Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Selection
2.2. Trial Protocol
2.3. Outcome Measures
2.4. Gene Expression Analysis in Blood
2.5. Fecal Analysis
2.6. Calprotectin Enzyme-Linked Immuno-Sorbent Assay
2.7. Fecal Short-Chain Fatty Acids and pH
2.8. Microbiota Sequencing
2.9. Clinical Outcomes
2.10. Statistical Analysis
3. Results
3.1. Recruitment and Demographics
3.2. Peripheral Blood Gene Expression
3.3. Fecal Outcomes
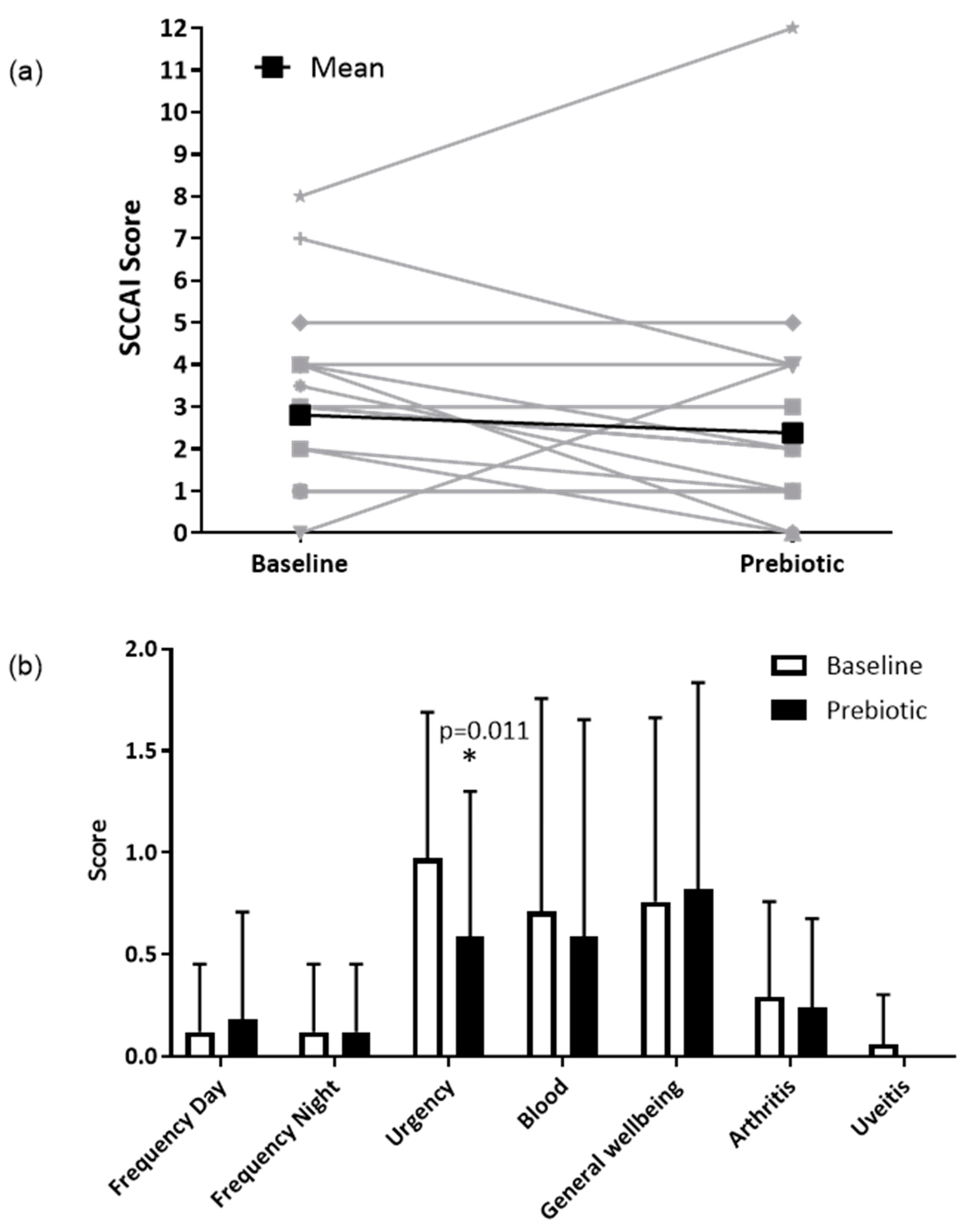
3.4. Clinical Outcomes
4. Discussion
4.1. Limitations
4.2. Clinical Relevance and Recommendations for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, L.; Meyer, D.; Pullens, G.; Faas, M.; Smelt, M.; Venema, K.; Ramasamy, U.; Schols, H.A.; De Vos, P. Immunological Properties of Inulin-Type Fructans. Crit. Rev. Food Sci. Nutr. 2015, 55, 414–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulevic, J.; Rastall, R.A.; Gibson, G.R. Developing a quantitative approach for determining the in vitro prebiotic potential of dietary oligosaccharides. FEMS Microbiol. Lett. 2004, 236, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.; Benjamin, J.; McCarthy, N.; Hedin, C.; Koutsoumpas, A.; Plamondon, S.; Price, C.; Hart, A.; Kamm, M.; Forbes, A. Relationship between human intestinal dendritic cells, gut microbiota, and disease activity in Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 2027–2037. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, I.B.; O’Toole, P.W.; Ohman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.; Simren, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Brint, E.K.; MacSharry, J.; Fanning, A.; Shanahan, F.; Quigley, E.M. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2011, 106, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2013, 63, 1275–1283. [Google Scholar] [CrossRef]
- Casellas, F.; Borruel, N.; Torrejon, A.; Varela, E.; Antolin, M.; Guarner, F.; Malagelada, J.R. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment. Pharmacol. Ther. 2007, 25, 1061–1067. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Guan, X.-X.; Tang, Y.-J.; Sun, J.-F.; Wang, X.-K.; Wang, W.-D.; Fan, J.-M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Rossi, M.; Dimidi, E.; Whelan, K. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Ramasamy, U.; Meyer, D.; Pullens, G.; Venema, K.; Faas, M.M.; Schols, H.A.; de Vos, P. Immune Modulation by Different Types of β2→1-Fructans Is Toll-Like Receptor Dependent. PLoS ONE 2013, 8, e68367. [Google Scholar] [CrossRef] [Green Version]
- Vogt, L.M.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Ramasamy, U.; Schols, H.A.; de Vos, P. Toll-Like Receptor 2 Activation by β2→ 1-Fructans Protects Barrier Function of T84 Human Intestinal Epithelial Cells in a Chain Length–Dependent Manner. J. Nutr. 2014, 144, 1002–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015, 3, e12327. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Prabhu, P.N.; Benefiel, A.C.; Miller, M.J.; Chow, J.; Davis, S.R.; Gaskins, H.R. Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Mol. Nutr. Food Res. 2015, 59, 566–573. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Bermejo, F.; Pérez-Calle, J.-L.; Taxonera, C.; Vera, I.; McNicholl, A.G.; Algaba, A.; López, P.; López-Palacios, N.; Calvo, M. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm. Bowel Dis. 2009, 15, 1190–1198. [Google Scholar] [CrossRef]
- Walmsley, R.; Ayres, R.; Pounder, R.; Allan, R. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Wiklund, I.; Fullerton, S.; Hawkey, C.; Jones, R.; Longstreth, G.; Mayer, E.; Peacock, R.; Wilson, I.; Naesdal, J. An irritable bowel syndrome-specific symptom questionnaire: Development and validation. Scand. J. Gastroenterol. 2003, 38, 947–954. [Google Scholar]
- Blake, M.; Raker, J.; Whelan, K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Dalman, M.R.; Deeter, A.; Nimishakavi, G.; Duan, Z.-H. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinform. 2012, 13, S11. [Google Scholar] [CrossRef] [Green Version]
- Primec, M.; Mičetić-Turk, D.; Langerholc, T. Analysis of short-chain fatty acids in human feces: A scoping review. Anal. Biochem. 2017, 526, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Nat. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Löwenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015, 149, 110–118.e114. [Google Scholar] [CrossRef]
- Depeint, F.; Tzortzis, G.; Vulevic, J.; I’Anson, K.; Gibson, G.R. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: A randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr. 2008, 87, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Rossi, M.; Kanno, T.; Parkes, G.C.; Anderson, S.; Mason, A.J.; Irving, P.M.; Lomer, M.C.; Whelan, K. β-Galactooligosaccharide in conjunction with low FODMAP diet improves irritable bowel syndrome symptoms but reduces fecal Bifidobacteria. Am. J. Gastroenterol. 2020, 115, 906–915. [Google Scholar] [CrossRef]
- Fransen, F.; Sahasrabudhe, N.; Elderman, M.; Bosveld, M.; El Aidy, S.; Hugenholtz, F.; Borghuis, T.; Kousemaker, B.; Winkel, S.; van der Gaast-de Jongh, C. β2→ 1-fructans modulate the immune system in vivo by direct interaction with the mucosa in a microbiota-independent fashion. Front. Immunol. 2017, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Voermans, N.; Preisler, N.; Madsen, K.; Janssen, M.; Kusters, B.; Bakar, N.A.; Conte, F.; Lamberti, V.; Nusman, F.; van Engelen, B. PGM1 deficiency: Substrate use during exercise and effect of treatment with galactose. Neuromuscul. Disord. 2017, 27, 370–376. [Google Scholar] [CrossRef]
- Sun, Y.; Long, H.; Sun, L.; Sun, X.; Pang, L.; Chen, J.; Yi, Q.; Liang, T.; Shen, Y. PGM5 is a promising biomarker and may predict the prognosis of colorectal cancer patients. Cancer Cell Int. 2019, 19, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotton, J.; Platnich, J.; Muruve, D.; Jijon, H.; Buret, A.; Beck, P. Interleukin-8 ingastrointestinal inflammation and malignancy: Induction and clinical consequences. Int. J. Interferon Cytokine Mediat. Res. 2016, 8, 13–34. [Google Scholar]
- Beaulieu, L.M.; Clancy, L.; Tanriverdi, K.; Benjamin, E.J.; Kramer, C.D.; Weinberg, E.O.; He, X.; Mekasha, S.; Mick, E.; Ingalls, R.R. Specific inflammatory stimuli lead to distinct platelet responses in mice and humans. PLoS ONE 2015, 10, e0131688. [Google Scholar] [CrossRef] [PubMed]
- Drakoularakou, A.; Tzortzis, G.; Rastall, R.; Gibson, G. A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers’ diarrhoea. Eur. J. Clin. Nutr. 2009, 64, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, R.; Cela, D.; Swann, J.R.; Vulevic, J.; Gibson, G.R.; Tzortzis, G.; Costabile, A. In vitro fermentation of B-GOS: Impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [Green Version]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [Green Version]
- Hedin, C.R.; McCarthy, N.E.; Louis, P.; Farquharson, F.M.; McCartney, S.; Stagg, A.J.; Lindsay, J.O.; Whelan, K. Prebiotic fructans have greater impact on luminal microbiology and CD3+ T cells in healthy siblings than patients with Crohn’s disease: A pilot study investigating the potential for primary prevention of inflammatory bowel disease. Clin. Nutr. 2021, 40, 5009–5019. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Matsumoto, S.; Ohashi, Y.; Imaoka, A.; Setoyama, H.; Umesaki, Y.; Tanaka, R.; Otani, T. Beneficial Effects of Probiotic Bifidobacterium and Galacto-Oligosaccharide in Patients with Ulcerative Colitis: A Randomized Controlled Study. Digestion 2011, 84, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Accarino, A.; Tzortzis, G.; Vulevic, J.; Gibson, G.; Guarner, F.; Azpiroz, F. Colonic gas homeostasis: Mechanisms of adaptation following HOST-G904 galactooligosaccharide use in humans. Neurogastroenterol. Motil. 2017, 29, e13080. [Google Scholar] [CrossRef] [PubMed]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.; Walsh, S.; O’neil, D.; Macfarlane, G. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]


| Public Gene IDs | Gene Symbol | Description | Chr | Strand | Start | Stop | Group | Signal Intensity, Mean (SD) (1) | Log2 Fold Change | p-Value (2) | FDR p-Value (3) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Prebiotic | |||||||||||
| - | - | Phosphoglucomutase (PGM5) | Chr9 | + | 68328308 | 68531061 | Coding | 5.17 (0.53) | 6.46 (0.22) | +2.5 | 0.0007 | 0.979 |
| AJ227913 | CXCL8 | Chemokine (C-X-C motif) ligand 8 | Chr4 | + | 73740506 | 73743716 | Noncoding | 6.88 (0.39) | 7.77 (0.44) | +1.9 | 0.0068 | 0.979 |
| NR_031684; uc021uim.1 | MIR302F | MicroRNA 302f | Chr18 | + | 30298910 | 30298960 | Coding | 3.82 (0.2) | 4.47 (0.26) | +1.6 | 0.0092 | 0.979 |
| - | RPL21; RPL21P28 | Ribosomal protein L21; ribosomal protein L21 pseudogene 28 | Chr13 | + | 27251309 | 27256691 | Coding | 9.87 (0.28) | 10.47 (0.26) | +1.5 | 0.0176 | 0.979 |
| Chr1 (4) | + | 212051524 | 212052006 | Noncoding | ||||||||
| BC070185 | RPL21 | Ribosomal protein L21 | Chr13 | + | 27251309 | 27256691 | Coding | 16.03 (0.3) | 16.64 (0.3) | +1.5 | 0.0317 | 0.979 |
| NR_026911 | RPL21P28 | Ribosomal protein L21 pseudogene 28 | Chr1 | + | 212051524 | 212052006 | Noncoding | 14.23 (0.29) | 14.82 (0.3) | +1.5 | 0.0327 | 0.979 |
| - | - | GRCh38.p12 primary assembly | Chr11 | - | 45689068 | 45689092 | Coding | 7.04 (0.21) | 6.43 (0.18) | −1.5 | 0.0051 | 0.979 |
| - | - | GRCh38.p12 primary assembly | Chr22 | - | 23610591 | 23610615 | Coding | 6.92 (0.29) | 6.29 (0.24) | −1.6 | 0.0081 | 0.979 |
| Mean (SD) | ||||||
|---|---|---|---|---|---|---|
| Per Protocol Population (n = 13) | Baseline | Prebiotic | Z-Score | p-Value * | ||
| p__Firmicutes_g__Dialister | 3.39 | (3.84) | 1.83 | (1.88) | −2.411 b | 0.016 |
| p__Firmicutes_g__Anaerostipes | 0.17 | (0.17) | 0.55 | (0.79) | −2.040 c | 0.041 |
| p__Firmicutes_g__Oscillospira | 3.81 | (3.03) | 2.80 | (2.58) | −1.992 b | 0.046 |
| p__Firmicutes_f_[Mogibacteriaceae] | 0.38 | (0.43) | 0.24 | (0.24) | −1.883 b | 0.06 |
| p__Firmicutes_g__Dorea | 1.24 | (0.79) | 1.56 | (0.91) | −1.852 c | 0.064 |
| p__Bacteroidetes_g__Paraprevotella | 0.04 | (0.08) | 0.14 | (0.27) | −1.826 c | 0.068 |
| p__Proteobacteria_g__Haemophilus | 2.86 | (9.53) | 0.26 | (0.74) | −1.782 b | 0.075 |
| p__Firmicutes_f__Erysipelotrichaceae | 0.86 | (1.54) | 1.58 | (3.20) | −1.642 c | 0.101 |
| p__Actinobacteria_g__Corynebacterium | 0.00 | (0.00) | 0.01 | (0.04) | −1.604 c | 0.109 |
| p__Firmicutes_g__Anaerotruncus | 0.03 | (0.05) | 0.02 | (0.04) | −1.599 b | 0.11 |
| Patients in remission at baseline (SCCAI≤2) (n = 6) | ||||||
| p__Firmicutes_ f__Christensenellaceae | 0.58 | (1.27) | 1.25 | (2.47) | −2.023 c | 0.043 |
| p__Actinobacteria_g__Bifidobacterium | 1.05 | (1.27) | 3.99 | (5.37) | −1.992 c | 0.046 |
| p__Firmicutes_g__Anaerostipes | 0.17 | (0.14) | 0.49 | (0.44) | −1.782 c | 0.075 |
| p__Firmicutes_f__[Mogibacteriaceae] | 0.24 | (0.30) | 0.18 | (0.29) | −1.753 b | 0.08 |
| p__Bacteroidetes_g__Prevotella | 2.46 | (3.81) | 0.43 | (0.93) | −1.604 b | 0.109 |
| p__Firmicutes_g__Holdemania | 0.03 | (0.04) | 0.01 | (0.02) | −1.604 b | 0.109 |
| p__Firmicutes_f__Ruminococcaceae | 3.66 | (4.56) | 6.88 | (7.66) | −1.572 c | 0.116 |
| p__Firmicutes_g__Dorea | 0.93 | (0.62) | 1.49 | (0.88) | −1.572 c | 0.116 |
| p__Firmicutes_g__Dialister | 3.25 | (5.04) | 1.72 | (1.88) | −1.363 b | 0.173 |
| p__Actinobacteria_g__Slackia | 0.03 | (0.08) | 0.05 | (0.08) | −1.342 c | 0.18 |
| Patients not in remission at baseline (SCCAI>2) (n = 7) | ||||||
| p__Firmicutes_g__Dialister | 3.50 | (2.87) | 1.92 | (2.02) | −2.028 b | 0.043 |
| p__Firmicutes_g__Roseburia | 3.27 | (2.35) | 5.86 | (3.78) | −1.859 c | 0.063 |
| p__Firmicutes_g__Blautia | 2.74 | (1.83) | 4.60 | (3.38) | −1.859 c | 0.063 |
| p__Proteobacteria_g__Sutterella | 0.22 | (0.20) | 0.41 | (0.28) | −1.859 c | 0.063 |
| p__Firmicutes_g__Oxobacter | 0.06 | (0.08) | 0.02 | (0.04) | −1.826 b | 0.068 |
| p__Cyanobacteria_o__Streptophyta | 0.03 | (0.04) | 0.01 | (0.03) | −1.753 b | 0.08 |
| p__Proteobacteria_g__Bilophila | 0.07 | (0.08) | 0.22 | (0.22) | −1.753 c | 0.08 |
| p__Firmicutes_g__Oscillospira | 3.81 | (3.11) | 2.47 | (1.86) | −1.690 b | 0.091 |
| p__Firmicutes_g__Anaerofilum | 0.01 | (0.01) | 0.00 | (0.01) | −1.604 b | 0.109 |
| p__Proteobacteria_g__Haemophilus | 5.03 | (13.02) | 0.41 | (1.02) | −1.521 b | 0.128 |
| Incidence (Days Out of 7) (n = 17) | Severity (^ = 17) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Baseline | Prebiotic | p * | Baseline | Prebiotic | p * | ||||
| Pain | 2.9 | (2.5) | 3.1 | (2.9) | 0.650 | 0.6 | (0.6) | 0.6 | (0.7) | 0.592 |
| Heartburn | 0.5 | (1.2) | 0.6 | (1.3) | 0.854 | 0.1 | (0.3) | 0.1 | (0.2) | 0.854 |
| Acid reflux | 0.3 | (0.8) | 0.1 | (0.3) | 0.257 | 0.1 | (0.2) | 0.0 | (0.1) | 0.197 |
| Nausea | 0.9 | (1.0) | 1.4 | (2.1) | 0.478 | 0.2 | (0.2) | 0.2 | (0.4) | 0.776 |
| Gurgling | 4.0 | (2.8) | 3.3 | (2.7) | 0.084 | 0.7 | (0.6) | 0.6 | (0.6) | 0.105 |
| Bloating | 2.8 | (2.7) | 3.3 | (2.9) | 0.361 | 0.6 | (0.6) | 0.6 | (0.6) | 0.443 |
| Belching | 2.7 | (3.0) | 3.1 | (3.0) | 0.429 | 0.4 | (0.5) | 0.4 | (0.4) | 0.794 |
| Flatulence | 4.2 | (2.8) | 3.8 | (3.0) | 0.228 | 0.9 | (0.6) | 0.7 | (0.6) | 0.089 |
| Constipation | 1.1 | (1.9) | 1.1 | (1.6) | 1.000 | 0.2 | (0.4) | 0.2 | (0.3) | 0.917 |
| Diarrhea | 1.5 | (2.8) | 0.9 | (2.1) | 0.180 | 0.4 | (0.8) | 0.3 | (0.8) | 0.285 |
| Loose stool | 3.2 | (2.4) | 1.6 | (2.0) | 0.012 | 0.7 | (0.7) | 0.5 | (0.8) | 0.046 |
| Hard stool | 0.5 | (1.0) | 0.1 | (0.2) | 0.066 | 0.1 | (0.3) | 0.0 | (0.1) | 0.068 |
| Urgency | 2.8 | (2.4) | 2.4 | (2.5) | 0.319 | 0.6 | (0.6) | 0.5 | (0.7) | 0.277 |
| Incomplete evacuation | 1.6 | (2.1) | 1.3 | (2.0) | 0.339 | 0.3 | (0.5) | 0.2 | (0.3) | 0.182 |
| Tiredness | 3.5 | (2.9) | 3.3 | (3.0) | 0.760 | 0.8 | (0.8) | 0.8 | (0.9) | 0.681 |
| Overall symptoms | 3.8 | (3.0) | 3.8 | (2.8) | 0.609 | 0.7 | (0.7) | 0.8 | (0.7) | 0.964 |
| Stool output | ||||||||||
| Frequency, /d, mean (SEM) | 2.0 | (1.2) | 2.1 | (1.4) | 0.833 | |||||
| Form, % of stools, mean (SEM) | ||||||||||
| Hard stool | 13% | (29) | 1% | (3) | 0.117 | |||||
| Normal stool | 49% | (34) | 70% | (36) | 0.026 | |||||
| Soft stool | 39% | (35) | 29% | (36) | 0.132 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, B.; Eyice, Ö.; Koumoutsos, I.; Lomer, M.C.; Irving, P.M.; Lindsay, J.O.; Whelan, K. Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients 2021, 13, 3598. https://doi.org/10.3390/nu13103598
Wilson B, Eyice Ö, Koumoutsos I, Lomer MC, Irving PM, Lindsay JO, Whelan K. Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients. 2021; 13(10):3598. https://doi.org/10.3390/nu13103598
Chicago/Turabian StyleWilson, Bridgette, Özge Eyice, Ioannis Koumoutsos, Miranda C. Lomer, Peter M. Irving, James O. Lindsay, and Kevin Whelan. 2021. "Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms" Nutrients 13, no. 10: 3598. https://doi.org/10.3390/nu13103598
APA StyleWilson, B., Eyice, Ö., Koumoutsos, I., Lomer, M. C., Irving, P. M., Lindsay, J. O., & Whelan, K. (2021). Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients, 13(10), 3598. https://doi.org/10.3390/nu13103598






