Maternal Nutrition and Neurodevelopment: A Scoping Review
Abstract
:1. Introduction
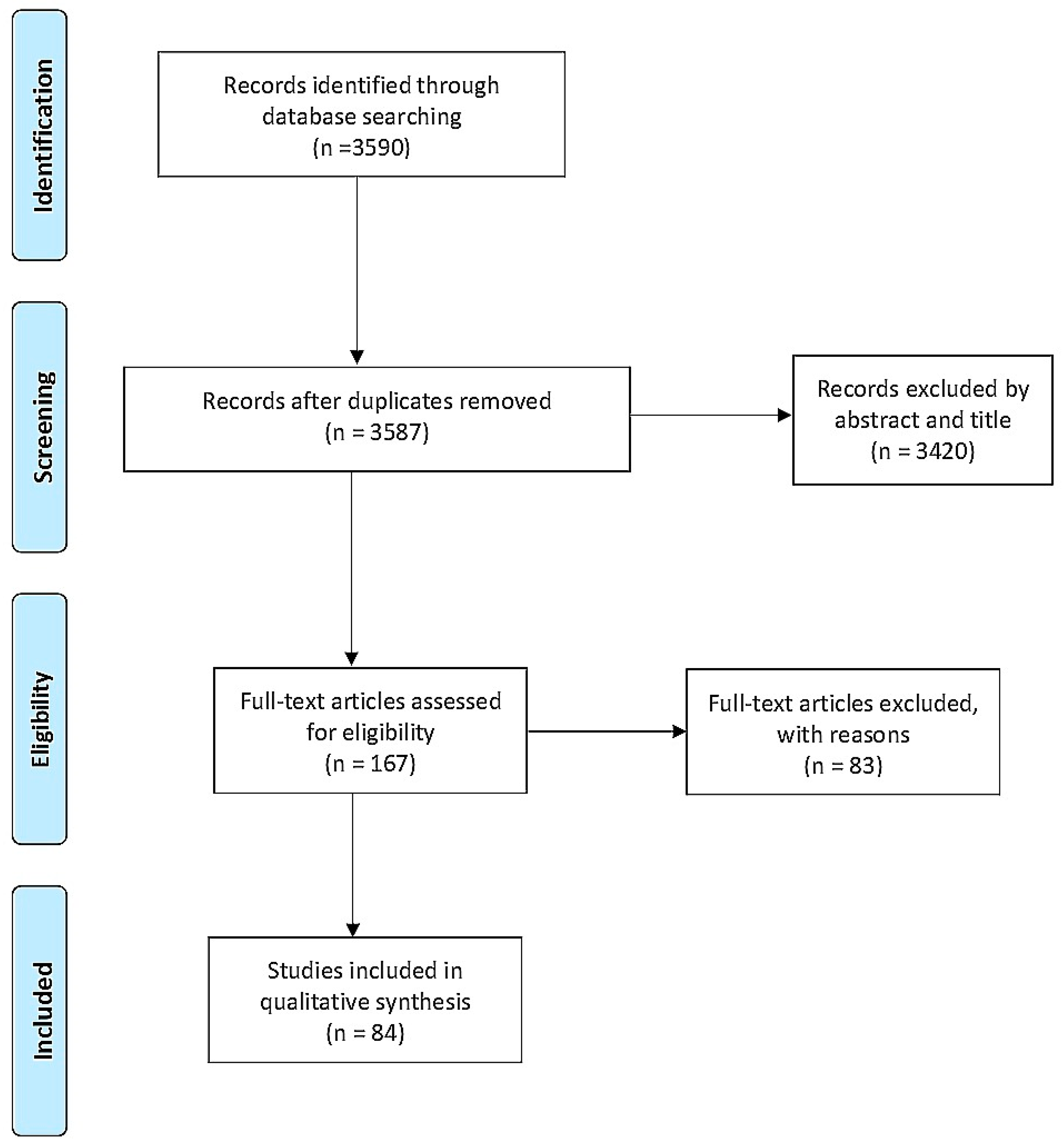
2. Materials and Methods
3. Results
4. Discussion
4.1. Diet
4.1.1. Undernutrition
4.1.2. Overnutrition
High Fat Diet
Obesity
Ketogenic Diet
4.2. Macronutrients
4.2.1. Fatty Acids
4.2.2. Proteins
4.3. Micronutrients
4.3.1. Iron
4.3.2. Copper
4.3.3. Creatine
4.3.4. Choline
4.3.5. Zinc
4.3.6. Iodine
4.3.7. Vitamin B12
4.3.8. Folate
4.3.9. Vitamin D
4.3.10. Vitamin A
4.3.11. Vitamins E and K
4.4. Other Elements
4.4.1. Gangliosides
4.4.2. Caffeine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Morgan, E. PLASMA-IRON AND HÆMOGLOBIN LEVELS IN PREGNANCY: The Effect of Oral Iron. Lancet 1961, 277, 9–12. [Google Scholar] [CrossRef]
- Allen, L.H. Pregnancy and Iron Deficiency: Unresolved Issues. Nutr. Rev. 1997, 55, 91–101. [Google Scholar] [CrossRef]
- Barker, D. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115. [Google Scholar] [CrossRef]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disor-ders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, M.; Robker, R.; Robertson, S.A. Parenting from before conception. Science 2014, 345, 756–760. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [Green Version]
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2009, 35, 147–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vohr, B.R.; Poggi Davis, E.; Wanke, C.A.; Krebs, N.F. Neurodevelopment: The Impact of Nutrition and Inflammation During Pre-conception and Pregnancy in Low-Resource Settings. Pediatrics 2017, 139 (Suppl. S1), S38–S49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, A.; Flynn, A.C.; Pasupathy, D. Nutrition in pregnancy. Obstet. Gynaecol. Reprod. Med. 2016, 26, 259–264. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef]
- Rivell, A.; Mattson, M.P. Intergenerational Metabolic Syndrome and Neuronal Network Hyperexcitability in Autism. Trends Neurosci. 2019, 42, 709–726. [Google Scholar] [CrossRef] [PubMed]
- DeCapo, M.; Thompson, J.R.; Dunn, G.; Sullivan, E.L. Perinatal Nutrition and Programmed Risk for Neuropsychiatric Disorders: A Focus on Animal Models. Biol. Psychiatry 2019, 85, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Yamada, H.; Munetsuna, E.; Ishikawa, H.; Mizuno, G.; Mukuda, T.; Mouri, A.; Nabeshima, T.; Saito, K.; Suzuki, K.; et al. Excess maternal fructose consumption impairs hippocampal function in offspring via epigenetic modification of BDNF promoter. FASEB J. 2018, 32, 2549–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, D.; Ellul, S.; Watters, L.; Lee, D.; Haluska, R.; White, R. The ketogenic diet in disease and development. Int. J. Dev. Neurosci. 2018, 68, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.P.; Bandarra, N.M.; Figueiredo-Braga, M. The role of marine omega-3 in human neurodevelopment, including Autism Spectrum Disorders and Attention-Deficit/Hyperactivity Disorder—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1431–1446. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Prenatal and early postnatal supplementation with long-chain polyunsaturated fatty acids: Neurodevel-opmental considerations. Am. J. Clin. Nutr. 2011, 94, 1874S–1879S. Available online: https://pubmed.ncbi.nlm.nih.gov/21525202/ (accessed on 5 August 2021). [CrossRef] [Green Version]
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef]
- Jensen, C.L. Effects of n−3 fatty acids during pregnancy and lactation. Am. J. Clin. Nutr. 2006, 83, 1452S–1457S. [Google Scholar] [CrossRef] [Green Version]
- Gould, J.; Anderson, A.; Yelland, L.; Gibson, R.; Makrides, M. Maternal characteristics influence response to DHA during pregnancy. Prostagland. Leukot. Essent. Fat. Acids 2016, 108, 5–12. [Google Scholar] [CrossRef]
- Meldrum, S.; Simmer, K. Docosahexaenoic Acid and Neurodevelopmental Outcomes of Term Infants. Ann. Nutr. Metab. 2016, 69, 23–28. [Google Scholar] [CrossRef]
- Sahay, A.; Kale, A.; Joshi, S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides 2020, 83, 102075. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013, 16, 1340–1353. [Google Scholar] [CrossRef]
- Prado, E.; Dewey, K. Nutrition and Brain Development in Early Life. Prenat. Child. Nutr. 2015, 72, 267–284. [Google Scholar] [CrossRef]
- Monk, C.; Georgieff, M.K.; Osterholm, E.A. Research Review: Maternal prenatal distress and poor nutrition-mutually influencing risk factors affecting infant neurocognitive development. J. Child Psychol. Psychiatry 2013, 54, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Lupu, D.S. High fat diet-induced maternal obesity alters fetal hippocampal development. Int. J. Dev. Neurosci. 2009, 27, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, A.; de Vega, W.; Sivanathan, S.; St-Cyr, S.; McGowan, P.O. Maternal high-fat diet alters anxiety behavior and glucocorti-coid signaling in adolescent offspring. Neuroscience 2014, 272, 92–101. [Google Scholar] [CrossRef]
- Khambadkone, S.G.; Cordner, Z.A.; Tamashiro, K.L. Maternal stressors and the developmental origins of neuropsychiatric risk. Front. Neuroendocr. 2020, 57, 100834. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Nousen, E.K.; Chamlou, K.A. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol. Behav. 2014, 123, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Pan, Y.X. Pathophysiological basis for compromised health beyond generations: Role of maternal high-fat diet and low-grade chronic inflammation. J. Nutr. Biochem. 2015, 26, 1–8. [Google Scholar] [CrossRef]
- Jiménez-Chillarón, J.C.; Díaz, R.; Martínez, D.; Pentinat, T.; Ramón-Krauel, M.; Ribó, S.; Plösch, T. The role of nutrition on epigenetic modifications and their implications on health. Biochimie 2012, 94, 2242–2263. [Google Scholar] [CrossRef]
- Cirulli, F.; Musillo, C.; Berry, A. Maternal Obesity as a Risk Factor for Brain Development and Mental Health in the Offspring. Neuroscience 2020, 447, 122–135. [Google Scholar] [CrossRef]
- Maldonado-Ruiz, R.; Garza-Ocañas, L.; Camacho, A. Inflammatory domains modulate autism spectrum disorder susceptibility during maternal nutritional programming. Neurochem. Int. 2019, 126, 109–117. [Google Scholar] [CrossRef]
- Andersen, C.H.; Thomsen, P.H.; Nohr, E.A.; Lemcke, S. Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. Eur. Child Adolesc. Psychiatry 2017, 27, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bilder, D.A.; Bakian, A.V.; Viskochil, J.; Clark, E.A.; Botts, E.L.; Smith, K.R.; Pimentel, R.; McMahon, W.M.; Coon, H. Maternal Prenatal Weight Gain and Autism Spectrum Disorders. Pediatrics 2013, 132, e1276–e1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krakowiak, P.; Walker, C.K.; Bremer, A.A.; Baker, A.S.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Maternal Metabolic Conditions and Risk for Autism and Other Neurodevelopmental Disorders. Pediatrics 2012, 129, e1121–e1128. [Google Scholar] [CrossRef] [Green Version]
- Christ, A.; Günther, P.; Lauterbach, M.A.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.-J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, M.D.; Graham, A.M.; Feczko, E.; Miranda-Dominguez, O.; Rasmussen, J.M.; Nardos, R.; Entringer, S.; Wadhwa, P.D.; Buss, C.; Fair, D.A. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 2018, 21, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, R.J. Role of maternal adiposity prior to and during pregnancy in cognitive and psychiatric problems in offspring. Nutr. Rev. 2013, 71, S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Reynolds, R.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, R.J.; Taylor, V.; Boyle, M.H. Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: A systematic review. Obes. Rev. 2011, 12, e548–e559. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef] [PubMed]
- Hannou, S.; Haslam, D.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Debnath, M.; Venkatasubramanian, G.; Berk, M. Fetal programming of schizophrenia: Select mechanisms. Neurosci. Biobehav. Rev. 2015, 49, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Van den Bergh, B.R.H.; de Rooij, S.R.; Kroegel, N.; Nathanielsz, P.W.; Rakers, F.; Roseboom, T.J.; Witte, O.W.; Schwab, M. Effects of maternal stress and nutrient restriction during gestation on offspring neuroanatomy in humans. Neurosci. Biobehav. Rev. 2020, 117, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Antonow-Schlorke, I.; Schwab, M.; Cox, L.A.; Li, C.; Stuchlik, K.; Witte, O.W.; Nathanielsz, P.W.; McDonald, T.J. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc. Natl. Acad. Sci. USA 2011, 108, 3011–3016. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.A. Handbook of Developmental Cognitive Neuroscience; MIT Press: Cambridge, MA, USA, 2001; 685p. [Google Scholar]
- Ke, X.; Schober, M.E.; McKnight, R.A.; O’Grady, S.; Caprau, D.; Yu, X.; Callaway, C.W.; Lane, R.H. Intrauterine growth retardation affects expression and epigenetic characteristics of the rat hippocampal glucocorticoid receptor gene. Physiol. Genom. 2010, 42, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Stevens, A.; Begum, G.; Cook, A.; Connor, K.; Rumball, C.; Oliver, M.; Challis, J.; Bloomfield, F.; White, A. Epigenetic Changes in the Hypothalamic Proopiomelanocortin and Glucocorticoid Receptor Genes in the Ovine Fetus after Periconceptional Undernutrition. Endocrinology 2010, 151, 3652–3664. [Google Scholar] [CrossRef]
- Coupé, B.; Amarger, V.; Grit, I.; Benani, A.; Parnet, P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 2010, 151, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jenkins, S.; Mattern, V.; Comuzzie, A.G.; Cox, L.A.; Huber, H.F.; Nathanielsz, P.W. Effect of moderate, 30 percent global maternal nutrient reduction on fetal and postnatal baboon phenotype. J. Med. Primatol. 2017, 46, 293–303. [Google Scholar] [CrossRef]
- Hulshoff Pol, H.E.; Hoek, H.W.; Susser, E.; Brown, A.S.; Dingemans, A.; Schnack, H.G.; van Haren, N.E.; Pereira Ramos, L.M.; Gispen-de Wied, C.C.; Kahn, R.S. Prenatal exposure to famine and brain morphology in schizophrenia. Am. J. Psychiatry 2000, 157, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.J.; Fischer, L.K.; Kundakovic, M.; Garg, P.; Jakovcevski, M.; Dincer, A.; Amaral, A.C.; Ginns, E.I.; Galdzicka, M.; Bryce, C.P.; et al. DNA Methylation Signatures of Early Childhood Malnutrition Associated With Impairments in Attention and Cognition. Biol. Psychiatry 2016, 80, 765–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, N.; Bautista, C.J.; Tovar, A.R.; Ordáz, G.; Rodríguez-Cruz, M.; Ortiz, V.; Granados, O.; Nathanielsz, P.W.; Larrea, F.; Zambrano, E. Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am. J. Physiol. Metab. 2010, 298, E270–E277. [Google Scholar] [CrossRef] [Green Version]
- de Rooij, S.R.; Wouters, H.; Yonker, J.; Painter, R.C.; Roseboom, T.J. Prenatal undernutrition and cognitive function in late adulthood. Proc. Natl. Acad. Sci. USA 2010, 107, 16881–16886. [Google Scholar] [CrossRef] [Green Version]
- Berti, C.; Biesalski, H.; Gärtner, R.; Lapillonne, A.; Pietrzik, K.; Poston, L.; Redman, C.; Koletzko, B.; Cetin, I. Micronutrients in pregnancy: Current knowledge and unresolved questions. Clin. Nutr. 2011, 30, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Radziejewska, A.; Chmurzynska, A. Folate and choline absorption and uptake: Their role in fetal development. Biochimie 2019, 158, 10–19. [Google Scholar] [CrossRef]
- Guéant, J.-L.; Namour, F.; Guéant-Rodriguez, R.-M.; Daval, J.-L. Folate and fetal programming: A play in epigenomics? Trends Endocrinol. Metab. 2013, 24, 279–289. [Google Scholar] [CrossRef]
- Silva, C.; Keating, E.; Pinto, E. The impact of folic acid supplementation on gestational and long term health: Critical temporal windows, benefits and risks. Porto Biomed. J. 2017, 2, 315–332. [Google Scholar] [CrossRef]
- Hamza, M.; Halayem, S.; Mrad, R.; Bourgou, S.; Charfi, F.; Belhadj, A. Epigenetics’ implication in autism spectrum disorders: A review. Encephale 2017, 43, 374–381. [Google Scholar] [CrossRef]
- Pepper, M.R.; Black, M.M. B12 in fetal development. Semin. Cell Dev. Biol. 2011, 22, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Tous, M.; Villalobos, M.; Iglesias, L.; Fernández-Barrés, S.; Arija, V. Vitamin D status during pregnancy and offspring outcomes: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2019, 74, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, D.; Anderson, G.H.; Poon, A.N.; Pannia, E.; Cho, C.E.; Huot, P.S.; Kubant, R. Maternal fat-soluble vitamins, brain development, and regulation of feeding behavior: An overview of research. Nutr. Res. 2016, 36, 1045–1054. [Google Scholar] [CrossRef]
- Larqué, E.; Morales, E.; Leis, R.; Blanco-Carnero, J.E. Maternal and Foetal Health Implications of Vitamin D Status during Pregnancy. Ann. Nutr. Metab. 2018, 72, 179–192. [Google Scholar] [CrossRef]
- Spiegler, E.; Kim, Y.-K.; Wassef, L.; Shete, V.; Quadro, L. Maternal–fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 88–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breymann, C. Iron Deficiency Anemia in Pregnancy. Semin. Hematol 2015, 52, 339–347. [Google Scholar] [CrossRef]
- Lozoff, B.; Georgieff, M.K. Iron Deficiency and Brain Development. Semin. Pediatr. Neurol. 2006, 13, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Jackson, A.A.; Robinson, S.M. Dietary guidelines for pregnancy: A review of current evidence. Public Health Nutr. 2001, 4, 625–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine status and supplementation before, during, and after pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101430. [Google Scholar] [CrossRef]
- Eastman, C.J.; Ma, G.; Li, M. Optimal Assessment and Quantification of Iodine Nutrition in Pregnancy and Lactation: Laboratory and Clinical Methods, Controversies and Future Directions. Nutrients 2019, 11, 2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skeaff, S.A. Iodine Deficiency in Pregnancy: The Effect on Neurodevelopment in the Child. Nutrients 2011, 3, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gonzalez, P.; Zhang, L. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: Mechanisms and possible interventions. Prog. Neurobiol. 2012, 98, 145–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, O.E.F.; Yager, J.Y. Preventing childhood and lifelong disability: Maternal dietary supplementation for perinatal brain injury. Pharm. Res. 2019, 139, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; West, A.A.; Caudill, M.A. Maternal choline supplementation: A nutritional approach for improving offspring health? Trends Endocrinol. Metab. 2014, 25, 263–273. [Google Scholar] [CrossRef]
- Ross, R.G.; Hunter, S.K.; McCarthy, L.; Beuler, J.; Hutchison, A.K.; Wagner, B.; Leonard, S.; Stevens, K.E.; Freedman, R. Perinatal Choline Effects on Neonatal Pathophysiology Related to Later Schizophrenia Risk. Am. J. Psychiatry 2013, 170, 290–298. [Google Scholar] [CrossRef]
- Richard, K.; Holland, O.; Landers, K.; Vanderlelie, J.J.; Hofstee, P.; Cuffe, J.S.; Perkins, A.V. Review: Effects of maternal micronutrient supplementation on placental function. Placenta 2017, 54, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Gambling, L.; Kennedy, C.; McArdle, H.J. Iron and copper in fetal development. Semin. Cell Dev. Biol. 2011, 22, 637–644. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, Q.; Ward, S.M.; Duan, E.; Zhang, Y. Impacts of Caffeine during Pregnancy. Trends Endocrinol. Metab. 2020, 31, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehlig, A.; Debry, G. Potential teratogenic and neurodevelopmental consequences of coffee and caffeine exposure: A review on human and animal data. Neurotoxicol. Teratol. 1994, 16, 531–543. [Google Scholar] [CrossRef]
- Linnet, K.M.; Wisborg, K.; Secher, N.J.; Thomsen, P.H.; Obel, C.; Dalsgaard, S.; Henriksen, T.B. Coffee consumption during pregnancy and the risk of hyperkinetic disorder and ADHD: A prospective cohort study. Acta Paediatr. 2009, 98, 173–179. [Google Scholar] [CrossRef]
- Galéra, C.; Bernard, J.Y.; van der Waerden, J.; Bouvard, M.-P.; Lioret, S.; Forhan, A.; De Agostini, M.; Melchior, M.; Heude, B. Prenatal Caffeine Exposure and Child IQ at Age 5.5 Years: The EDEN Mother-Child Cohort. Biol. Psychiatry 2016, 80, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Tsai, Y.-T.; Ariga, T. Functional Roles of Gangliosides in Neurodevelopment: An Overview of Recent Advances. Neurochem. Res. 2012, 37, 1230–1244. [Google Scholar] [CrossRef] [Green Version]
- Skaper, S.D.; Leon, A.; Toffano, G. Ganglioside function in the development and repair of the nervous system. Mol. Neurobiol. 1989, 3, 173–199. [Google Scholar] [CrossRef]
- Willinger, M.; Schachner, M. GM1 ganglioside as a marker for neuronal differentiation in mouse cerebellum. Dev. Biol. 1980, 74, 101–117. [Google Scholar] [CrossRef]
- Svennerholm, L.; Rynmark, B.-M.; Vilbergsson, G.; Fredman, P.; Gottfries, J.; Månsson, J.-E.; Percy, A. Gangliosides in Human Fetal Brain. J. Neurochem. 1991, 56, 1763–1768. [Google Scholar] [CrossRef]
- Ryan, J.M.; Rice, G.; Mitchell, M. The role of gangliosides in brain development and the potential benefits of perinatal supplementation. Nutr. Res. 2013, 33, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Ohmi, Y.; Ohkawa, Y.; Tokuda, N.; Kondo, Y.; Tajima, O.; Furukawa, K. Regulatory Mechanisms of Nervous Systems with Glycosphingolipids. Neurochem. Res. 2011, 36, 1578–1586. [Google Scholar] [CrossRef]
- Lapp, H.E.; Hunter, R.G. Early life exposures, neurodevelopmental disorders, and transposable elements. Neurobiol. Stress 2019, 11, 100174. [Google Scholar] [CrossRef] [PubMed]
- The Role of Nutrition in Cognitive Development. In Handbook of Developmental Cognitive Neuroscience; MIT Press: Cambridge, MA, USA, 2008.
- Iglesias, L.; Canals, J.; Arija, V. Effects of prenatal iron status on child neurodevelopment and behavior: A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1604–1614. [Google Scholar] [CrossRef]
- Ando, S. GANGLIOSIDES IN THE NERVOUS SYSTEM. Sel. Top. Neurochem. 1985, 5, 439–486. [Google Scholar] [CrossRef]
- Ledeen, R.W. Ganglioside structures and distribution: Are they localized at the nerve ending? J. Supramol. Struct. 1978, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]

| Nutrient Category | Nutrient | Reference Citation | |
|---|---|---|---|
| Macronutrients | Fatty acids | [5,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] | |
| Proteins | [10,11,13,25] | ||
| Diets | Overnutrition | High fat diet | [26,27,28,29,30,31,32,33,34,35,36,37,38] |
| Obesity | [12,13,14,29,30,39,40,41,42,43,44,45] | ||
| Ketogenic diet | [15] | ||
| Undernutrition | Maternal malnutrition/famine | [10,11,25,26,46,47,48,49,50,51,52,53,54,55,56,57] | |
| Vitamins | Folate | [5,58,59,60,61,62] | |
| B12 | [9,11,58,63] | ||
| Vitamin D | [46,62,64,65,66] | ||
| Vitamin A | [10,44,67] | ||
| Vitamin E | |||
| Vitamin K | |||
| Micronutrients | Iron | [5,26,58,68,69,70] | |
| Iodine | [9,10,71,72,73,74] | ||
| Choline | [25,46,75,76,77,78] | ||
| Zinc | [11,79] | ||
| Copper | [71,80,81,82] | ||
| Creatine | [76,83] | ||
| Other | Caffeine | [5,84,85,86,87] | |
| Gangliosides | [88,89,90,91,92,93] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. https://doi.org/10.3390/nu13103530
Cortés-Albornoz MC, García-Guáqueta DP, Velez-van-Meerbeke A, Talero-Gutiérrez C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients. 2021; 13(10):3530. https://doi.org/10.3390/nu13103530
Chicago/Turabian StyleCortés-Albornoz, María Camila, Danna Paola García-Guáqueta, Alberto Velez-van-Meerbeke, and Claudia Talero-Gutiérrez. 2021. "Maternal Nutrition and Neurodevelopment: A Scoping Review" Nutrients 13, no. 10: 3530. https://doi.org/10.3390/nu13103530
APA StyleCortés-Albornoz, M. C., García-Guáqueta, D. P., Velez-van-Meerbeke, A., & Talero-Gutiérrez, C. (2021). Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients, 13(10), 3530. https://doi.org/10.3390/nu13103530







