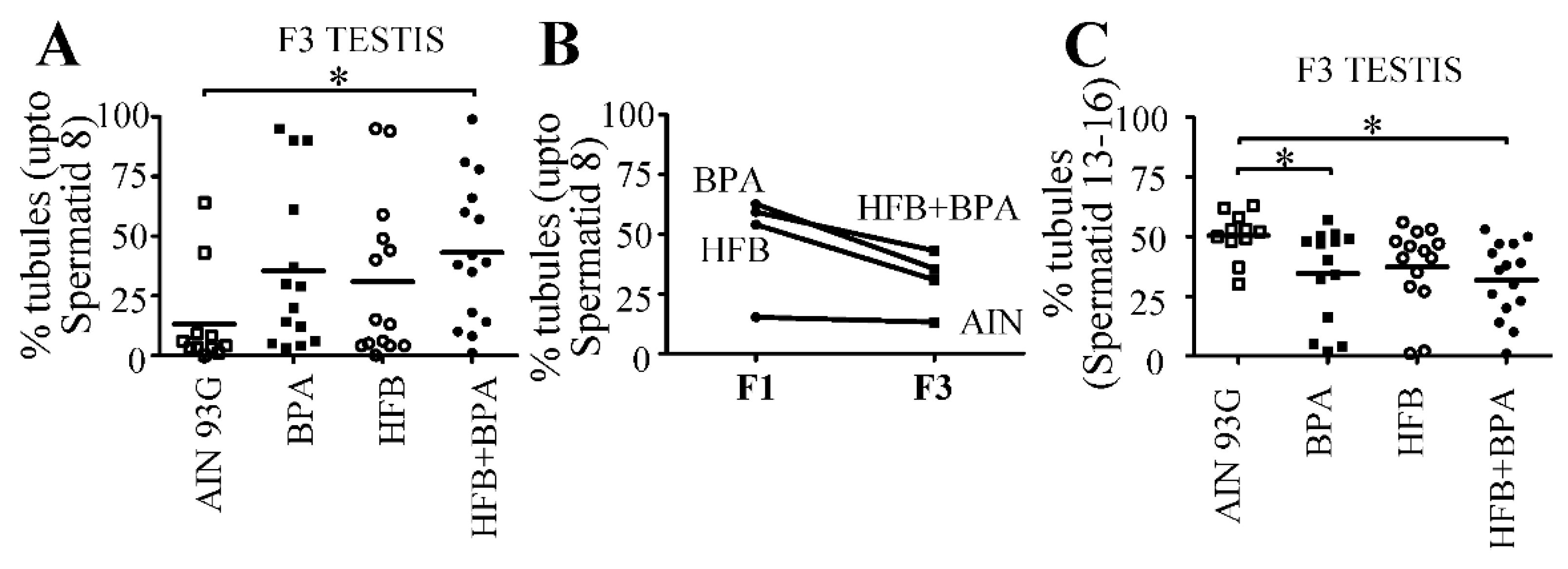
3.1. Prenatal HFB + BPA Exposure Induces Significant Spermatogenesis Arrest in T + E2 Implanted Offspring
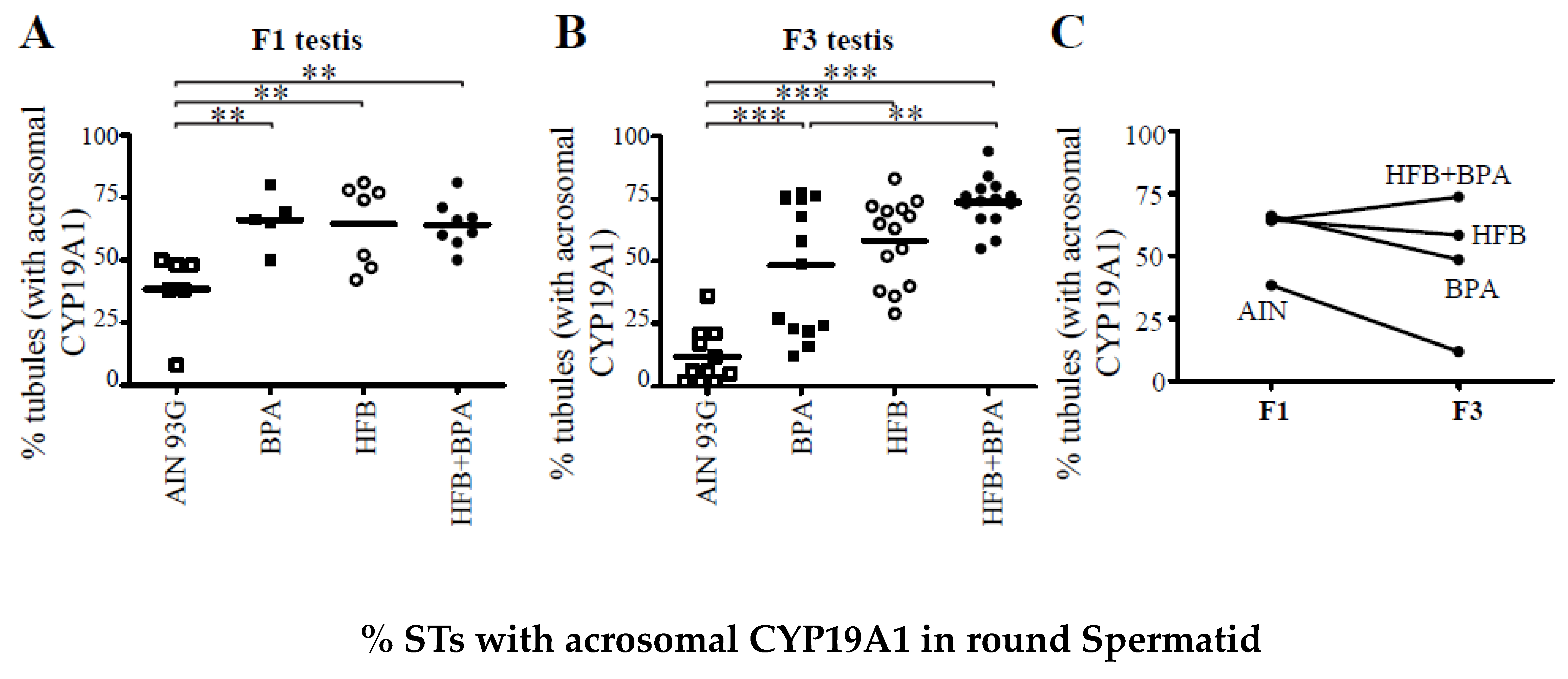
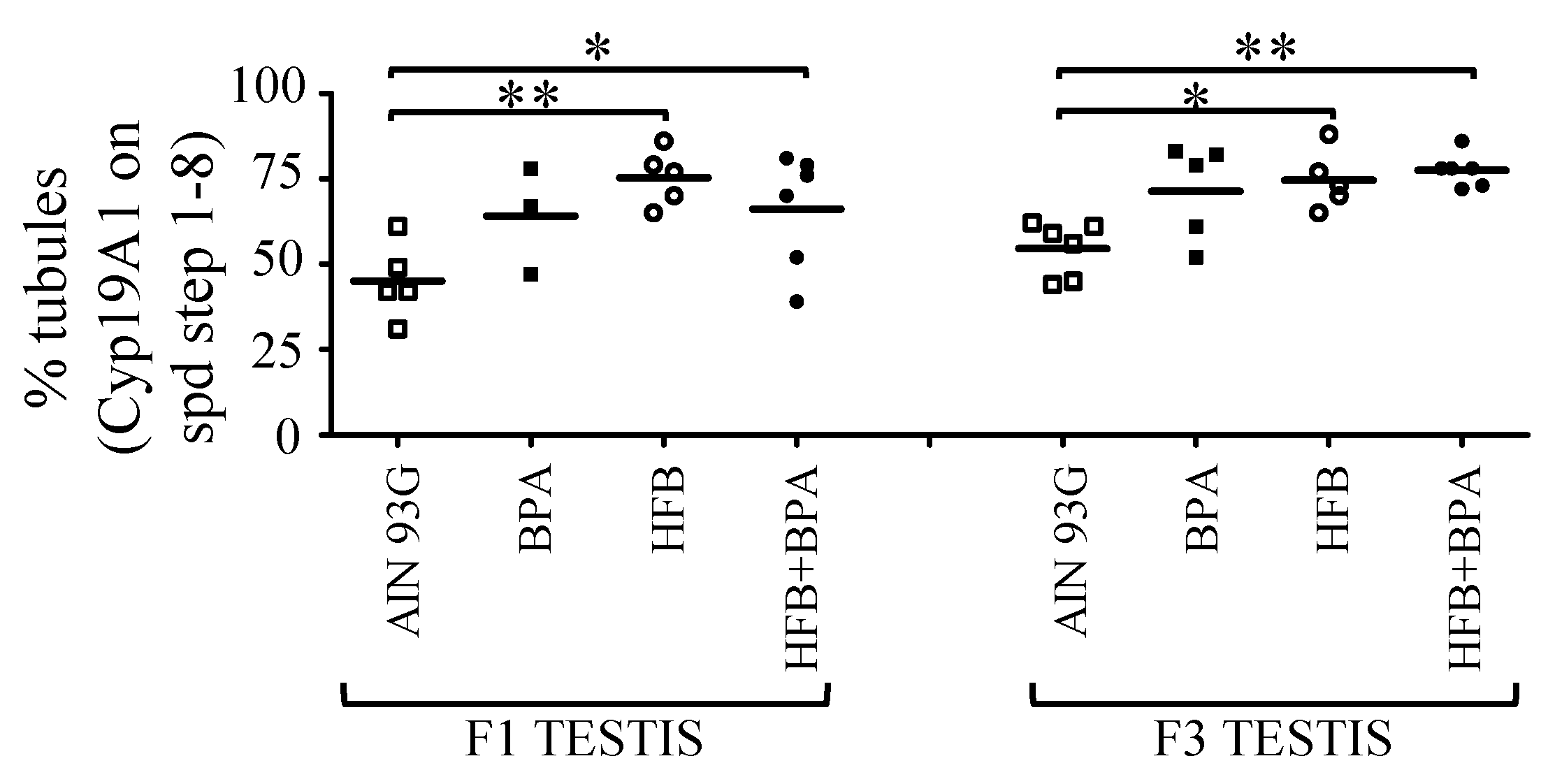
3.2. CYP19A1/Aromatase Expression during Spermatogenesis in T + E2-Implanted Offspring
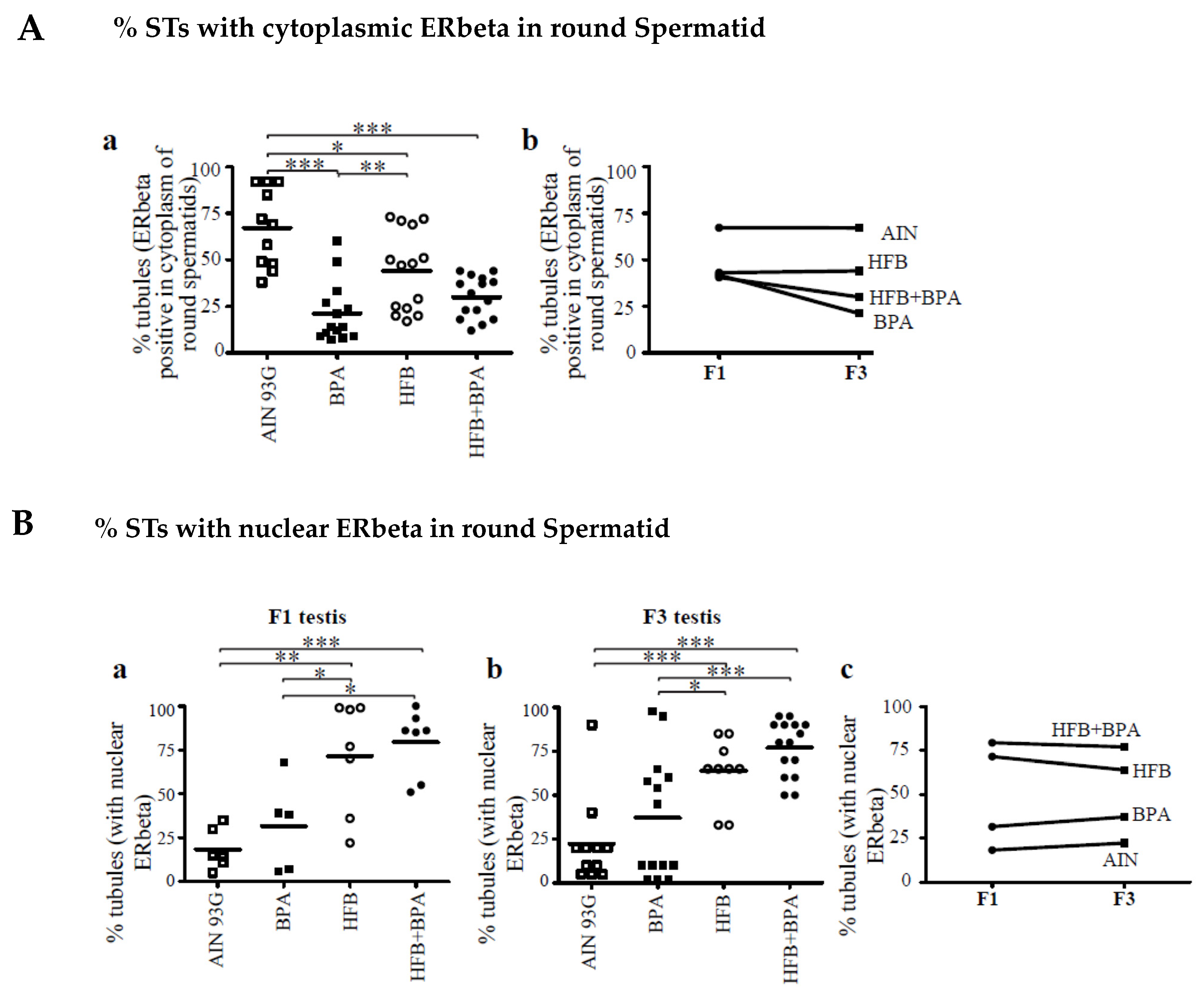
3.3. Decrease in Cytoplasmic ERbeta Expression in Round Spermatids of T + E2-Implanted Offspring
3.4. Nuclear ERbeta Localization in Round Spermatids in T + E2-Implanted Offspring
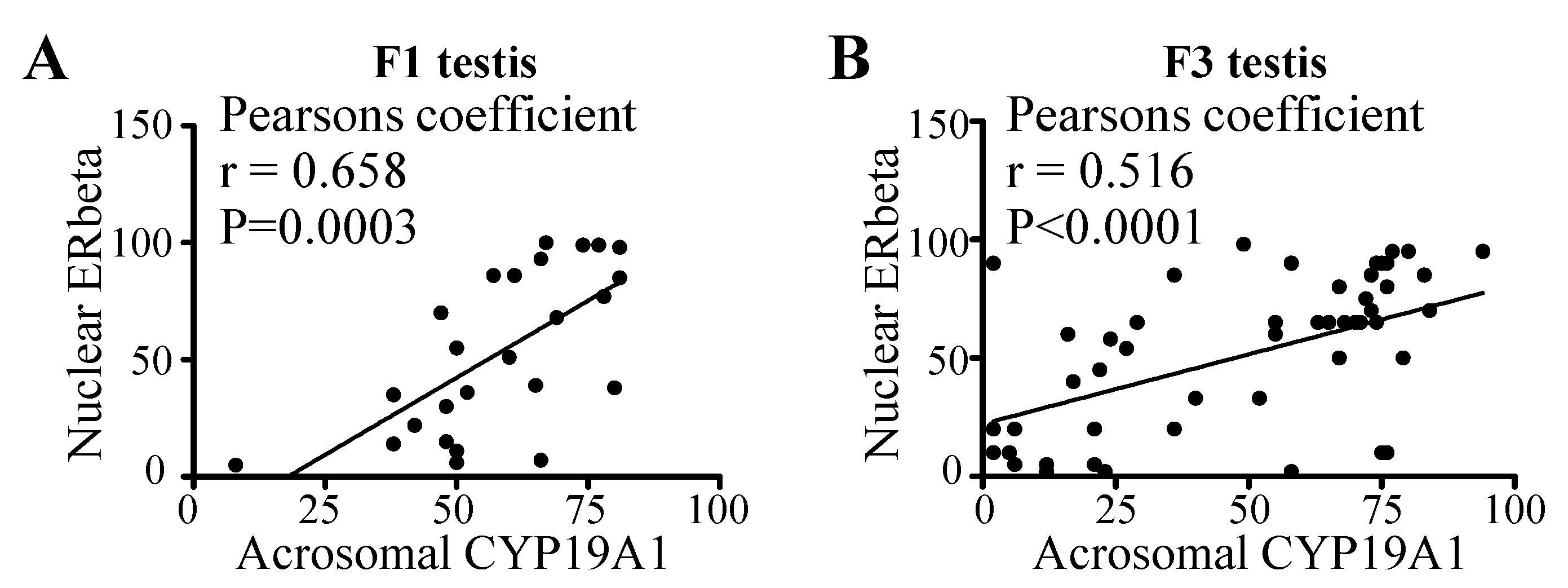
3.5. Correlation between Acrosomal CYP19A1 and Nuclear ERbeta Localization in Round Spermatids
3.6. 18-Month-Old F3-Generation Testes Exhibited Decreased Spermatozoa Numbers
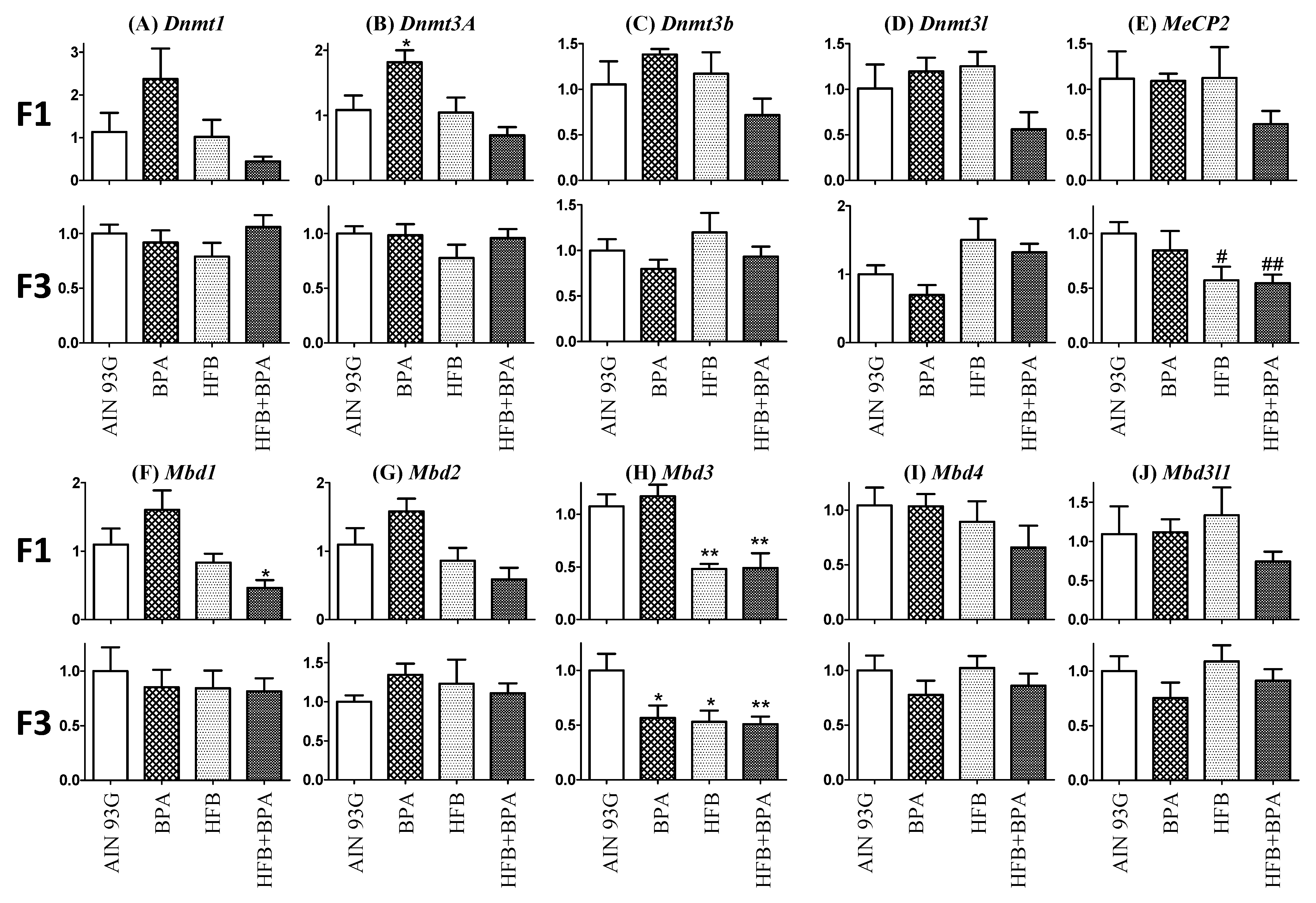
3.7. Methyl-CpG-Binding Domain (MBD3) Levels Are Reduced in HFB and HFB + BPA Group Offspring (T + E2-Treated)
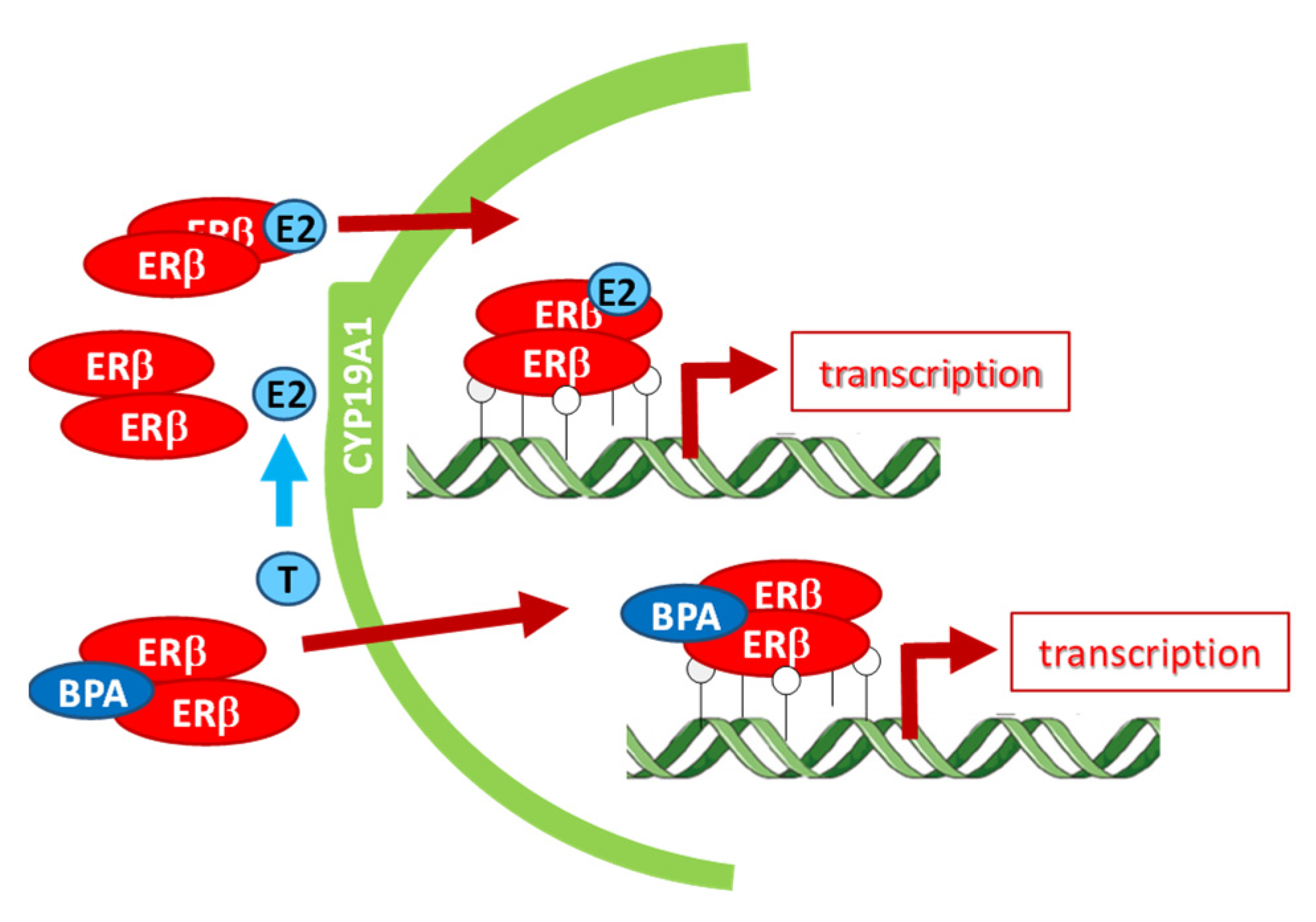
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Maternal constraint of fetal growth and its consequences. Semin. Fetal. Neonatal Med. 2004, 9, 419–425. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Roseboom, T.J.; van der Meulen, J.H.; Ravelli, A.C.; Osmond, C.; Barker, D.J.; Bleker, O.P. Effects of prenatal exposure to the Dutch famine on adult disease in later life: An overview. Mol. Cell Endocrinol. 2001, 185, 93–98. [Google Scholar] [CrossRef]
- Sun, B.; Purcell, R.H.; Terrillion, C.E.; Yan, J.; Moran, T.H.; Tamashiro, K.L. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 2012, 61, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell Endocrinol. 2006, 254-255, 179–186. [Google Scholar] [CrossRef]
- Murono, E.P.; Derk, R.C. The reported active metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, inhibits testosterone formation by cultured Leydig cells from neonatal rats. Reprod. Toxicol. 2005, 20, 503–513. [Google Scholar] [CrossRef]
- Gray, L.E., Jr.; Ostby, J.; Monosson, E.; Kelce, W.R. Environmental antiandrogens: Low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol. Ind. Health 1999, 15, 48–64. [Google Scholar] [CrossRef]
- Ho, S.M.; Cheong, A.; Adgent, M.A.; Veevers, J.; Suen, A.A.; Tam, N.N.C.; Leung, Y.K.; Jefferson, W.N.; Williams, C.J. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod. Toxicol. 2017, 68, 85–104. [Google Scholar] [CrossRef]
- Ho, S.M. Environmental epigenetics of asthma: An update. J. Allergy Clin. Immunol. 2010, 126, 453–465. [Google Scholar] [CrossRef]
- Ho, S.M.; Johnson, A.; Tarapore, P.; Janakiram, V.; Zhang, X.; Leung, Y.K. Environmental epigenetics and its implication on disease risk and health outcomes. ILAR J. 2012, 53, 289–305. [Google Scholar] [CrossRef]
- Zhang, X.; Ho, S.M. Epigenetics meets endocrinology. J. Mol. Endocrinol. 2011, 46, R11–R32. [Google Scholar] [CrossRef]
- Tarapore, P.; Hennessy, M.; Song, D.; Ying, J.; Ouyang, B.; Govindarajah, V.; Leung, Y.K.; Ho, S.M. High butter-fat diet and bisphenol A additively impair male rat spermatogenesis. Reprod. Toxicol. 2017, 68, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Damstra, T.; Barlow, S.; Bergman, A.; Kavlock, R.; Van Der Kraak, G. Global Assessment of the State-of-the-Science of Endocrine Disruptors; International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Tomza-Marciniak, A.; Stepkowska, P.; Kuba, J.; Pilarczyk, B. Effect of bisphenol A on reproductive processes: A review of in vitro, in vivo and epidemiological studies. J. Appl. Toxicol. 2018, 38, 51–80. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Sharpe, R.M. Environmental/lifestyle effects on spermatogenesis. Philos. Trans. R. Soc. Lond B Biol. Sci. 2010, 365, 1697–1712. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; Kim, G.H.; et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef]
- Mendonca, K.; Hauser, R.; Calafat, A.M.; Arbuckle, T.E.; Duty, S.M. Bisphenol A concentrations in maternal breast milk and infant urine. Int. Arch. Occup. Environ. Health 2014, 87, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Boudalia, S.; Berges, R.; Chabanet, C.; Folia, M.; Decocq, L.; Pasquis, B.; Abdennebi-Najar, L.; Canivenc-Lavier, M.C. A multi-generational study on low-dose BPA exposure in Wistar rats: Effects on maternal behavior, flavor intake and development. Neurotoxicol. Teratol. 2014, 41, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.K.; Govindarajah, V.; Cheong, A.; Veevers, J.; Song, D.; Gear, R.; Zhu, X.; Ying, J.; Kendler, A.; Medvedovic, M.; et al. Gestational high-fat diet and bisphenol A exposure heightens mammary cancer risk. Endocr. Relat Cancer 2017, 24, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Medwid, S.; Guan, H.; Yang, K. Prenatal exposure to bisphenol A disrupts adrenal steroidogenesis in adult mouse offspring. Environ. Toxicol. Pharmacol. 2016, 43, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Bu, P.; Li, F.; Lan, S.; Wu, H.; Yuan, L.; Wang, Y. Neonatal bisphenol A exposure induces meiotic arrest and apoptosis of spermatogenic cells. Oncotarget 2016, 7, 10606–10615. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.M.; Miller, R.L.; Perzanowski, M.S.; Just, A.C.; Hoepner, L.A.; Arunajadai, S.; Canfield, S.; Resnick, D.; Calafat, A.M.; Perera, F.P.; et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J. Allergy Clin. Immunol. 2013, 131, 736–742. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Edwards, M.; Shetty, S.R.; Gatewood, J.D.; Taylor, J.A.; Rissman, E.F.; Connelly, J.J. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology 2012, 153, 3828–3838. [Google Scholar] [CrossRef]
- Salian, S.; Doshi, T.; Vanage, G. Perinatal exposure of rats to Bisphenol A affects fertility of male offspring—An overview. Reprod. Toxicol. 2011, 31, 359–362. [Google Scholar] [CrossRef]
- Munoz-de-Toro, M.; Markey, C.M.; Wadia, P.R.; Luque, E.H.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology 2005, 146, 4138–4147. [Google Scholar] [CrossRef]
- Tarapore, P.; Hennessy, M.; Song, D.; Ying, J.; Ouyang, B.; Govindarajah, V.; Leung, Y.K.; Ho, S.M. Data on spermatogenesis in rat males gestationally exposed to bisphenol A and high fat diets. Data Brief. 2016, 9, 812–817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mirihagalle, S.; You, T.; Suh, L.; Patel, C.; Gao, L.; Rattan, S.; Qiao, H. Prenatal exposure to di-(2-ethylhexyl) phthalate and high-fat diet synergistically disrupts mouse fetal oogenesis and affects folliculogenesis. Biol. Reprod. 2019, 100, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Oshio, L.T.; Andreazzi, A.E.; Lopes, J.F.; Sá, J.P.; Bolotari, M.; Costa, V.M.G.; Guerra, M.O.; Peters, V.M. A paternal hypercaloric diet affects the metabolism and fertility of F1 and F2 Wistar rat generations. J. Dev. Orig. Health Dis. 2020, 11, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Ramaiyan, B.; Zarei, M.; Acharya, P.; Talahalli, R.R. Dietary n-3 but not n-6 fatty acids modulate anthropometry and fertility indices in high-fat diet fed rats: A two-generation study. J. Food Sci. Technol. 2021, 58, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Uddin, G.M.; Youngson, N.A.; Agapiou, D.; Walters, K.A.; Sinclair, D.A.; Morris, M.J.; Gilchrist, R.B. Multigenerational obesity-induced perturbations in oocyte-secreted factor signalling can be ameliorated by exercise and nicotinamide mononucleotide. Hum. Reprod. Open. 2018, 2018, hoy010. [Google Scholar] [CrossRef]
- Finger, B.J.; Harvey, A.J.; Green, M.P.; Gardner, D.K. Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics, morphology and metabolism. Hum. Reprod. 2015, 30, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Obesity and Overweight. Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 September 2021).
- Chavatte-Palmer, P.; Tarrade, A.; Levy, R. [Developmental origins of health and disease in adults: Role of maternal environment]. Gynecol. Obstet. Fertil. 2012, 40, 517–519. [Google Scholar] [CrossRef]
- Lane, M.; Robker, R.L.; Robertson, S.A. Parenting from before conception. Science 2014, 345, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Blatt, J.; Van, L.L.; Weiner, T.; Sailer, S. Ovarian carcinoma in an adolescent with transgenerational exposure to diethylstilbestrol. J. Pediatr. Hematol. Oncol. 2003, 25, 635–636. [Google Scholar] [CrossRef]
- Drake, A.J.; Walker, B.R.; Seckl, J.R. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am. J. Physiol Regul. Integr. Comp Physiol 2005, 288, R34–R38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, S.; Li, S.; Feng, R.; Na, L.; Chu, X.; Wu, X.; Niu, Y.; Sun, Z.; Han, T.; et al. Prenatal exposure to famine and the development of hyperglycemia and type 2 diabetes in adulthood across consecutive generations: A population-based cohort study of families in Suihua, China. Am. J. Clin. Nutr. 2017, 105, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lumey, L.H.; Stein, A.D. In utero exposure to famine and subsequent fertility: The Dutch Famine Birth Cohort Study. Am. J. Public Health 1997, 87, 1962–1966. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynska, M.M.; Tyrkiel, E.J. The effect of preconceptional exposure of F0 male mice to di(2-ethylhexyl)phthalate on the induction of reproductive toxicity in F2 generation. Drug Chem. Toxicol. 2018, 1–6. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Wen, S.; Shen, L.; Peng, J.; Yan, C.; Cao, X.; Zhou, Y.; Long, C.; Lin, T.; et al. The Mechanism of Environmental Endocrine Disruptors (DEHP) Induces Epigenetic Transgenerational Inheritance of Cryptorchidism. PLoS ONE 2015, 10, e0126403. [Google Scholar] [CrossRef]
- Guerrero-Bosagna, C.; Covert, T.R.; Haque, M.M.; Settles, M.; Nilsson, E.E.; Anway, M.D.; Skinner, M.K. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod. Toxicol. 2012, 34, 694–707. [Google Scholar] [CrossRef]
- Guerrero-Bosagna, C.M.; Skinner, M.K. Epigenetic transgenerational effects of endocrine disruptors on male reproduction. Semin. Reprod. Med. 2009, 27, 403–408. [Google Scholar] [CrossRef]
- Zambrano, E.; Martinez-Samayoa, P.M.; Bautista, C.J.; Deas, M.; Guillen, L.; Rodriguez-Gonzalez, G.L.; Guzman, C.; Larrea, F.; Nathanielsz, P.W. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J. Physiol 2005, 566, 225–236. [Google Scholar] [CrossRef]
- Skinner, M.K.; Anway, M.D. Seminiferous cord formation and germ-cell programming: Epigenetic transgenerational actions of endocrine disruptors. Ann. N. Y. Acad. Sci. 2005, 1061, 18–32. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Tain, Y.L. Maternal Exposure to Bisphenol A Combined with High-Fat Diet-Induced Programmed Hypertension in Adult Male Rat Offspring: Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 4382. [Google Scholar] [CrossRef]
- Ding, S.; Fan, Y.; Zhao, N.; Yang, H.; Ye, X.; He, D.; Jin, X.; Liu, J.; Tian, C.; Li, H.; et al. High-fat diet aggravates glucose homeostasis disorder caused by chronic exposure to bisphenol A. J. Endocrinol. 2014, 221, 167–179. [Google Scholar] [CrossRef]
- Andreollo, N.A.; Santos, E.F.; Araujo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Arq Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.M.; Tang, W.Y.; Belmonte de, F.J.; Prins, G.S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006, 66, 5624–5632. [Google Scholar] [CrossRef]
- Lee, C.; Prins, G.S.; Henneberry, M.O.; Grayhack, J.T. Effect of Estradiol on the Rat Prostate in the Presence and Absence of Testosterone and Pituitary. J Androl. 1981, 2, 293–299. [Google Scholar] [CrossRef]
- Clarke, M.; Pearl, C.A. Alterations in the estrogen environment of the testis contribute to declining sperm production in aging rats. Syst. Biol. Reprod. Med. 2014, 60, 89–97. [Google Scholar] [CrossRef]
- Tang, W.Y.; Morey, L.M.; Cheung, Y.Y.; Birch, L.; Prins, G.S.; Ho, S.M. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 2012, 153, 42–55. [Google Scholar] [CrossRef]
- Thompson, C.J.; Tam, N.N.; Joyce, J.M.; Leav, I.; Ho, S.M. Gene expression profiling of testosterone and estradiol-17 beta-induced prostatic dysplasia in Noble rats and response to the antiestrogen ICI 182,780. Endocrinology 2002, 143, 2093–2105. [Google Scholar] [CrossRef]
- Lombo, M.; Fernandez-Diez, C.; Gonzalez-Rojo, S.; Herraez, M.P. Genetic and epigenetic alterations induced by bisphenol A exposure during different periods of spermatogenesis: From spermatozoa to the progeny. Sci. Rep. 2019, 9, 18029. [Google Scholar] [CrossRef]
- Dumasia, K.; Kumar, A.; Deshpande, S.; Sonawane, S.; Balasinor, N.H. Differential roles of estrogen receptors, ESR1 and ESR2, in adult rat spermatogenesis. Mol. Cell Endocrinol. 2016, 428, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Delbes, G.; Levacher, C.; Pairault, C.; Racine, C.; Duquenne, C.; Krust, A.; Habert, R. Estrogen receptor beta-mediated inhibition of male germ cell line development in mice by endogenous estrogens during perinatal life. Endocrinology 2004, 145, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, Z.; Wang, G.; Li, Z.; Liang, A.; Wang, H.; Dai, Y.; Huang, X.; Chen, X.; Ma, Y.; et al. Overexpression of Human-Derived DNMT3A Induced Intergenerational Inheritance of Active DNA Methylation Changes in Rat Sperm. Front Genet. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Salian, S.; Doshi, T.; Vanage, G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009, 85, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Ziv-Gal, A.; Wang, W.; Zhou, C.; Flaws, J.A. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol. Appl. Pharmacol. 2015, 284, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, J.T.; Goldsby, J.A.; Rissman, E.F. Transgenerational effects of prenatal bisphenol A on social recognition. Horm. Behav. 2013, 64, 833–839. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef] [PubMed]
- Eddy, E.M.; Washburn, T.F.; Bunch, D.O.; Goulding, E.H.; Gladen, B.C.; Lubahn, D.B.; Korach, K.S. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 1996, 137, 4796–4805. [Google Scholar] [CrossRef]
- Fisher, C.R.; Graves, K.H.; Parlow, A.F.; Simpson, E.R. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc. Natl. Acad. Sci. USA 1998, 95, 6965–6970. [Google Scholar] [CrossRef]
- Robertson, K.M.; O’Donnell, L.; Jones, M.E.; Meachem, S.J.; Boon, W.C.; Fisher, C.R.; Graves, K.H.; McLachlan, R.I.; Simpson, E.R. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc. Natl. Acad. Sci. USA 1999, 96, 7986–7991. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Jia, L.; Li, X.; Rahman, N. Oestrogen action and male fertility: Experimental and clinical findings. Cell Mol. Life Sci. 2015, 72, 3915–3930. [Google Scholar] [CrossRef]
- Li, X.; Strauss, L.; Kaatrasalo, A.; Mayerhofer, A.; Huhtaniemi, I.; Santti, R.; Makela, S.; Poutanen, M. Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology 2006, 147, 1271–1277. [Google Scholar] [CrossRef]
- Carreau, S.; Hess, R.A. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. Lond B Biol. Sci. 2010, 365, 1517–1535. [Google Scholar] [CrossRef]
- Selva, D.M.; Tirado, O.M.; Toran, N.; Suarez-Quian, C.A.; Reventos, J.; Munell, F. Estrogen receptor beta expression and apoptosis of spermatocytes of mice overexpressing a rat androgen-binding protein transgene. Biol. Reprod. 2004, 71, 1461–1468. [Google Scholar] [CrossRef]
- Chimento, A.; Sirianni, R.; Zolea, F.; Bois, C.; Delalande, C.; Ando, S.; Maggiolini, M.; Aquila, S.; Carreau, S.; Pezzi, V. Gper and ESRs are expressed in rat round spermatids and mediate oestrogen-dependent rapid pathways modulating expression of cyclin B1 and Bax. Int. J. Androl. 2011, 34, 420–429. [Google Scholar] [CrossRef]
- Fowler, K.A.; Gill, K.; Kirma, N.; Dillehay, D.L.; Tekmal, R.R. Overexpression of aromatase leads to development of testicular leydig cell tumors: An in vivo model for hormone-mediated TesticularCancer. Am. J. Pathol. 2000, 156, 347–353. [Google Scholar] [CrossRef]
- Laing, L.V.; Viana, J.; Dempster, E.L.; Uren Webster, T.M.; van Aerle, R.; Mill, J.; Santos, E.M. Sex-specific transcription and DNA methylation profiles of reproductive and epigenetic associated genes in the gonads and livers of breeding zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 222, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rojo, S.; Lombo, M.; Fernandez-Diez, C.; Herraez, M.P. Male exposure to bisphenol a impairs spermatogenesis and triggers histone hyperacetylation in zebrafish testes. Environ. Pollut. 2019, 248, 368–379. [Google Scholar] [CrossRef]
- Santangeli, S.; Maradonna, F.; Gioacchini, G.; Cobellis, G.; Piccinetti, C.C.; Dalla, V.L.; Carnevali, O. BPA-Induced Deregulation of Epigenetic Patterns: Effects on Female Zebrafish Reproduction. Sci. Rep. 2016, 6, srep21982. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Y.; Gai, Z.; Li, R.; Zhu, Z.; Bai, C.; Tanguay, R.L.; Xu, X.; Huang, C.; Dong, Q. Reproductive toxicity of low level bisphenol A exposures in a two-generation zebrafish assay: Evidence of male-specific effects. Aquat. Toxicol. 2015, 169, 204–214. [Google Scholar] [CrossRef]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [PubMed]
- Blin, G.; Liand, M.; Mauduit, C.; Chehade, H.; Benahmed, M.; Simeoni, U.; Siddeek, B. Maternal Exposure to High-Fat Diet Induces Long-Term Derepressive Chromatin Marks in the Heart. Nutrients 2020, 12, 181. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, Y.Z.; Wang, C.H.; Lin, F.J. The nonalcoholic fatty liver disease-like phenotype and lowered serum VLDL are associated with decreased expression and DNA hypermethylation of hepatic ApoB in male offspring of ApoE deficient mothers fed a with Western diet. J. Nutr. Biochem. 2020, 77, 108319. [Google Scholar] [CrossRef] [PubMed]
- Keleher, M.R.; Zaidi, R.; Shah, S.; Oakley, M.E.; Pavlatos, C.; El, I.S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J.M. Maternal high-fat diet associated with altered gene expression, DNA methylation, and obesity risk in mouse offspring. PLoS ONE 2018, 13, e0192606. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.J.; Lim, D.S.; Lee, J.S. A novel role for methyl CpG-binding domain protein 3, a component of the histone deacetylase complex, in regulation of cell cycle progression and cell death. Biochem. Biophys. Res. Commun. 2009, 378, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ozmadenci, D.; Feraud, O.; Markossian, S.; Kress, E.; Ducarouge, B.; Gibert, B.; Ge, J.; Durand, I.; Gadot, N.; Plateroti, M.; et al. Netrin-1 regulates somatic cell reprogramming and pluripotency maintenance. Nat. Commun. 2015, 6, 7398. [Google Scholar] [CrossRef]
- Das, P.M.; Ramachandran, K.; Vanwert, J.; Ferdinand, L.; Gopisetty, G.; Reis, I.M.; Singal, R. Methylation mediated silencing of TMS1/ASC gene in prostate cancer. Mol. Cancer 2006, 5, 28. [Google Scholar] [CrossRef]

| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog Number | Species, Monoclonal or Polyclonal | Dilution |
|---|---|---|---|---|---|
| CYP19A1 | 209-503 | H-300 | Santa Cruz Biotechnology, sc-30086 | Rabbit polyclonal | 1:50 |
| ESR2/ERbeta | 17-mer, close to C-terminus | anti-ESR2 | BioGeneX, AR385 | Rabbit polyclonal | 1:100 |
| Primer Name | Primer Sequence | Amplicon Size (bps) | Annealing Temperature | |
|---|---|---|---|---|
| rDNMT1 | Forward: | 5′-GAGGTGGGCGACTGCGTCTC-3′ | 214 | 60 |
| Reverse: | 5′-TGTGGATGTAGGAAAGTTGCA-3′ | |||
| rDNMT3a | Forward: | 5′-CAGAATAGCCAAGTTCAGCAAAGTGA-3′ | 68 | 58 |
| Reverse: | 5′-CTTTGCCCTGCTTTATGGAG-3′ | |||
| rDNMT3b | Forward: | 5′-GTTAAAGAAAGTACAGACAATAACCAC-3′ | 220 | 57 |
| Reverse: | 5′-TCTGATGACTGGCACACTCC-3′ | |||
| rDNMT3l | Forward: | 5’-AATGGCCGAAATCAGCCCCA-3’ | 139 | 60 |
| Reverse: | 5’-CGCTGGTTCACGTTGACTTC-3’ | |||
| rMeCP2 | Forward: | 5′-GTCGCTCTGCTGGAAAGTAT-3′ | 189 | 57 |
| Reverse: | 5′-TGGGCTTCTTAGGTGGTTTC-3′ | |||
| rMBD1 | Forward: | 5′-CAGCAGTCACAACCTTCCTG-3′ | 182 | 58 |
| Reverse: | 5′-GGTGCCAATCCCTCCTATCT-3′ | |||
| rMBD2 | Forward: | 5′-GTCGGCCCAGGTAGTAATGAT-3′ | 195 | 60 |
| Reverse: | 5′-GACTCGCTCTTCCTGTTTCCT-3′ | |||
| rMBD3 | Forward: | 5′-CTGAACACTGCACTGCCTGTA-3′ | 145 | 58 |
| Reverse: | 5′-GTTTCTTCTCCCAGAAAAGCTG-3′ | |||
| rMBD4 | Forward: | 5′-CCTACCGGATCTTTTGTGTCA-3′ | 90 | 58 |
| Reverse: | 5′-GATTTTCCCAAAGCCAGTCAT-3′ | |||
| rMBP3l1 | Forward: | 5’-GCTGGTTGGAGACTGGCAAT-3’ | 96 | 60 |
| Reverse: | 5’-TTGCCCATCTGACTCCGTTC-3’ | |||
| rRpl19 | Forward: | 5′-GCATATGGGCATAGGGAAGA-3′ | 197 | 58 |
| Reverse: | 5′-CCATGAGAATCCGCTTGTTT-3′ |
| Number of Animals | % Animals (Normal) | Odds Ratio | p-Value | |
|---|---|---|---|---|
| AIN | 10/11 | 91% | ||
| BPA | 10/14 | 71% | 4 | 0.340 |
| HFB | 9/14 | 64% | 5.6 | 0.180 |
| HFB + BPA | 6/15 | 40% | 15 | 0.014 * |
| Number of Animals | % Animals (Normal) | Odds Ratio | p-Value | |
|---|---|---|---|---|
| AIN | 6/7 | 85.7% | ||
| BPA25 | 4/5 | 80.0% | 1.5 | 1 |
| HFB | 4/5 | 80.0% | 1.5 | 1 |
| HFB + BPA | 3/6 | 50.0% | 6.0 | 0.265 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, S.-M.; Rao, R.; Ouyang, B.; Tam, N.N.C.; Schoch, E.; Song, D.; Ying, J.; Leung, Y.-K.; Govindarajah, V.; Tarapore, P. Three-Generation Study of Male Rats Gestationally Exposed to High Butterfat and Bisphenol A: Impaired Spermatogenesis, Penetrance with Reduced Severity. Nutrients 2021, 13, 3636. https://doi.org/10.3390/nu13103636
Ho S-M, Rao R, Ouyang B, Tam NNC, Schoch E, Song D, Ying J, Leung Y-K, Govindarajah V, Tarapore P. Three-Generation Study of Male Rats Gestationally Exposed to High Butterfat and Bisphenol A: Impaired Spermatogenesis, Penetrance with Reduced Severity. Nutrients. 2021; 13(10):3636. https://doi.org/10.3390/nu13103636
Chicago/Turabian StyleHo, Shuk-Mei, Rahul Rao, Bin Ouyang, Neville N. C. Tam, Emma Schoch, Dan Song, Jun Ying, Yuet-Kin Leung, Vinothini Govindarajah, and Pheruza Tarapore. 2021. "Three-Generation Study of Male Rats Gestationally Exposed to High Butterfat and Bisphenol A: Impaired Spermatogenesis, Penetrance with Reduced Severity" Nutrients 13, no. 10: 3636. https://doi.org/10.3390/nu13103636
APA StyleHo, S.-M., Rao, R., Ouyang, B., Tam, N. N. C., Schoch, E., Song, D., Ying, J., Leung, Y.-K., Govindarajah, V., & Tarapore, P. (2021). Three-Generation Study of Male Rats Gestationally Exposed to High Butterfat and Bisphenol A: Impaired Spermatogenesis, Penetrance with Reduced Severity. Nutrients, 13(10), 3636. https://doi.org/10.3390/nu13103636








