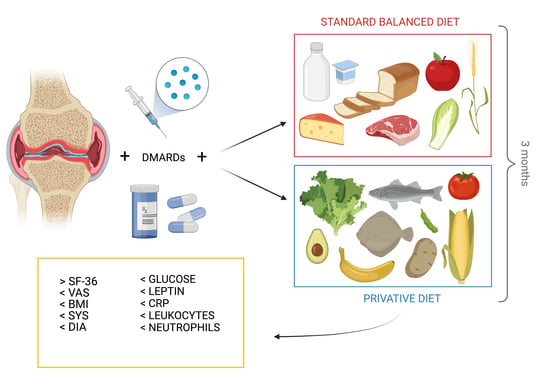
Improvement of Inflammation and Pain after Three Months’ Exclusion Diet in Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Enrollment
2.2. Study Design and Dietary Regimens Protocol
2.3. Disease Monitoring
2.4. Laboratory Data and Anthropometric Measurements
2.5. Detection of Adipokines and Cytokines
2.6. Statistical Analysis
3. Results
3.1. Patients’ Adherence
3.2. VAS Score
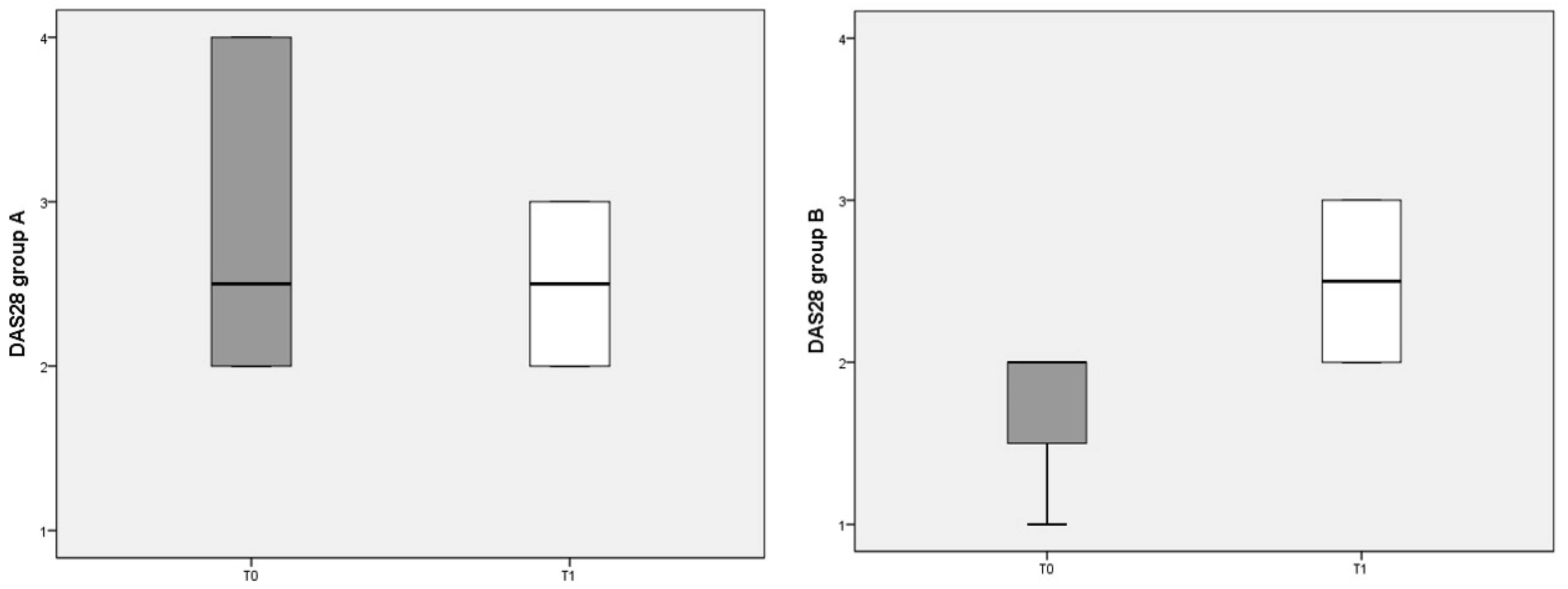
3.3. DAS 28 Score
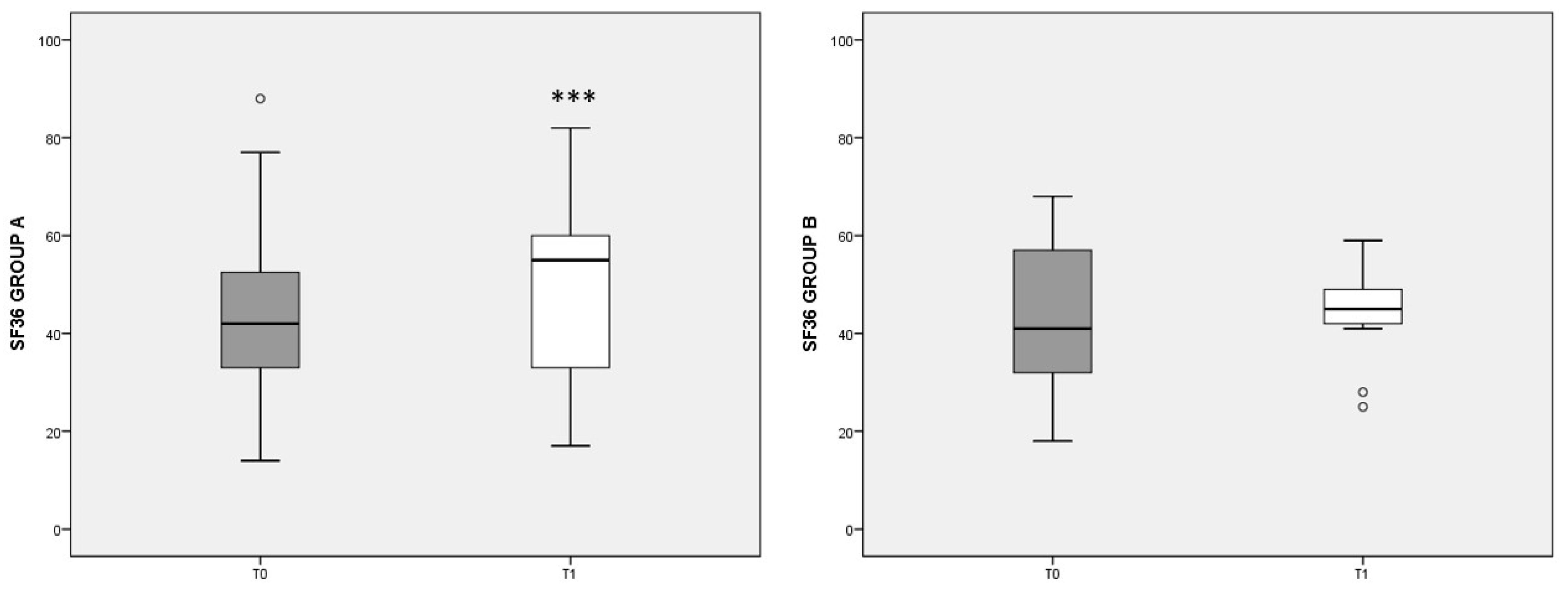
3.4. SF-36 Score
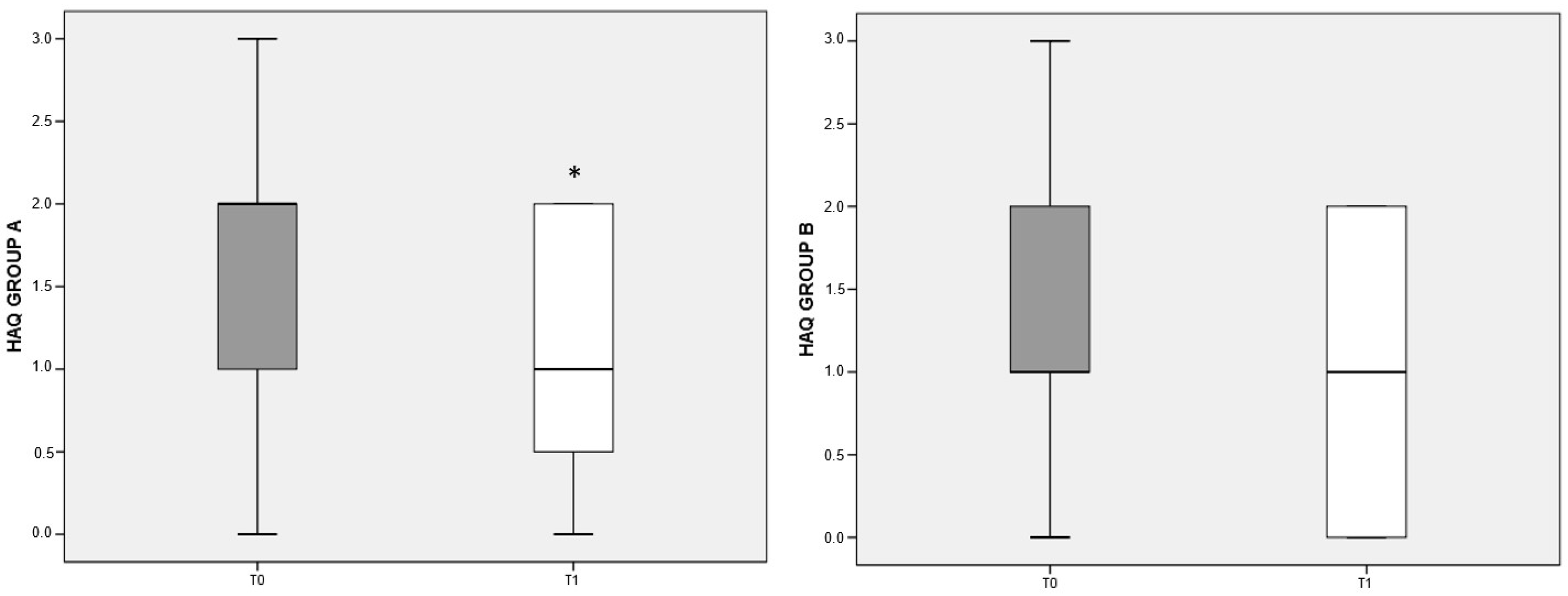
3.5. HAQ Score
3.6. Anthropometric Measures
3.7. Laboratory Parameters
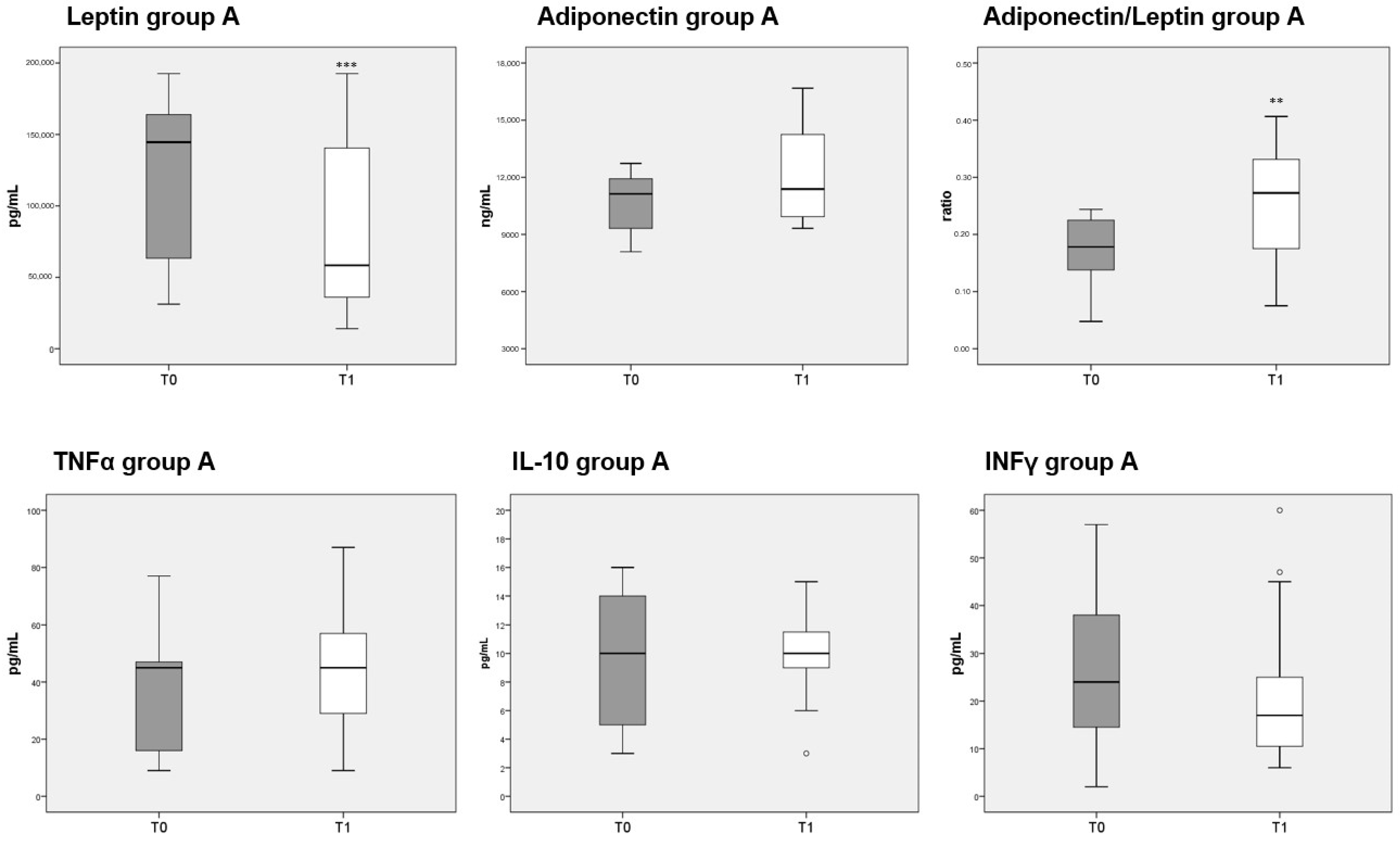
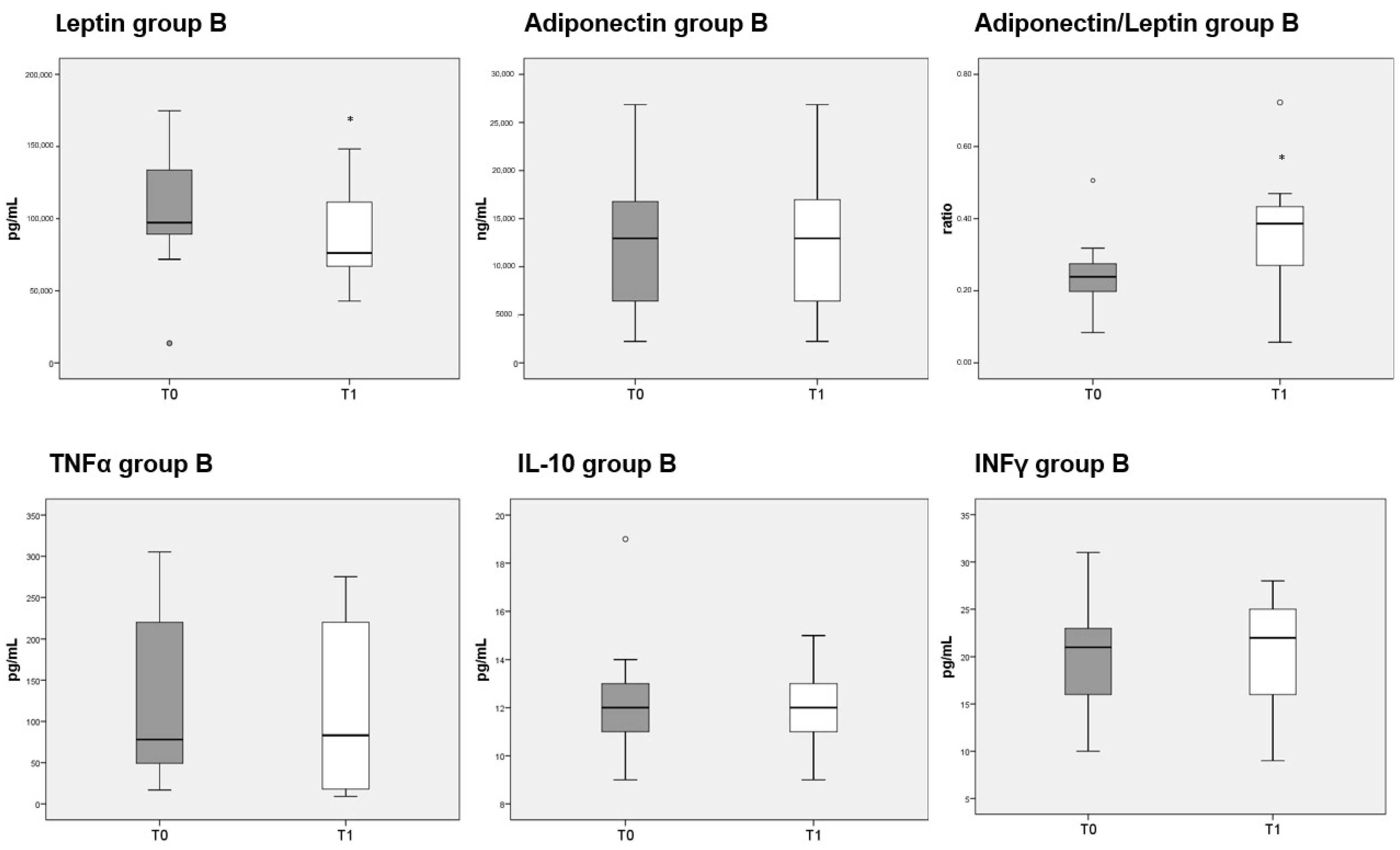
3.8. Adipokines and Cytokines Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, ITC1–ITC16. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [Green Version]
- Philippou, E.; Nikiphorou, E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun. Rev. 2018, 17, 1074–1077. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Spinella, P.; Fiocco, U.; Ramonda, R.; Sfriso, P.; Punzi, L. How the Mediterranean diet and some of its components modulate inflammatory pathways in arthritis. Swiss Med. Wkly. 2015, 145, w14190. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, D.; van der Helm-van Mil, A.H.M. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B.; Paganelli, R. Aging, Obesity, and Inflammatory Age-Related Diseases. Front. Immunol. 2017, 8, 1745. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5725402/ (accessed on 8 April 2020). [CrossRef]
- Petta, I.; Fraussen, J.; Somers, V.; Kleinewietfeld, M. Interrelation of Diet, Gut Microbiome, and Autoantibody Production. Front. Immunol. 2018, 9, 439. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, C.; Reale, M.; Costantini, E. Microbiota and Probiotics in Health and HIV Infection. Nutrients 2017, 9, 615. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.Y.; Devkota, S.; Moscoso, D.; Chang, E.B.; Leone, V.A. The role of diet in triggering human inflammatory disorders in the modern age. Microbes Infect. 2013, 15, 765–774. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Brandl, C.; Bucci, L.; Schett, G.; Zaiss, M.M. Crossing the barriers: Revisiting the gut feeling in rheumatoid arthritis. Eur. J. Immunol. 2021, 51, 798–810. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Wu, H.-J.J.; Mauro, D.; Schett, G.; Ciccia, F. The gut–joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 224–237. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Horta-Baas, G.; Sandoval-Cabrera, A.; Romero-Figueroa, M.D.S. Modificatin of Gut Microbiota in Inflammatory Arthritis: Highlights and Future Challenges. Curr. Rheumatol. Rep. 2021, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J.; Hvatum, M.; Haugen, M.; Førre, O.; Scott, H. Antibodies against dietary antigens in rheumatoid arthritis patients treated with fasting and a one-year vegetarian diet. Clin. Exp. Rheumatol. 1995, 13, 167–172. [Google Scholar] [PubMed]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, CD006400. [Google Scholar] [CrossRef]
- Oliviero, F.; Punzi, L.; Spinella, P. Mediterranean Food Pattern in Rheumatoid Arthritis. Curr. Rheumatol. Rev. 2009, 5, 233–240. Available online: http://www.eurekaselect.com/85519/article (accessed on 30 January 2020). [CrossRef]
- Petersson, S.; Philippou, E.; Rodomar, C.; Nikiphorou, E. The Mediterranean diet, fish oil supplements and Rheumatoid arthritis outcomes: Evidence from clinical trials. Autoimmun. Rev. 2018, 17, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Philippou, E.; Petersson, S.D.; Rodomar, C.; Nikiphorou, E. Rheumatoid arthritis and dietary interventions: Systematic review of clinical trials. Nutr. Rev. 2021, 79, 410–428. [Google Scholar] [CrossRef]
- Grygielska, J.; Kłak, A.; Raciborski, F.; Mańczak, M. Nutrition and quality of life referring to physical abilities—A comparative analysis of a questionnaire study of patients with rheumatoid arthritis and osteoarthritis. Reumatologia 2017, 55, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeschi, S.K.; Frits, M.; Cui, J.; Zhang, Z.Z.; Mahmoud, T.; Iannaccone, C.; Lin, T.-C.; Yoshida, K.; Weinblatt, M.E.; Shadick, N.A.; et al. Diet and Rheumatoid Arthritis Symptoms: Survey Results from a Rheumatoid Arthritis Registry. Arthritis Care Res. 2017, 69, 1920–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, W.B. The role of meat in the expression of rheumatoid arthritis. Br. J. Nutr. 2000, 84, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.K. Diet and rheumatoid arthritis: Red meat and beyond. Arthritis Rheum. 2004, 50, 3745–3747. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.K.; Waud, J.P.; Matthews, S.B. The Molecular basis of Lactose Intolerance. Sci. Prog. 2005, 88, 157–202. Available online: https://journals.sagepub.com/doi/10.3184/003685005783238408?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 22 September 2020). [CrossRef] [PubMed]
- Melnik, B.C. Milk--the promoter of chronic Western diseases. Med. Hypotheses 2009, 72, 631–639. [Google Scholar] [CrossRef]
- El-Chammas, K.; Danner, E. Gluten-Free Diet in Nonceliac Disease. Nutr. Clin. Pract. 2011, 26, 294–299. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. Available online: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2010.1461 (accessed on 29 June 2020).
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Maska, L.; Anderson, J.; Michaud, K. Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ-II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res. 2011, 63 (Suppl. 11), S4–S13. [Google Scholar]
- Van Riel, P.L.C.M.; Renskers, L. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, S40–S44. [Google Scholar]
- Jabłonowska-Lietz, B.; Wrzosek, M.; Włodarczyk, M.; Nowicka, G. New indexes of body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiol. Pol. 2017, 75, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6889899/ (accessed on 18 March 2021). [CrossRef] [PubMed]
- Innala, L.; Möller, B.; Ljung, L.; Magnusson, S.; Smedby, T.; Södergren, A.; Öhman, M.-L.; Rantapää-Dahlqvist, S.; Wållberg-Jonsson, S. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: A five year prospective study. Arthritis Res. Ther. 2011, 13, R131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Filippo, L.; De Lorenzo, R.; Sciorati, C.; Capobianco, A.; Lorè, N.I.; Giustina, A.; Manfredi, A.A.; Rovere-Querini, P.; Conte, C. Adiponectin to leptin ratio reflects inflammatory burden and survival in COVID-19. Diabetes Metab. 2021, 47, 101268. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gomez-Arbelaez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Veru-Lesmes, F.; Guay, S.; Shah, J.L.; Schmitz, N.; Giguère, C.É.; Joober, R.; Iyer, S.N.; Malla, A.K. Adipose tissue dysregulation at the onset of psychosis: Adipokines and social determinants of health. Psychoneuroendocrinology 2021, 123, 104915. [Google Scholar] [CrossRef]
- Häger, J.; Bang, H.; Hagen, M.; Frech, M.; Träger, P.; Sokolova, M.V.; Steffen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, J.M.; Lawrence, D.A.; Jubiz, W.; DiGiacomo, R.; Rynes, R.; Bartholomew, L.E.; Sherman, M. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990, 33, 810–820. [Google Scholar] [CrossRef]
- James, M.J.; Cleland, L.G. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin. Arthritis Rheum. 1997, 27, 85–97. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.; Laerum, E.; Eek, M.; Mowinkel, P.; Hovi, K.; Førre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Sköldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Dourado, E.; Ferro, M.; Guerreiro, C.S.; Fonseca, J. Diet as a Modulator of Intestinal Microbiota in Rheumatoid Arthritis. Nutrients 2020, 12, 3504. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, A.P.; Panebianco, C.; Salerno, G.; Di Rosa, R.; Salemi, S.; Sorgi, M.L.; Meneguzzi, G.; Mariani, M.B.; Rai, A.; Iacono, D.; et al. Impact of Mediterranean Diet on Disease Activity and Gut Microbiota Composition of Rheumatoid Arthritis Patients. Microorganisms 2020, 8, 1989. [Google Scholar] [CrossRef]
- Venetsanopoulou, A.I.; Voulgari, P.V.; Drosos, A.A. Fasting mimicking diets: A literature review of their impact on inflammatory arthritis. Mediterr. J. Rheumatol. 2019, 30, 201–206. [Google Scholar] [CrossRef]
- Rubin, K.H.; Rasmussen, N.F.; Petersen, I.; Kopp, T.I.; Stenager, E.; Magyari, M.; Hetland, M.L.; Bygum, A.; Glintborg, B.; Andersen, V. Intake of dietary fibre, red and processed meat and risk of late-onset Chronic Inflammatory Diseases: A prospective Danish study on the “diet, cancer and health” cohort. Int. J. Med. Sci. 2020, 17, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2991. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.J.; Symmons, D.P.M.; Lunt, M.; Welch, A.; Luben, R.; Bingham, S.A.; Khaw, K.T.; Day, N.E.; Silman, A.J. Dietary risk factors for the development of inflammatory polyarthritis: Evidence for a role of high level of red meat consumption. Arthritis Rheum. 2004, 50, 3804–3812. [Google Scholar] [CrossRef]
- Skoczyńska, M.; Świerkot, J. The role of diet in rheumatoid arthritis. Reumatologia 2018, 56, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hazlewood, G.S.; Kaplan, G.G.; Eksteen, B.; Barnabe, C. Impact of Obesity on Remission and Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2017, 69, 157–165. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.-C. Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: A meta-analysis. Z. Rheumatol. 2016, 75, 1021–1027. [Google Scholar] [CrossRef]
- James, M.; Proudman, S.; Cleland, L. Fish oil and rheumatoid arthritis: Past, present and future. Proc. Nutr. Soc. 2010, 69, 316–323. [Google Scholar] [CrossRef] [Green Version]

| Group A (n = 15) | p-Value a | Group B (n = 13) | p-Value a | p-Value b | p-Value c | |||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |||||
| Weight (Kg) | 73 (68–87) | 72 (64–82) | <0.001 | 72 (65–80) | 67 (64–75.5) | 0.003 | 0.270 | 0.420 |
| BMI (kg/m2) | 29 (26–36) | 27 (25–33) | <0.001 | 29 (25–33.5) | 26 (24–31.5) | 0.012 | 0.355 | 0.677 |
| BIA: | ||||||||
| Muscle mass (Kg) | 44 (41–45) | 43 (41–45) | 0.188 | 41 (39.5–42.5) | 40 (38.5–42) | 0.070 | 0.058 | 0.030 |
| Fat mass (Kg) | 26 (23–39) | 25 (20–35) | <0.001 | 28 (22.5–35) | 25 (20–32) | 0.015 | 0.533 | 0.926 |
| Bone mass (Kg) | 2 (2–2) | 2 (2–2) | 0.564 | 2 (2–3) | 2 (1–2) | 0.157 | 0.097 | 0.636 |
| Water (Kg) | 33 (31–34) | 32 (30–34) | 0.256 | 31 (29–33) | 29 (28.5–31) | 0.028 | 0.138 | 0.045 |
| Basal metabolism (Kcal) | 1375 (1305–1456) | 1368 (1294–1445) | 0.379 | 1308 (1258–1380) | 1293 (1241–1364) | 0.084 | 0.093 | 0.096 |
| Waist circumference (cm) | 101 (97–105) | 93 (82–106) | <0.001 | 101 (97–104) | 95 (92–98) | 0.005 | 0.890 | 0.948 |
| Hips circumference (cm) | 110 (102–119) | 104 (98–110) | <0.001 | 111 (108–114) | 107 (104–110) | 0.013 | 0.945 | 0.746 |
| Waist Hips Ratio (WHR) | 0.92 (0.89–0.94) | 0.91 (0.85–0.94) | 0.543 | 0.91 (0.89–0.93) | 0.88 (0.86–0.91) | 0.059 | 1.000 | 0.504 |
| SYS (mmHg) | 131 (127–136) | 120 (116–124) | 0.003 | 125 (122–128) | 127 (123–130) | 0.103 | 0.856 | 0.036 |
| DIA (mmHg) | 80 (79–84) | 74 (71–76) | 0.025 | 80 (70–80) | 75 (70–80) | 0.253 | 0.316 | 0.964 |
| Group A (n = 15) | p-Value a | Group B (n = 13) | p-Value a | p-Value b | p-Value c | |||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |||||
| OGTT: | ||||||||
| Basal (mg/dL) | 93 (81–98) | 85 (80–90) | <0.001 | 90 (85–94.5) | 85 (80–90) | 0.003 | 0.419 | 0.888 |
| After 120 min (mg/dL) | 129 (112–139) | - | 131 (109–145.5) | - | 0.872 | - | ||
| Insulin (μUL/mL) | 11 (6–18) | 9 (6–11) | 0.070 | 8 (6–10) | 9 (5–11.5) | 0.632 | 0.343 | 0.817 |
| HOMA index | 3 (1–4) | 2 (1–2) | 0.044 | 2 (1–2) | 2 (1–2.5) | 0.705 | 0.201 | 0.939 |
| Total Cholesterol (mg/dL) | 202 (177–226) | 196 (186–208) | 0.426 | 203 (181.5–229) | 213 (174.5–237) | 0.456 | 0.712 | 0.300 |
| HDL-Cholesterol (mg/dL) | 60 (46–77) | 60 (47–76) | 0.858 | 58 (50.5–67.5) | 60 (54–67.5) | 0.452 | 0.963 | 0.945 |
| Triglycerides (mg/dL) | 137 (64–195) | 102 (72–163) | 0.064 | 102 (76–171) | 101 (67–136.5) | 0.147 | 0.747 | 0.565 |
| Leukocytes (n × 103/μL) | 8 (6–9) | 6 (6–8) | 0.003 | 7 (6–8) | 6 (5–7.5) | 0.114 | 0.270 | 0.419 |
| Neutrophils (n × 103/μL) | 5 (3–5) | 3 (3–4) | 0.006 | 4 (3–5) | 4 (2.5–4) | 0.103 | 0.408 | 0.566 |
| Lymphocytes (n × 103/μL) | 3 (2–3) | 2 (2–3) | 0.180 | 3 (2–3) | 2 (2–3) | 0.256 | 0.592 | 0.662 |
| Hemoglobin (g/dL) | 13 (13–14) | 13 (13–14) | 0.655 | 14 (13–14) | 13 (13–14) | 0.248 | 0.074 | 0.507 |
| Platelets (n × 103/μL) | 279 (234–323) | 269 (223–303) | 0.342 | 283 (241–321.5) | 267 (214.5–329.5) | 0.184 | 0.612 | 0.945 |
| ESR (mm/h) | 21 (18–25) | 21 (18–24) | 0.735 | 16 (13–20.5) | 22 (18–25) | 0.069 | 0.885 | |
| Total Proteins (g/dL) | 7 (7–8) | 7 (7–8) | 0.414 | 7 (7–8) | 7 (7–7.5) | 0.317 | 0.891 | 0.830 |
| hs-CRP (mg/L) | 0.7 (0.5–0.9) | 0.5 (0.4–0.5) | 0.032 | 0.6 (0.4–0.7) | 0.5 (0.4–0.55) | 0.306 | 0.960 | |
| Albumin (g/dL) | 4 (4–4) | 4 (4–5) | 0.346 | 4 (4–4) | 4 (4–5) | 0.564 | 0.856 | 0.413 |
| Transferrin (mg/dL) | 280 (263–304) | 266 (239–302) | 0.016 | 292 (264–300.5) | 284 (272–295) | 0.126 | 0.549 | 0.596 |
| GOT (U/L) | 25 (21–44) | 28 (21–33) | 0.222 | 23 (19–28.5) | 24 (20.5–28.5) | 0.875 | 0.249 | 0.344 |
| GPT (U/L) | 38 (24–54) | 34 (26–45) | 0.875 | 25 (21–41.5) | 28 (23.5–34) | 0.727 | 0.254 | 0.128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guagnano, M.T.; D’Angelo, C.; Caniglia, D.; Di Giovanni, P.; Celletti, E.; Sabatini, E.; Speranza, L.; Bucci, M.; Cipollone, F.; Paganelli, R. Improvement of Inflammation and Pain after Three Months’ Exclusion Diet in Rheumatoid Arthritis Patients. Nutrients 2021, 13, 3535. https://doi.org/10.3390/nu13103535
Guagnano MT, D’Angelo C, Caniglia D, Di Giovanni P, Celletti E, Sabatini E, Speranza L, Bucci M, Cipollone F, Paganelli R. Improvement of Inflammation and Pain after Three Months’ Exclusion Diet in Rheumatoid Arthritis Patients. Nutrients. 2021; 13(10):3535. https://doi.org/10.3390/nu13103535
Chicago/Turabian StyleGuagnano, Maria Teresa, Chiara D’Angelo, Daniela Caniglia, Pamela Di Giovanni, Eleonora Celletti, Emanuela Sabatini, Lorenza Speranza, Marco Bucci, Francesco Cipollone, and Roberto Paganelli. 2021. "Improvement of Inflammation and Pain after Three Months’ Exclusion Diet in Rheumatoid Arthritis Patients" Nutrients 13, no. 10: 3535. https://doi.org/10.3390/nu13103535
APA StyleGuagnano, M. T., D’Angelo, C., Caniglia, D., Di Giovanni, P., Celletti, E., Sabatini, E., Speranza, L., Bucci, M., Cipollone, F., & Paganelli, R. (2021). Improvement of Inflammation and Pain after Three Months’ Exclusion Diet in Rheumatoid Arthritis Patients. Nutrients, 13(10), 3535. https://doi.org/10.3390/nu13103535