Abstract
Background: Osteoprotegerin (OPG) belongs to the tumour necrosis factor superfamily and is known to accelerate endothelial dysfunction and vascular calcification. OPG concentrations are elevated in patients with chronic kidney disease. The aim of this study was to investigate the association between OPG levels and frequent complications of chronic kidney disease (CKD) such as anaemia, protein energy wasting (PEW), inflammation, overhydration, hyperglycaemia and hypertension. Methods: One hundred non-dialysis-dependent men with CKD stage 3–5 were included in the study. Bioimpedance spectroscopy (BIS) was used to measure overhydration, fat amount and lean body mass. We also measured the serum concentrations of haemoglobin, albumin, total cholesterol, C-reactive protein (CRP), fibrinogen and glycated haemoglobin (HgbA1c), as well as blood pressure. Results: We observed a significant, positive correlation between OPG and age, serum creatinine, CRP, fibrinogen, HgbA1c concentrations, systolic blood pressure and overhydration. Negative correlations were observed between OPG and glomerular filtration rate (eGFR), serum albumin concentrations and serum haemoglobin level. Logistic regression models revealed that OPG is an independent marker of metabolic complications such as anaemia, PEW, inflammation and poor renal prognosis (including overhydration, uncontrolled diabetes and hypertension) in the studied population. Conclusion: Our results suggest that OPG can be an independent marker of PEW, inflammation and vascular metabolic disturbances in patients with chronic kidney disease.
1. Introduction
Osteoprotegerin (OPG) is a molecule belonging to the tumour necrosis factor (TNF) superfamily. It is a decoy receptor for TNF-related apoptosis-inducing ligand (TRAIL). The TNF superfamily regulates immune responses, homeostasis as well as haematopoiesis [1]. Initially, OPG was identified as a bone resorption inhibitor that blocks the binding of RANK (receptor activator of nuclear factor kappa-β) to its ligand RANKL [2]. Further studies revealed that it has an additional impact on the cardiovascular and immune systems [3]. The OPG/RANKL/RANK system is involved in pathological angiogenesis, inflammatory states and cell survival. OPG is a glycoprotein released by vascular smooth muscle cells, endothelial cells, osteoblasts and immune cells. There is a signalling pathway between endothelial cells and osteoblasts during osteogenesis creating a connection between angiogenesis and osteogenesis [4]. The OPG/RANKL/RANK/TRAIL system, via receptors located on the cell surface, sends intracellular signals and modifies gene expression. As a result, monocytes, neutrophils and endothelial cells are recruited through cytokine production and receptor activation [5]. Serum OPG levels are associated with endothelial dysfunction and mediate vascular calcification [6]. Elevated OPG levels were observed in patients with atherosclerosis, heart failure, metabolic syndrome and diabetes [7,8,9].
The role of OPG in kidney pathologies is not well understood. OPG is expressed in kidney samples, cultured tubular cells and urinary exosome-like vesicles. It has been hypothesized that it can play a role in matrix deposition, inflammation and apoptosis [10]. Furthermore, the role of OPG in cardiovascular pathology and vascular calcification, which are common complications of chronic kidney disease, makes OPG an interesting marker also in CKD. When compared to healthy individuals, non-dialysis-dependent CKD patients, haemodialysis and peritoneal dialysis patients and renal transplant recipients present elevated levels of OPG [11,12,13]. As OPG is involved in vascular calcification, it is also associated with cardiovascular mortality in CKD patients [14]. Decreased levels of OPG are observed in nephrotic syndrome, which is probably caused by the loss with urine. Glucocorticosteroid treatment increases RANKL and reduces OPG expression, which also decreases the OPG levels in patients with nephrotic syndrome [15].
The purpose of our study was to investigate the association between OPG levels and the main complications of chronic kidney disease, including anaemia, protein energy wasting, inflammation and poor prognosis factors of CKD progression such as overhydration, hyperglycaemia and hypertension.
2. Methods
2.1. Design
A prospective, observational study in male patients with chronic kidney disease non-treated with dialysis was performed. The inclusion criterion was eGFR lower than 60 mL/min/1.73 m2.
2.2. Patients
Participants were recruited among patients visiting the Nephrological Outpatient Clinic for a routine control during the period between November 2018 and February 2020. If they fulfilled the inclusion criteria, a new visit was arranged for participation in the study. The participants were asked to avoid saunas, physical exertion and alcohol consumption the day before the examination. The visit took place after overnight fasting.
One hundred men with chronic kidney disease and eGFR lower than 60 mL/min/1.73 m2 were included in the study. The exclusion criteria were: renal replacement therapy or its requirement within the following 3 months, clinical signs of infection and presence of metal parts in the body. The study protocol was accepted by the local ethics committee (Bioethics Committee in Military Institute of Medicine, IRB acceptance number 120/WIM/2018 obtained 22 August 2018). All participants signed an informed consent.
Body composition including overhydration (OH), fat amount and lean body mass were measured by bioimpedance spectroscopy with the use of a Body Composition Monitor (Fresenius Medical Care). While being measured, patients stayed in a supine position after a 5 min rest. Electrodes were placed in a tetrapolar configuration (on one hand and one foot).
Blood samples for standard measurements were collected after an overnight fast and were transported immediately to the local Department of Laboratory Diagnostics. Concentrations of high-sensitivity C-reactive protein were determined by a nephelometry assay (BNII Siemens) with a cut-off point of 0.8 mg/dL. Serum creatinine concentrations were measured using the Jaffe method (Gen.2; Roche Diagnostics GmbH, Risch-Rotkreuz, Switzerland), and serum albumin levels using a BCP Albumin Assay Kit (Roche Diagnostics GmbH, Risch-Rotkreuz, Switzerland). Samples for measuring osteoprotegerin (OPG) levels were kept frozen at −80 °C. OPG levels were assessed using the Luminex MAGPIX platform.
GFR was calculated according to the short Modification of Diet in Renal Disease (MDRD) formula.
GFR in mL/min per 1.73 m2 = 175 × SerumCr−1.154 × age−0.203 × 1.212 (if patient is black) × 0.742 (if female)
2.3. Defining the Complications of Chronic Kidney Disease
Complications of chronic kidney disease were defined as follows:
- -
- Anaemia, when serum haemoglobin level was lower than 12 g/dL [16]
- -
- Protein energy wasting (PEW), when one of the following occurred: serum albumin level was lower than 3.8 g/dL, total cholesterol level was lower than 100 mg/dL, BMI was lower than 23 kg/m2 or Fat was lower than 10% [17]
- -
- Inflammatory state, when CRP >0.8 mg/dL or Fbg >400 mg/dL [17]
- -
- Poor prognostic factors of CKD progression, when HbA1c >8% or SBP >140 mmHg or presence of overhydration, i.e., (OH) >4 L [18]
2.4. Statistical Analysis
The characteristics of the study population are presented using medians with interquartile ranges (for continuous data with distribution other than normal, tested with the Shapiro–Wilk test). The correlation between OPG and clinical and laboratory parameters was assessed using the Spearman rank correlation coefficient. Logistic regression was used to investigate the significance of OPG levels as a marker of metabolic complications of CKD. Given the known relationship between OPG, age and GFR, we compared the value of logistic models, with and without OPG, for each metabolic complication (anaemia, PEW, inflammation, uncontrolled factors of CKD progression). Suitability of fit of each model was assessed using the area under the curve (AUC) with standard error (SE) and presented using the Receiver Operating Characteristic (ROC) curve. The models were compared using Hanley’s algorithm [19]. The analysis was performed with Statistica 13.1 with p-values < 0.05 considered statistically significant.
3. Results
The study population consisted of 100 male patients with chronic kidney disease and eGFR lower than 60 mL/min/1.73 m2, non-treated with dialysis. The median age of the studied population was 66 years (59–72). Clinical data are presented in Table 1.

Table 1.
Clinical characteristics of the study population.
We observed significant, positive correlations between OPG and age, serum creatinine concentration, CRP, fibrinogen, HgBA1C, systolic blood pressure and overhydration. Significant, negative correlations were observed between OPG and eGFR, serum albumin concentration as well as serum haemoglobin level. Table 2.

Table 2.
Correlations between OPG and the studied parameters.
Logistic regression models, adjusted for age and GFR, were created to evaluate the influence of the OPG level as an independent marker of complications in chronic kidney disease patients, such as anaemia, PEW, an inflammatory state, poor prognostic factors of CKD progression.
3.1. Anaemia
The OPG level was a significant, independent marker of anaemia in the studied population (p < 0.001).
The model including OPG, age and GFR (AUC=0.84 ± 0.05) identified CKD patients with anaemia better than the model including only age and GFR (AUC=0.76 ± 0.06), as shown in Table 3. However, the difference was not of statistical significance (p = 0.096), as shown in Figure 1.

Table 3.
Markers associated with anaemia in chronic kidney disease patients based on a logistic regression model.
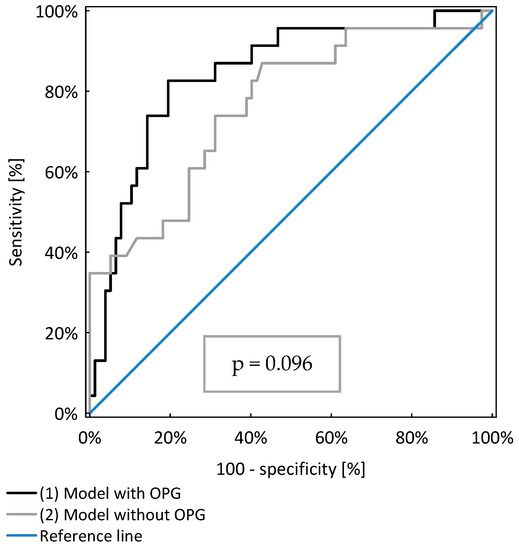
Figure 1.
Comparison of two models of logistic regression analysis identifying CKD patients with anaemia.
3.2. Protein Energy Wasting (PEW)
OPG was a significant, independent risk factor for PEW occurrence in the study population (p < 0.001). However, the model including OPG, age and GFR was not significantly better (AUC = 0.79 ± 0.05) than those considering only age and GFR (AUC = 0.71 ± 0.06, p = 0.142), as shown in Table 4 and Figure 2.

Table 4.
Markers associated with protein energy wasting in chronic kidney disease patients based on a logistic regression model.
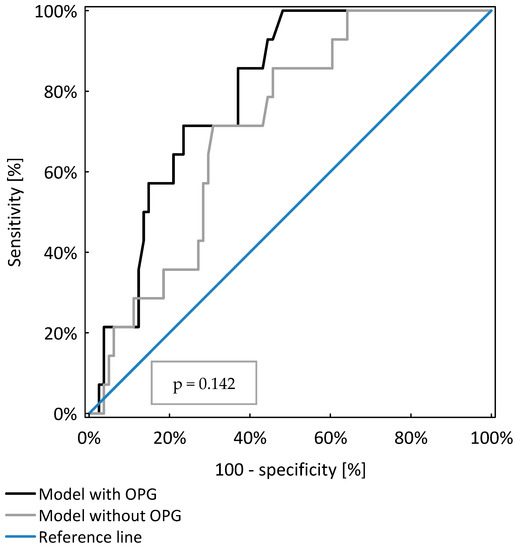
Figure 2.
Comparison of two models of logistic regression analysis identifying CKD patients with protein energy wasting.
3.3. Inflammatory State
OPG was a significant, independent risk factor for subclinical inflammation in the study population (p < 0.001). The model including OPG, age and GFR was significantly better (AUC = 0.77 ± 0.05) than the model including only age and GFR (AUC = 0.67 ± 0.06; p = 0.041), as shown in Table 5 and Figure 3.

Table 5.
Markers associated with inflammation in chronic kidney disease patients based on a logistic regression model.

Figure 3.
Comparison of two models of logistic regression analysis identifying CKD patients with protein energy wasting.
3.4. Poor Prognostic Factors (Overhydration, Hyperglycaemia and Hypertension)
Overhydration, hyperglycaemia and hypertension were chosen as poor prognostic factors of chronic kidney disease progression and were analysed together in logistic regression analysis. OPG was an independent marker that identified patients with poor prognostic factors (p < 0.001). The model including OPG, age and GFR was significantly better (AUC = 0.77 ± 0.05) than the model which included only age and GFR (AUC = 0.67 ± 0.06; p = 0.041), as shown in Table 6 and Figure 4.

Table 6.
Markers associated with poor prognostic factors in chronic kidney disease patients based on a logistic regression model.
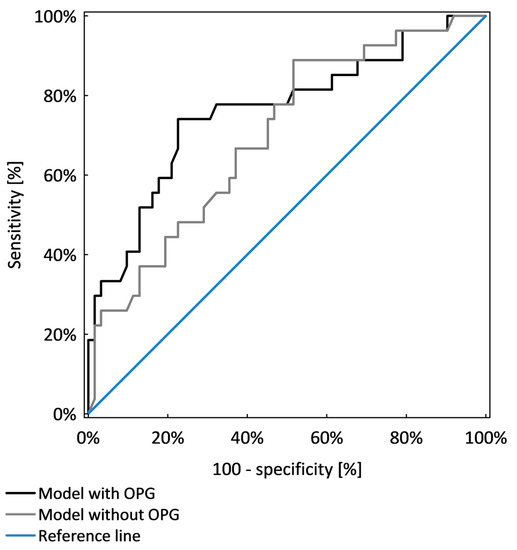
Figure 4.
Comparison of two models of logistic regression analysis identifying CKD patients with poor prognostic factors.
4. Discussion
The role of OPG in chronic kidney disease patients is insufficiently comprehended. We know that the OPG level increases together with a decrease in the glomerular filtration rate, as proved in many studies [20,21]. The results of our study also confirm these observations. In a population of non-dialysis-dependent men with stage 3–5 chronic kidney disease, a significant, inverse correlation between OPG levels and eGFR was noted (R = −0.36; p < 0.001).
In our study, we also observed significant correlations between OPG and parameters which indicate CKD complications. The OPG level was correlated with the level of haemoglobins and acted as a marker of anaemia occurrence in the studied population. The logistic regression model including OPG was superior to that including only age and GFR in identifying patients with anaemia. However, the difference was not of statistical significance. OPG, as a member of the TNF superfamily, can influence haematopoiesis. Some researchers have observed that TNF superfamily members can regulate the growth of hematopoietic stem cells and progenitor cells [1,22].
Aside from anaemia, protein energy wasting (PEW) is also a complication of chronic kidney disease. The components of PEW are low serum albumin, cholesterol levels as well as low BMI and fat amount [17]. In our study, the OPG level was a marker of PEW in non-dialysis-dependent CKD men. PEW aetiology in CKD patients is associated with many factors such as hormonal disorders, insulin resistance and a subclinical inflammatory state. In our study, the OPG levels also correlated with the CRP and fibrinogen levels. OPG was also a significant marker of instances of inflammation. The logistic regression model that included OPG proved significantly better in in the identification of subclinical inflammation than the model which only included age and GFR. This finding is unsurprising, because OPG is known for its pro-inflammatory action. OPG has been described as a marker of endothelial damage associated with the inflammatory process, and in vitro studies have shown that OPG is involved in inflammatory cell chemotaxis [23].
Most studies concerning OPG in CKD patients concentrate on the association between OPG and vascular calcification. OPG levels correlate with vascular calcification in non-dialysis-dependent CKD patients as well as in patients treated with renal replacement therapy [24,25]. In animal studies, mice without OPG developed severe vascular calcification [26]. Therefore, it is hypothesized that OPG has a protective role in vascular injury, and the elevation of OPG levels inhibits vascular calcification. However, vascular calcification can also occur in the medial as well as in the intimal layer. In atherosclerosis, intima is thickened, inflamed and calcified, forming plaques with diffuse localisation along the vessel walls, whereas medial calcification occurs along the elastic lamina, leading to stiffness of the artery wall. Studies in animal models, including Apolipoprotein E-deficient mice, suggest that OPG plays a protective, anti-calcification role in both the medial and the intimal (atherosclerotic) calcification process [27].
The OPG levels increase rapidly in the early stages of vascular calcification and subsequently remain almost invariant [21]. Therefore, OPG appears to be a marker of the onset of atherosclerosis. However, the development of cardiovascular disease (CVD) begins with endothelial dysfunction. The earliest manifestation of CVD is observed in the area of microcirculation, and in CKD patients, this area is affected by the loss of homeostasis, even in cases of non-severe renal impairment [28]. OPG, as a factor released by endothelial cells, can be a marker of endothelial dysfunction and may probably be involved in a complicated chain of pathophysiological connections between chronic kidney disease and CVD.
In our study, we analysed factors of poor prognosis of CKD progression which are associated with vascular damage, such as overhydration, hypertension and glycaemic disturbances expressed by HgbA1c. Positive, significant correlations were observed between OPG levels and all three parameters. In other studies, OPG levels were associated with glycaemic status, and higher OPG levels were observed in patients with poor glycaemic control [29]. In our study, OPG was identified independently, associated with the presence of poor prognostic factors (overhydration, hypertension and glycaemic disturbances) irrespective of age and eGFR.
The limitation of our study is its relatively small sample size. An increase in the number of participants could enable a division of the study population into various subgroups according to the different stages of chronic kidney disease. Complications of CKD are more severe in more advanced stages of CKD, and OPG influence could also be more significant.
5. Conclusions
CKD is in itself a cardiovascular risk factor, and cardiovascular mortality in CKD patients is high. CVD mortality in CKD patients is even greater in the presence of diabetes and hypertension [30]. Early detection of such complications and the identification of patients at risk of developing them is vital. Therefore, new markers that can predict metabolic disorders and have an unfavourable effect on vascular damage are necessary. OPG seems to be a possible marker associated with PEW, inflammation and vascular metabolic disturbances which is worth further study.
Author Contributions
A.R. and S.N. conceived and designed the study; K.R. performed the procedures; A.M. performed statistical analysis. A.R. and K.R. performed the database; A.R. analysed the data, preformed the literature review, prepared the manuscript. Z.B. performed and analysed the OPG measurements. A.R., K.R., A.M., Z.B. and S.N. corrected and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The preparation of this paper was supported by an internal grant of the Military Institute of Medicine, Warsaw, Poland.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Military Institute of Medicine (protocol code 120/WIM/2018 obtained 22 August 2018).
Informed Consent Statement
The study protocol was accepted by the local ethics committee. All participants signed an informed consent.
Data Availability Statement
All relevant data analyzed during the current trial are included in the article. Access to raw datasets may be provided upon reasonable request to the corresponding author following permission by the Ethics Committee and the Institute at which the study was conducted.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Simonet, W.; Lacey, D.; Dunstan, C.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The Role of Osteoprotegerin and Its Ligands in Vascular Function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, A.; Lin, P.; Colina, C.M. Unraveling Binding Interactions between Human RANKL and Its Decoy Receptor Osteoprotegerin. J. Phys. Chem. B 2017, 121, 9141–9148. [Google Scholar] [CrossRef] [PubMed]
- Barbu, C.G.; Arsene, A.L.; Florea, S.; Albu, A.; Sirbu, A.; Martin, S.; Nicolae, A.C.; Burcea-Dragomiroiu, G.T.; Popa, D.E.; Velescu, B.S.; et al. Cardiovascular risk assessment in osteoporotic patients using osteoprotegerin as a reliable predictive biochemical marker. Mol. Med. Rep. 2017, 16, 6059–6067. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Schett, G.; Wenning, G.; Redlich, K.; Oberhollenzer, M.; Mayr, A.; Santer, P.; Smolen, J.; Poewe, W.; Willeit, J. Osteoprotegerin Is a Risk Factor for Progressive Atherosclerosis and Cardiovascular Disease. Circulation 2004, 109, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Yndestad, A.; Øie, E.; Florholmen, G.; Halvorsen, B.; Frøland, S.S.; Simonsen, S.; Christensen, G.; Gullestad, L.; Aukrust, P. Dysregulated Osteoprotegerin/RANK Ligand/RANK Axis in Clinical and Experimental Heart Failure. Circulation 2005, 111, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Fabris, B.; Thomas, M.; Toffoli, B.; Tikellis, C.; Candido, R.; Catena, C.; Mulatero, P.; Barbone, F.; Radillo, O.; et al. Osteoprotegerin increases in metabolic syndrome and promotes adipose tissue proinflammatory changes. Mol. Cell. Endocrinol. 2014, 394, 13–20. [Google Scholar] [CrossRef]
- Martin, A.B.; Ucero, A.; Zubiri, I.; Posada-Ayala, M.; Fernandez-Fernandez, B.; Cannata-Ortiz, P.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Egido, J.; Alvarez-Llamas, G.; et al. Osteoprotegerin in Exosome-Like Vesicles from Human Cultured Tubular Cells and Urine. PLoS ONE 2013, 8, e72387. [Google Scholar] [CrossRef]
- Ford, M.L.; Smith, E.R.; Tomlinson, L.; Chatterjee, P.K.; Rajkumar, C.; Holt, S. FGF-23 and osteoprotegerin are independently associated with myocardial damage in chronic kidney disease stages 3 and 4. Another link between chronic kidney disease-mineral bone disorder and the heart. Nephrol. Dial. Transplant. 2011, 27, 727–733. [Google Scholar] [CrossRef]
- Rashtchizadeh, N.; Ghorbanihaghjo, A.; Argani, H.; Meimand, S.M.; Safa, J.; Vatankhahan, H.; Shahidi, M. Serum Receptor Activator of Nuclear Factor-κ B Ligand, Osteoprotegrin, and Intact Parathyroid Hormone in Hemodialysis and Renal Transplant Patients. Ther. Apher. Dial. 2012, 16, 600–604. [Google Scholar] [CrossRef]
- Janda, K.; Krzanowski, M.; Chowaniec, E.; Kuśnierz-Cabala, B.; Dumnicka, P.; Kraśniak, A.; Podolec, P.; Sułowicz, W. Osteoprotegerin as a marker of cardiovascular risk in patients on peritoneal dialysis. Pol. Arch. Intern. Med. 2013, 123, 149–155. [Google Scholar] [CrossRef]
- Huang, Q.-X.; Li, J.-B.; Huang, N.; Huang, X.-W.; Li, Y.-L.; Huang, F.-X. Elevated Osteoprotegerin Concentration Predicts Increased Risk of Cardiovascular Mortality in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2020, 45, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.B.; Abdel-Latif, E.A. Serum osteoprotegerin (OPG) in children with primary nephrotic syndrome. Saudi J. Kidney Dis. Transplant. 2011, 22, 955–962. [Google Scholar]
- Tonelli, M.; Wanner, C.; Cass, A.; Amit, G.; Holdaas, H.; Jardine, A.; Jiang, L.; Kronenberg, F.; Parekh, R.; Shoji, T.; et al. Summary of Recommendation Statements. Kidney Int. Suppl. 2012, 2, 283–287. [Google Scholar] [CrossRef][Green Version]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment. Kidney Int. 2020, 98, 839–848. [Google Scholar] [CrossRef]
- Hanley, J.A.; Hajian-Tilaki, K.O. Sampling variability of nonparametric estimates of the areas under receiver operating characteristic curves: An update. Acad. Radiol. 1997, 4, 49–58. [Google Scholar] [CrossRef]
- Kamińska, J.; Stopiński, M.; Mucha, K.; Pac, M.; Gołębiowski, M.; Niewczas, M.A.; Pączek, L.; Foroncewicz, B. Circulating Osteoprotegerin in Chronic Kidney Disease and All-Cause Mortality. Int. J. Gen. Med. 2021, 14, 2413–2420. [Google Scholar] [CrossRef]
- Moréna, M.; Jaussent, I.; Halkovich, A.; Dupuy, A.-M.; Bargnoux, A.-S.; Chenine, L.; Leray-Moragues, H.; Klouche, K.; Vernhet, H.; Canaud, B.; et al. Bone Biomarkers Help Grading Severity of Coronary Calcifications in Non Dialysis Chronic Kidney Disease Patients. PLoS ONE 2012, 7, e36175. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, M.; Ma, D. TNF-α, a good or bad factor in hematological diseases? Stem Cell Investig. 2014, 1, 12. [Google Scholar] [CrossRef]
- Zauli, G.; Corallini, F.; Bossi, F.; Fischetti, F.; Durigutto, P.; Celeghini, C.; Tedesco, F.; Secchiero, P. Osteoprotegerin increases leukocyte adhesion to endothelial cells both in vitro and in vivo. Blood 2007, 110, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, M.K.; Levin, A.; Er, L.; McIntyre, C.W. Elevated osteoprotegerin is associated with all-cause mortality in CKD stage 4 and 5 patients in addition to vascular calcification. Nephrol. Dial. Transplant. 2009, 24, 3157–3162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bargnoux, A.-S.; Dupuy, A.-M.; Garrigue, V.; Jaussent, I.; Gahide, G.; Badiou, S.; Szwarc, I.; Deleuze, S.; Vernhet, H.; Cristol, J.-P.; et al. Evolution of Coronary Artery Calcifications Following Kidney Transplantation: Relationship with Osteoprotegerin Levels. Arab. Archaeol. Epigr. 2009, 9, 2571–2579. [Google Scholar] [CrossRef]
- Bucay, N.; Sarosi, I.; Dunstan, C.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Golledge, J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 2009, 204, 321–329. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial Dysfunction. Arter. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- De Ciriza, C.P.; Lawrie, A.; Varo, N. Osteoprotegerin in Cardiometabolic Disorders. Int. J. Endocrinol. 2015, 2015, 564934. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).