Development and Relative Validity of the Chronic Kidney Disease Short Food Frequency Questionnaire (CKD SFFQ) to Determine Diet Quality and Dietary Habits among Adults with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
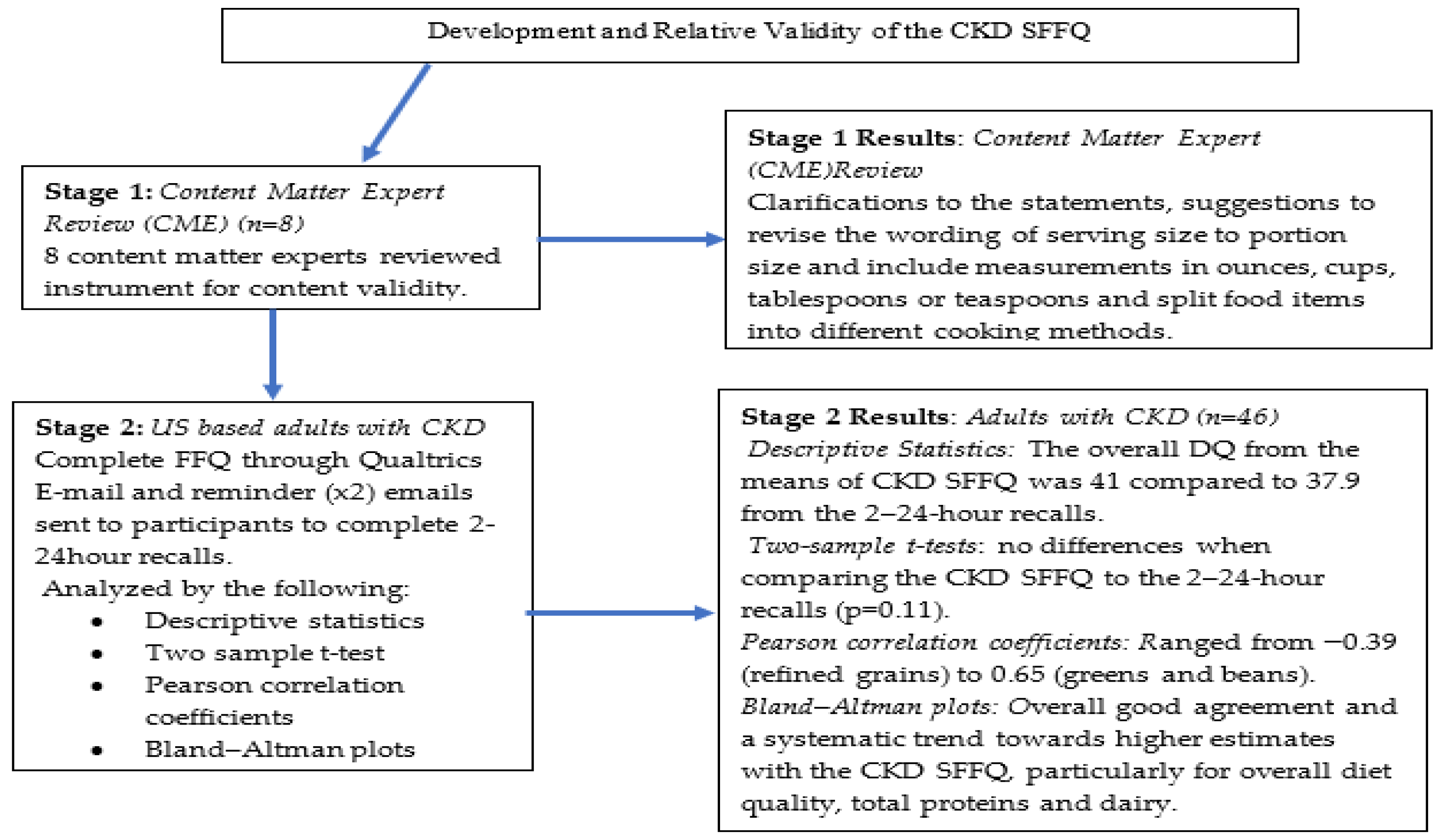
2.1. Stage 1: Development of the CKD SFFQ
Design and Participants
2.2. Stage 2: Criterion Validation Process
2.3. Diet Quality (DQ) Analysis
2.3.1. HEI-2015 Scores
2.3.2. CKD SFFQ
2.4. Statistical Analysis
3. Results
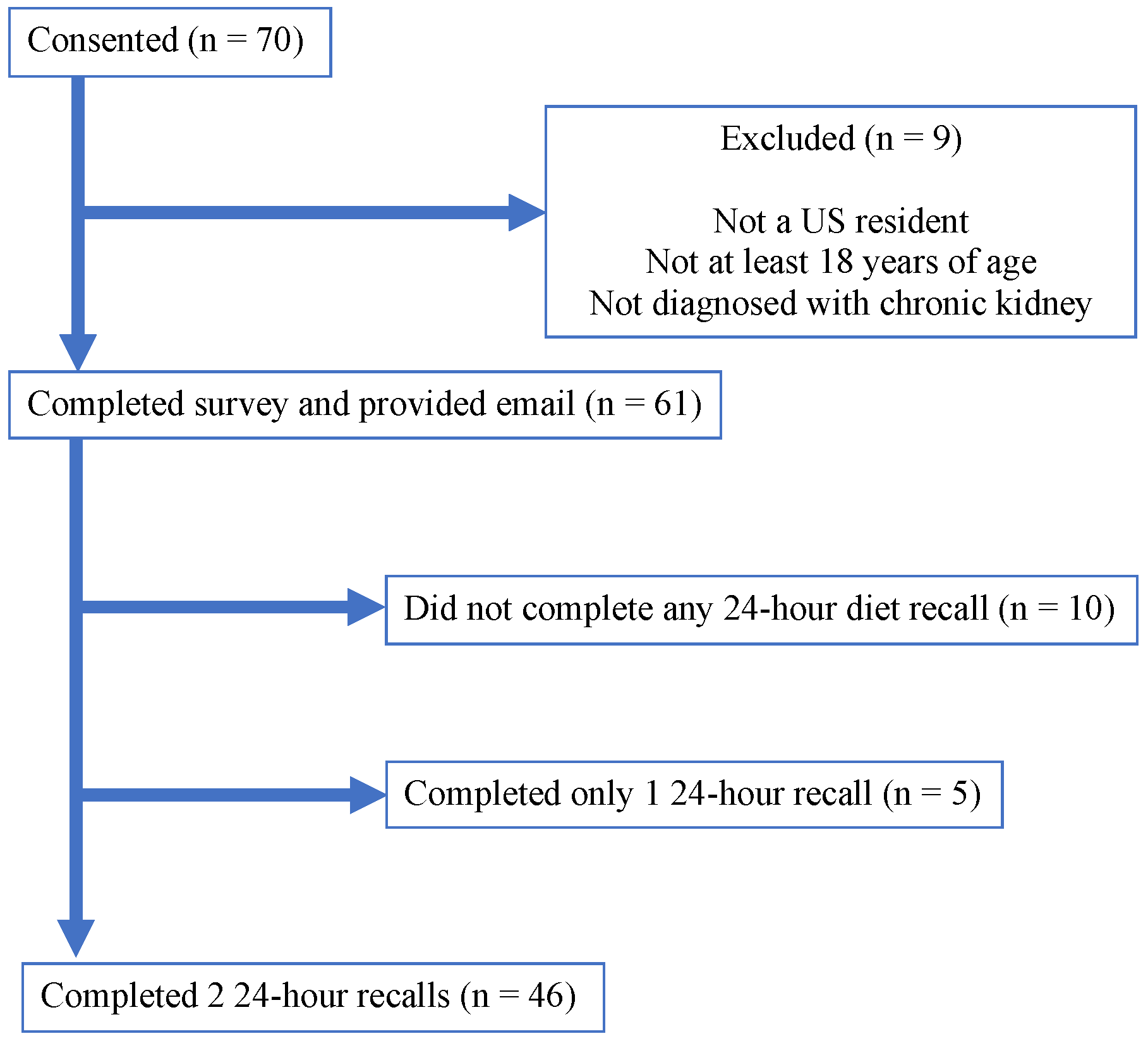
3.1. Study Participants
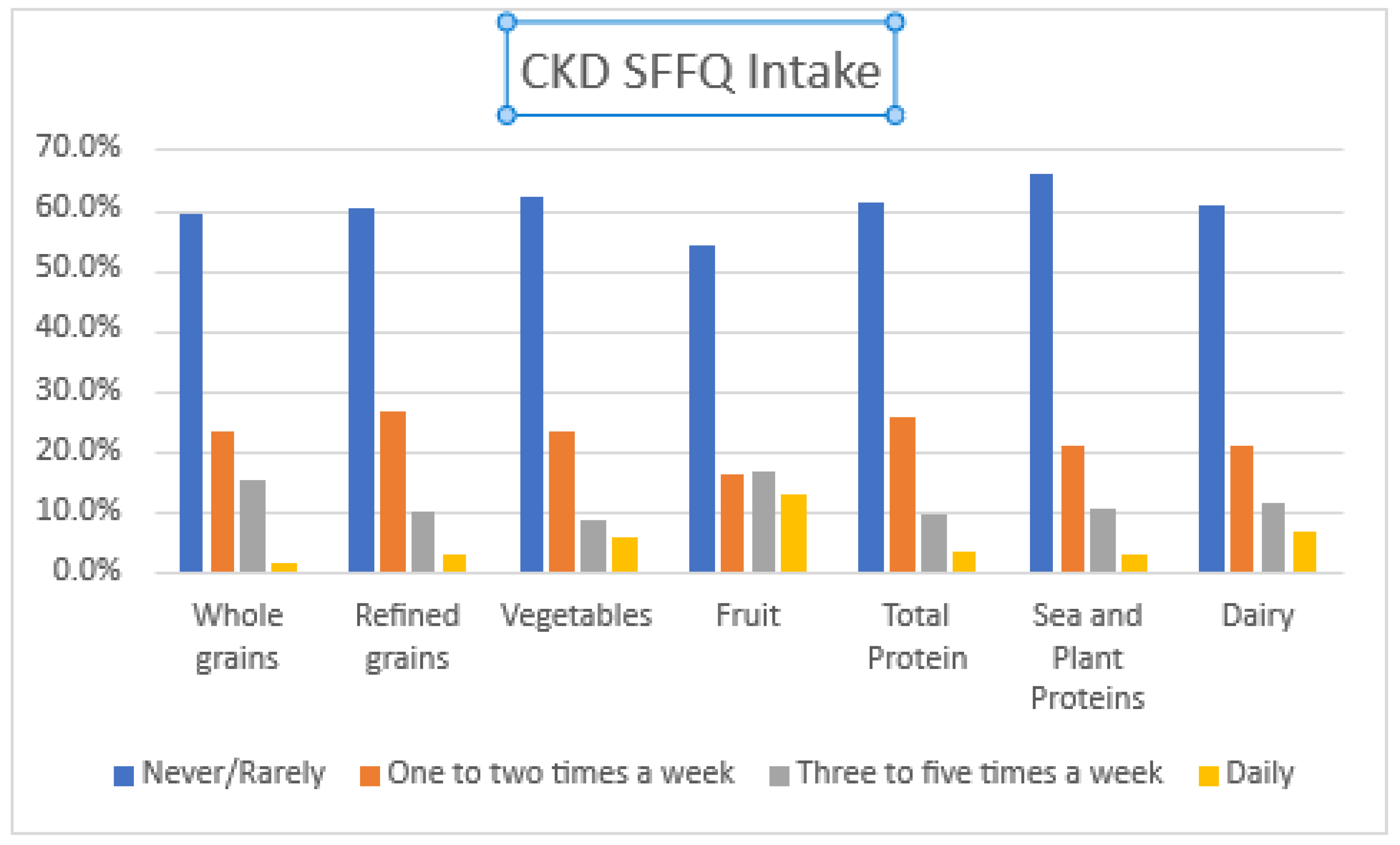
3.2. Dietary Intake
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Chronic Kidney Disease in the United States, 2019. Centers for Disease Control and Prevention; 2019. Available online: https://www.cdc.gov/kidneydisease/publications-resources/2019-national-facts.html (accessed on 8 September 2021).
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The cytoprotective protein C pathway. Blood 2006, 109, 3161–3172. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Palmer, S.C.; Maggo, J.K.; Campbell, K.L.; Craig, J.C.; Johnson, D.W.; Sutanto, B.; Ruospo, M.; Tong, A.; Strippoli, G.F. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2017, 2017, CD011998. [Google Scholar] [CrossRef] [Green Version]
- USRDS. National Institute of Diabetes Digestive Kidney Disease. USRDS 2018 Annual Date Report—Chapter 5: Mortality. Available online: https://www.usrds.org/media/1730/v2_c05_mortality_18_usrds.pdf (accessed on 8 September 2021).
- Nazar, C.M.J. Significance of diet in chronic kidney disease. J. Nephropharmacol. 2013, 2, 37–43. [Google Scholar]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikizler, T.A.; Cuppari, L. The 2020 Updated KDOQI Clinical Practice Guidelines for Nutrition in Chronic Kidney Disease. Blood Purif. 2021, 50, 1–5. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Ramos, C.I.; Nerbass, F.B.; Cuppari, L. Diet Quality of Chronic Kidney Disease Patients and the Impact of Nutritional Counseling. J. Ren. Nutr. 2018, 28, 403–410. [Google Scholar] [CrossRef]
- Kramer, H. Diet and Chronic Kidney Disease. Adv. Nutr. 2019, 10, S367–S379. [Google Scholar] [CrossRef] [PubMed]
- Santin, F.; Canella, D.; Borges, C.; Lindholm, B.; Avesani, C.M. Dietary Patterns of Patients with Chronic Kidney Disease: The Influence of Treatment Modality. Nutrients 2019, 11, 1920. [Google Scholar] [CrossRef] [Green Version]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Goraya, N.; Wesson, D.E. Dietary interventions to improve outcomes in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 505–510. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. Kidney Int. Suppl. 2013, 3, 1–150. Available online: www.publicationethics.org (accessed on 8 September 2021).
- World Kidney on Behalf of the World Kidney Day Steering Committee; Kovesdy, C.P.; Furth, S.; Zoccali, C. Obesity and kidney disease: Hidden consequences of the epidemic. Indian J. Nephrol. 2017, 27, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [Green Version]
- Olokor, A.B.; Ojogwu, I.L.; Ugbodaga, P.F. Hyperhomocysteinemia in Chronic Kidney Disease Patients in a Teaching Hospital in Nigeria. Br. J. Med. Med. Res. 2016, 18, 1–7. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Moore, L.W. Does Kidney Longevity Mean Healthy Vegan Food and Less Meat or Is Any Low-Protein Diet Good Enough? J. Ren. Nutr. 2019, 29, 79–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lew, Q.-L.J.; Jafar, T.H.; Koh, H.W.L.; Jin, A.; Chow, K.Y.; Yuan, J.-M.; Koh, W.-P. Red Meat Intake and Risk of ESRD. J. Am. Soc. Nephrol. 2016, 28, 304–312. [Google Scholar] [CrossRef] [PubMed]
- USDA. 2015 Dietary Guidelines|Dietary Guidelines for Americans. Published 2016. Available online: https://www.dietaryguidelines.gov/about-dietary-guidelines/previous-editions/2015-dietary-guidelines (accessed on 8 September 2021).
- Onvani, S.; Haghighatdoost, F.; Surkan, P.J.; Larijani, B.; Azadbakht, L. Adherence to the Healthy Eating Index and Alternative Healthy Eating Index dietary patterns and mortality from all causes, cardiovascular disease and cancer: A meta-analysis of observational studies. J. Hum. Nutr. Diet. 2016, 30, 216–226. [Google Scholar] [CrossRef]
- Colby, S.; Zhou, W.; Allison, C.; Mathews, A.E.; Olfert, M.D.; Morrell, J.S.; Byrd-Bredbenner, C.; Greene, G.; Brown, O.; Kattelmann, K.; et al. Development and Validation of the Short Healthy Eating Index Survey with a College Population to Assess Dietary Quality and Intake. Nutrients 2020, 12, 2611. [Google Scholar] [CrossRef] [PubMed]
- Beto, J.A.; Schury, K.; Bansal, V. Strategies to promote adherence to nutritional advice in patients with chronic kidney disease: A narrative review and commentary. Int. J. Nephrol. Renov. Dis. 2016, 9, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Carroll, R.J.; Midthune, D.; Subar, A.F.; Shumakovich, M.; Freedman, L.S.; Thompson, F.; Kipnis, V. Taking Advantage of the Strengths of Two Different Dietary Assessment Instru-ments to Improve Intake Estimates for Nutritional Epidemiology. 2010. Available online: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.227.6967 (accessed on 8 September 2021).
- Solbak, N.M.; Robson, P.J.; Siou, G.L.; Al Rajabi, A.; Paek, S.; Vena, J.E.; Kirkpatrick, S.I. Administering a combination of online dietary assessment tools, the Automated Self-Administered 24-Hour Dietary Assessment Tool, and Diet History Questionnaire II, in a cohort of adults in Alberta’s Tomorrow Project. J. Acad. Nutr. Diet. 2021, 121, 1312–1326. [Google Scholar] [CrossRef]
- Procter-Gray, E.; Olendzki, B.; Kane, K.; Churchill, L.; Hayes, R.B.; Aguirre, A.; Kang, H.-J.; Li, W. Comparison of Dietary Quality Assessment Using Food Frequency Questionnaire and 24-hour-recalls in Older Men and Women. AIMS Public Health 2017, 4, 326–346. [Google Scholar] [CrossRef]
- Newby, P.K.; Hu, F.B.; Rimm, E.B.; Smith-Warner, S.A.; Feskanich, D.; Sampson, L.; Willett, W.C. Reproducibility and validity of the Diet Quality Index Revised as assessed by use of a food-frequency questionnaire. Am. J. Clin. Nutr. 2003, 78, 941–949. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, R.; Ajayan, K.; Bharathi, A.V.; Zhang, X.; Islam, S.; Soman, C.R.; Merchant, A.T. Refinement and validation of an FFQ developed to estimate macro- and micronutrient intakes in a south Indian population. Public Health Nutr. 2009, 12, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masip, G.; Keski-Rahkonen, A.; Pietiläinen, K.H.; Kujala, U.M.; Rottensteiner, M.; Väisänen, K.; Kaprio, J.; Bogl, L.H. Development of a food-based diet quality score and associations with eating styles and nutrient intakes in Finnish twins. Proc. Nutr Soc. 2020, 79, E419. [Google Scholar] [CrossRef]
- Affret, A.; Wagner, S.; El Fatouhi, D.; Dow, C.; Correia, E.; Niravong, M.; Clavel-Chapelon, F.; De Chefdebien, J.; Fouque, D.; Stengel, B.; et al. Validity and reproducibility of a short food frequency questionnaire among patients with chronic kidney disease. BMC Nephrol. 2017, 18, 297. [Google Scholar] [CrossRef]
- Steinemann, N.; Grize, L.; Ziesemer, K.; Kauf, P.; Probst-Hensch, N.; Brombach, C. Relative validation of a food frequency questionnaire to estimate food intake in an adult population. Food Nutr. Res. 2017, 61, 1305193. [Google Scholar] [CrossRef] [Green Version]
- Dahl, L.; Mæland, C.A.; Bjørkkjær, T. A short food frequency questionnaire to assess intake of seafood and n-3 supplements: Validation with biomarkers. Nutr. J. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadifard, N.; Sajjadi, F.; Maghroun, M.; Alikhasi, H.; Nilforoushzadeh, F.; Sarrafzadegan, N. Validation of a simplified food frequency questionnaire for the assessment of dietary habits in Iranian adults: Isfahan Healthy Heart Program, Iran. ARYA Atheroscler. 2015, 11, 139–146. [Google Scholar]
- Huang, Y.; Lee, M.; Pan, W.; Wahlqvist, M.L. Validation of a Simplified Food Frequency Questionnaire as Used in the Nutrition and Health Survey in Taiwan (NAHSIT) for the Elderly. Asia Pac. J. Clin. Nutr. 2011, 20, 134–140. [Google Scholar] [PubMed]
- Crocker, L.; Llabre, M.; Miller, M.D. The Generalizability of Content Validity Ratings. J. Educ. Meas. 1988, 25, 287–299. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, E.F.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The automated self-administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the national cancer institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Developing the Healthy Eating Index. Developing the Healthy Eating Index (HEI)|EGRP/DCCPS/NCI/NIH. Available online: https://epi.grants.cancer.gov/hei/developing.html (accessed on 8 September 2021).
- ASA24® Evaluation & Validation. ASA24® Evaluation & Validation|EGRP/DCCPS/NCI/NIH. Available online: https://epi.grants.cancer.gov/asa24/respondent/validation.html (accessed on 8 September 2021).
- Reedy, J.; Lerman, J.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Leppälä, J.; Lagström, H.; Kaljonen, A.; Laitinen, K. Construction and evaluation of a self-contained index for assessment of diet quality. Scand. J. Public Health 2010, 38, 794–802. [Google Scholar] [CrossRef]
- FDA: Interactive Food Lable-Sodium. Available online: https://www.accessdata.fda.gov/scripts/interactivenutritionfactslabel/sodium.cfm (accessed on 8 September 2021).
- FDA: Added Sugars on the New Food Label. Available online: https://www.fda.gov/food/new-nutrition-facts-label/added-sugars-new-nutrition-facts-label (accessed on 8 September 2021).
- FDA: Interactive Food Label Saturated Fat. Available online: https://www.accessdata.fda.gov/scripts/interactivenutritionfactslabel/saturated-fat.cfm (accessed on 8 September 2021).
- FDA: Interactive Food Label D Fiber. Available online: https://www.accessdata.fda.gov/scripts/interactivenutritionfactslabel/dietary-fiber.cfm (accessed on 8 September 2021).
- Lombard, M.J.; Steyn, N.P.; Charlton, K.E.; Senekal, M. Application and interpretation of multiple statistical tests to evaluate validity of dietary intake assessment methods. Nutr. J. 2015, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Masson, L.; Mcneill, G.; Tomany, J.; Simpson, J.; Peace, H.; Wei, L.; Grubb, D.; Bolton-Smith, C. Statistical approaches for assessing the relative validity of a food-frequency questionnaire: Use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003, 6, 313–321. [Google Scholar] [CrossRef]
- Watson, J.F.; Collins, C.E.; Sibbritt, D.W.; Dibley, M.J.; Garg, M.L. Reproducibility and comparative validity of a food frequency questionnaire for Australian children and adolescents. Int. J. Behav. Nutr. Phys. Act. 2009, 6, 17–62. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Olendzki, B.; Pagoto, S.; Hurley, T.G.; Magner, R.P.; Ockene, I.S.; Schneider, K.L.; Merriam, P.A.; Hébert, J.R. Number of 24-Hour Diet Recalls Needed to Estimate Energy Intake. Ann. Epidemiol. 2009, 19, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamah-Levy, T.; Rodríguez-Ramírez, S.; Gaona-Pineda, E.B.; Cuevas-Nasu, L.; Carriquiry, A.L.; A Rivera, J. Three 24-Hour Recalls in Comparison with One Improve the Estimates of Energy and Nutrient Intakes in an Urban Mexican Population. J. Nutr. 2016, 146, 1043–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, R.J.; Midthune, D.; Subar, A.F.; Shumakovich, M.; Freedman, L.S.; Thompson, F.E.; Kipnis, V. Taking Advantage of the Strengths of 2 Different Dietary Assessment Instruments to Improve Intake Estimates for Nutritional Epidemiology. Am. J. Eidemiol. 2012, 175, 340–347. [Google Scholar] [CrossRef] [Green Version]
- DeBiasse, M.A.; Bowen, D.J.; Quatromoni, P.A.; Quinn, E.; Quintiliani, L.M. Feasibility and Acceptability of Dietary Intake Assessment Via 24-Hour Recall and Food Frequency Questionnaire among Women with Low Socioeconomic Status. J. Acad. Nutr. Diet. 2018, 118, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIDDK. Available online: https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd/eating-nutrition/nutrition-advanced-chronic-kidney-disease-adults (accessed on 7 September 2021).
- Aycart, D.; Acevedo, S.; Eguiguren-Jimenez, L.; Andrade, J. Influence of Plant and Animal Proteins on Inflammation Markers among Adults with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1660. [Google Scholar] [CrossRef]
- Mirmiran, P.; Yuzbashian, E.; Aghayan, M.; Mahdavi, M.; Asghari, G.; Azizi, F. A Prospective Study of Dietary Meat Intake and Risk of Incident Chronic Kidney Disease. J. Ren. Nutr. 2020, 30, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary Protein Sources and Risk for Incident Chronic Kidney Disease: Results from the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren. Nutr. 2017, 27, 233–242. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-Based Diets and Incident CKD and Kidney Function. Clin. J. Am. Soc. Nephrol. 2019, 14, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Clarke, R.E.; Coughlan, M.T. Stirring the Pot: Can Dietary Modification Alleviate the Burden of CKD? Nutrients 2017, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.L. Conducting Online Surveys. J. Hum. Lact. 2018, 35, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.R.; Waithaka, E.; Paudyal, A.; Simkhada, P.; van Teijlingen, E. Nepal Journal of Epidemiology Guide to the Design and Application of Online Questionnaire Surveys. 2016. Available online: www.nepjol.info/index.php/NJE (accessed on 7 September 2021).
| Variables | No. of Responses (%) |
|---|---|
| Sex | |
| Male | 14 (30.4%) |
| Female | 32 (69.6%) |
| Race/Ethnicity | |
| African American | 8 (17.4%) |
| Asian | 1 (2.2 %) |
| Caucasian | 37 (80.4%) |
| Age | |
| 18–24 years old | 0 |
| 25–29 years old | 1 (2.2%) |
| 30–49 years old | 13 (28.3%) |
| 50–59 years old | 8 (17.4%) |
| 60–69 years old | 8 (17.4%) |
| 70+ years old | 16 (34.7%) |
| Length of time with CKD | |
| <6 months ago | 0 |
| 1–2 years ago | 7 (15.2%) |
| 3–4 years ago | 9 (19.6%) |
| >5 years ago | 30 (65.2%) |
| Initial stage of CKD | |
| 1 | 6 (13.0%) |
| 2 | 8 (17.4%) |
| 3 | 21 (45.7%) |
| 4 | 6 (13.0%) |
| 5 | 1 (2.1%) |
| Don’t know | 4 (8.8%) |
| Conditions | |
| Cancer | 5 (10.9%) |
| Depression | 17 (37.0%) |
| Diabetes | 15 (32.6%) |
| Diverticulosis/Diverticulitis | 6 (13.0%) |
| Gastric reflux | 18 (39.1%) |
| Heart disease (includes high blood pressure, heart attack, artery disease, stroke, angina) | 31 (67.4%) |
| Irritable Bowels | 9 (19.6%) |
| Liver disease | 5 (10.9%) |
| Lung disease | 8 (17.4%) |
| Nausea/Vomiting | 9 (19.6%) |
| Other | 28 (60.9%) |
| Unknown | 1 (2.2%) |
| 1 condition | 3 (6.5%) |
| 2 conditions | 10 (21.7%) |
| 3 conditions | 10 (21.7%) |
| 4 or more conditions | 21 (45.7%) |
| Item | HEI-2015 | CKD SFFQ | t-Test | ||
|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | ||
| Overall Diet Quality | 37.89 | 62.15 | 41.08 | 57.54 | 0.11 |
| Total Vegetables | 3.20 | 8.57 | 3.83 | 1.66 | 0.09 |
| Greens and Beans | 0.76 | 2.44 | 0.72 | 0.74 | 0.44 |
| Total Fruit | 2.36 | 4.54 | 2.62 | 0.50 | 0.22 |
| Whole Fruit | 1.99 | 4.25 | 1.62 | 0.30 | 0.12 |
| Dairy | 3.04 | 6.20 | 3.68 | 2.04 | 0.07 |
| Total Protein | 11.76 | 56.89 | 13.48 | 10.09 | 0.08 |
| Seafood and Plant Proteins | 3.70 | 12.32 | 3.05 | 2.35 | 0.13 |
| Refined Grains | 9.65 | 25.60 | 10.71 | 7.24 | 0.11 |
| Whole Grains | 1.57 | 2.13 | 1.37 | 1.24 | 0.23 |
| Components | r | (CI 95%) * | p-Value ** |
|---|---|---|---|
| Overall Diet Quality | 0.21 | (−0.08–0.46) | 0.16 |
| Total Vegetables | 0.18 | (−0.12–0.44) | 0.24 |
| Greens and Beans | 0.60 | (0.37–0.76) | <0.001 ** |
| Total Fruit | 0.23 | (−0.12–0.43) | 0.17 |
| Whole Fruit | 0.21 | (−0.15–0.41) | 0.16 |
| Dairy | 0.41 | (0.03–0.55) | 0.01 ** |
| Total Protein | −0.02 | (−0.30–0.27) | 0.91 |
| Seafood and Plant Proteins | 0.29 | (0.01–0.53) | 0.04 ** |
| Refined Grains | −0.52 | (−0.52–0.02) | <0.001 ** |
| Whole Grains | 0.25 | (−0.08–0.46) | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Zarah, A.; Feraudo, M.C.; Andrade, J.M. Development and Relative Validity of the Chronic Kidney Disease Short Food Frequency Questionnaire (CKD SFFQ) to Determine Diet Quality and Dietary Habits among Adults with Chronic Kidney Disease. Nutrients 2021, 13, 3610. https://doi.org/10.3390/nu13103610
Bin Zarah A, Feraudo MC, Andrade JM. Development and Relative Validity of the Chronic Kidney Disease Short Food Frequency Questionnaire (CKD SFFQ) to Determine Diet Quality and Dietary Habits among Adults with Chronic Kidney Disease. Nutrients. 2021; 13(10):3610. https://doi.org/10.3390/nu13103610
Chicago/Turabian StyleBin Zarah, Aljazi, Mary Carissa Feraudo, and Jeanette Mary Andrade. 2021. "Development and Relative Validity of the Chronic Kidney Disease Short Food Frequency Questionnaire (CKD SFFQ) to Determine Diet Quality and Dietary Habits among Adults with Chronic Kidney Disease" Nutrients 13, no. 10: 3610. https://doi.org/10.3390/nu13103610
APA StyleBin Zarah, A., Feraudo, M. C., & Andrade, J. M. (2021). Development and Relative Validity of the Chronic Kidney Disease Short Food Frequency Questionnaire (CKD SFFQ) to Determine Diet Quality and Dietary Habits among Adults with Chronic Kidney Disease. Nutrients, 13(10), 3610. https://doi.org/10.3390/nu13103610








