Retrospective Evaluation on the Use of a New Polysaccharide Complex in Managing Paediatric Type 1 Diabetes with Metabolic Syndrome (MetS)
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Design
2.2. Study Protocol
2.3. Auxological and Clinical Methods
2.4. Laboratory Methods
2.5. Statistical Analysis
3. Results
3.1. Overall Baseline Data
3.2. Group 1 and 2 Baseline Data (T0)
3.3. Group 1 and 2 T1 vs. T2 Data
3.4. Safety Data
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, O.O. Recent advances in childhood diabetes mellitus. Ann. Ib. Postgrad. Med. 2008, 6, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.A. Diabetes Mellitus. In Pediatric Endocrinology, 2nd ed.; Sperling, M.A., Ed.; Saunders: Philadelphia, PA, USA, 2002; pp. 323–366. [Google Scholar]
- Nathan, D.M.; Zinman, B.; Cleary, P.A.; Backlund, J.Y.C.; Genuth, S.; Miller, R.; Orchard, T.J.; Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: The diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Arch. Intern. Med. 2009, 169, 1307–1316. [Google Scholar] [CrossRef]
- Soni, A.; Ng, S.M. Intensive diabetes management and goal setting are key aspects of improving metabolic control in children and young people with type 1 diabetes mellitus. World J. Diabetes 2014, 5, 877–881. [Google Scholar] [CrossRef]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- Choudhary, P. Review of dietary recommendations for diabetes mellitus. Diabetes Res. Clin. Pract. 2004, 65 (Suppl. 1), S9–S15. [Google Scholar] [CrossRef]
- Quirk, H.; Blake, H.; Tennyson, R.; Randell, T.L.; Glazebrook, C. Physical activity interventions in children and young people with Type 1 diabetes mellitus: A systematic review with meta-analysis. Diabet. Med. 2014, 31, 1163–1173. [Google Scholar] [CrossRef]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D.; et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31 (Suppl. 1), S61–S78. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Barclay, A.; Colagiuri, S.; Brand-Miller, J. Glycemic index and glycemic load of carbohydrates in the diabetes diet. Curr. Diab. Rep. 2011, 11, 120–127. [Google Scholar] [CrossRef]
- Lottenberg, A.M. Diet composition along the evolution of type 1 diabetes mellitus. Arq. Bras. Endocrinol. Metabol. 2008, 52, 250–259. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef]
- Burani, J.; Longo, P.J. Low-glycemic index carbohydrates: An effective behavioral change for glycemic control and weight management in patients with type 1 and 2 diabetes. Diabetes Educ. 2006, 32, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Opperman, A.M.; Venter, C.S.; Oosthuizen, W.; Thompson, R.L.; Vorster, H.H. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br. J. Nutr. 2004, 92, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Buyken, A.E.; Toeller, M.; Heitkamp, G.; Karamanos, B.; Rottiers, R.; Muggeo, M.; Fuller, J.H.; EURODIAB IDDM Complications Study Group. Glycemic index in the diet of European outpatients with type 1 diabetes: Relations to glycated hemoglobin and serum lipids. Am. J. Clin. Nutr. 2001, 73, 574–581. [Google Scholar] [CrossRef]
- Gilbertson, H.R.; Brand-Miller, J.C.; Thorburn, A.W.; Evans, S.; Chondros, P.; Werther, G.A. The effect of flexible low glycemic index dietary advice versus measured carbohydrate exchange diets on glycemic control in children with type 1 diabetes. Diabetes Care 2001, 24, 1137–1143. [Google Scholar] [CrossRef]
- Du, H.; van der A, D.L.; van Bakel, M.M.; van der Kallen, C.J.; Blaak, E.E.; van Greevenbroek, M.M.; Jansen, E.H.; Nijpels, G.; Stehouwer, C.D.; Dekker, J.M.; et al. Glycemic index and glycemic load in relation to food and nutrient intake and metabolic risk factors in a Dutch population. Am. J. Clin. Nutr. 2008, 87, 655–661. [Google Scholar] [CrossRef]
- Wolever, T.M.; Mehling, C.; Chiasson, J.L.; Josse, R.G.; Leiter, L.A.; Maheux, P.; Rabasa-Lhoret, R.; Rodger, N.W.; Ryan, E.A. Low glycaemic index diet and disposition index in type 2 diabetes (the Canadian trial of carbohydrates in diabetes): A randomised controlled trial. Diabetologia 2008, 51, 1607–1615. [Google Scholar] [CrossRef]
- Ghosh, S.; Collier, A.; Hair, M.; Malik, I.; Elhadd, T. Metabolic syndrome in type 1 diabetes. Int. J. Diabetes Mellit. 2010, 2, 38–42. [Google Scholar] [CrossRef][Green Version]
- Thorn, L.M.; Forsblom, C.; Fagerudd, J.; Thomas, M.C.; Pettersson-Fernholm, K.; Saraheimo, M.; Waden, J.; Ronnback, M.; Rosengard-Barlund, M.; Bjorkesten, C.-G.A.; et al. Metabolic syndrome in type 1 diabetes: Association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 2005, 28, 2019–2024. [Google Scholar] [CrossRef]
- Da Costa, V.M.; de Carvalho Padilha, P.; de Lima, G.C.; Ferreira, A.A.; Luescher, J.L.; Porto, L.; Peres, W.A.F. Overweight among children and adolescent with type I diabetes mellitus: Prevalence and associated factors. Diabetol. Metab. Syndr. 2016, 8, 39. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Levek-Motola, N.; Kaidar, K.; Boyko, V.; Tisch, E.; Mazor-Aronovitch, K.; Graf-Barel, C.; Landau, Z.; Lerner-Geva, L.; Ben-David, R.F. Prevalence of overweight, obesity and metabolic syndrome components in children, adolescents and young adults with type 1 diabetes mellitus. Diabetes Metab. Res. Rev. 2015, 31, 76–84. [Google Scholar] [CrossRef]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007, 30, 707–712. [Google Scholar] [CrossRef]
- Teupe, B.; Bergis, K. Epidemiological evidence for “double diabetes”. Lancet 1991, 337, 361–362. [Google Scholar] [CrossRef]
- Reinehr, T.; Holl, R.W.; Roth, C.L.; Wiesel, T.; Stachow, R.; Wabitsch, M.; Andler, W.; DPV-Wiss Study Group. Insulin resistance in children and adolescents with type 1 diabetes mellitus: Relation to obesity. Pediatr. Diabetes 2005, 6, 5–12. [Google Scholar] [CrossRef]
- BEACh Program: Prevalence of Metabolic Syndrome. Sydney: AgPSCC University of Sydney, Sydney, New South Wales. 2006. Available online: http://sydney.edu.au/medicine/fmrc/publications/sand-abstracts/92-Metabolic_syndrome.pdf (accessed on 20 June 2013).
- McGill, M.; Molyneaux, L.; Twigg, S.M.; Yue, D.K. The metabolic syndrome in type 1 diabetes: Does it exist and does it matter? J. Diabetes Complicat. 2008, 22, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; Maffeis, C.; Zucchini, S.; Lombardo, F.; Toni, S.; Rabbone, I.; Federico, G.; Scaramuzza, A.; Franzese, A.; Cherubini, V.; et al. Geographic variation in the frequency of abdominal adiposity and metabolic syndrome in Italian adolescents with type 1 diabetes. Acta Diabetol. 2014, 51, 163–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stagi, S.; Lapi, E.; Seminara, S.; Pelosi, P.; Del Greco, P.; Capirchio, L.; Strano, M.; Giglio, S.; Chiarelli, F.; De Martino, M. Policaptil Gel Retard significantly reduces body mass index and hyperinsulinism and may decrease the risk of type 2 diabetes mellitus (T2DM) in obese children and adolescents with family history of obesity and T2DM. Ital. J. Pediatr. 2015, 41, 10. [Google Scholar] [CrossRef] [PubMed]
- Stagi, S.; Ricci, F.; Bianconi, M.; Sammarco, M.A.; Municchi, G.; Toni, S.; Lenzi, L.; Verrotti, A.; De Martino, M. Retrospective Evaluation of Metformin and/or Metformin Plus a New Polysaccharide Complex in Treating Severe Hyperinsulinism and Insulin Resistance in Obese Children and Adolescents with Metabolic Syndrome. Nutrients 2017, 9, 524. [Google Scholar] [CrossRef]
- Fornari, E.; Morandi, A.; Piona, C.; Tommasi, M.; Corradi, M.; Maffeis, C. Policaptil Gel Retard Intake Reduces Postprandial Triglycerides, Ghrelin and Appetite in Obese Children: A Clinical Trial. Nutrients 2020, 12, 214. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008, 31 (Suppl. 1), S55–S60. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Fida, S.; Myers, M.; Mackay, I.R.; Zimmet, P.Z.; Mohan, V.; Deepa, R.; Rowley, M.J. Antibodies to diabetes-associated autoantigens in Indian patients with Type 1 diabetes: Prevalence of anti-ICA512/IA2 and anti-SOX13. Diabetes Res. Clin. Pract. 2001, 52, 205–211. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef]
- Zaccardi, F.; Pitocco, D.; Ghirlanda, G. Glycemic risk factors of diabetic vascular complications: The role of glycemic variability. Diabetes Metab. Res. Rev. 2009, 25, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Seaquist, E.R.; Anderson, J.; Childs, B.; Cryer, P.; Dagogo-Jack, S.; Fish, L.; Heller, S.R.; Rodriguez, H.; Rosenzweig, J.; Vigersky, R. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013, 36, 1384–1395. [Google Scholar] [CrossRef]
- Standl, E.; Schnell, O.; Ceriello, A. Postprandial hyperglycemia and glycemic variability: Should we care? Diabetes Care 2011, 34 (Suppl. 2), S120–S127. [Google Scholar] [CrossRef]
- Kovatchev, B.P.; Otto, E.; Cox, D.; Gonder-Frederick, L.; Clarke, W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care 2006, 29, 2433–2438. [Google Scholar] [CrossRef]
- Kovatche, B.P.; Cox, D.J.; Kumar, A.; Gonder-Frederick, L.; Clarke, W.L. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol. Ther. 2003, 5, 817–828. [Google Scholar] [CrossRef]
- Wójcicki, J.M. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm. Metab. Res. 1995, 27, 41–42. [Google Scholar] [CrossRef]
- Service, F.J.; Molnar, G.D.; Rosevear, J.W.; Ackerman, E.; Gatewood, L.C.; Taylor, W.F. Mean amplitude of glycemic excursions: A measure of diabetic instability. Diabetes 1970, 19, 644–655. [Google Scholar] [CrossRef]
- Rodbard, D. The challenges of measuring glycemic variability. J. Diabetes Sci. Technol. 2012, 6, 712–715. [Google Scholar] [CrossRef]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.V.; Erbey, J.R.; Becker, D.; Arslanian, S.; Orchard, T.J. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000, 49, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; D’Agostino, J.R.B.; Mason, C.C.; West, N.; Hamman, R.F.; Mayer-Davis, E.J.; Maahs, D.; Klingensmith, G.; Knowler, W.C.; Nadeau, K. Development, validation and use of an insulin sensitivity score in youths with diabetes: The search for diabetes in youth study. Diabetologia 2011, 54, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Mde, D.F.; Reis, J.S.; Ferrari, T.C.A.; de Castro, M.G.B.; Teixeira, B.P.; da Silva, A.I.C.; Bicalho, M.B.; Fóscolo, R.B. Insulin resistance and associated factors in patients with Type 1 Diabetes. Diabetol. Metab. Syndr. 2014, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Management of dyslipidemia in children and adolescents with diabetes. Diabetes Care 2003, 26, 2194–2197. [Google Scholar] [CrossRef]
- Fredriks, A.M.; van Buuren, S.; Fekkes, M.; Verloove-Vanhorick, S.P.; Wit, J.M. Are age references for waist circumference, hip circumference and waist-hip ratio in Dutch children useful in clinical practice? Eur. J. Pediatr. 2005, 164, 216–222. [Google Scholar] [CrossRef]
- Maffeis, C.; Grezzani, A.; Pietrobelli, A.; Provera, S.; Tatò, L. Does waist circumference predict fat gain in children? Int. J. Obes. Relat. Metab. Disord. 2001, 25, 978–983. [Google Scholar] [CrossRef]
- Stagi, S.; Galli, L.; Cecchi, C.; Chiappini, E.; Losi, S.; Gattinara, C.; Gabiano, C.; Tovo, P.A.; Bernardi, S.; Chiarelli, F.; et al. Final height in patients perinatally infected with the human immunodeficiency virus. Horm. Res. Paediatr. 2010, 74, 165–171. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114 (Suppl. 2), 555–576. [Google Scholar] [CrossRef]
- Patterson, C.C.; Gyürüs, E.; Rosenbauer, J.; Cinek, O.; Neu, A.; Schober, E.; Parslow, R.; Joner, G.; Svensson, J.; Castell, C.; et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: Evidence of non-uniformity over time in rates of increase. Diabetologia 2012, 55, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Castro-Correia, C.; Santos-Silva, R.; Pinheiro, M.; Costa, C.; Fontoura, M. Metabolic risk factors in adolescent girls with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2018, 31, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Billow, A.; Anjana, R.M.; Ngai, M.; Amutha, A.; Pradeepa, R.; Jebarani, S.; Unnikrishnan, R.; Michael, E.; Mohan, V. Prevalence and clinical profile of metabolic syndrome among type 1 diabetes. J. Diabetes Complicat. 2015, 29, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Łuczyński, W.; Szypowska, A.; Głowińska-Olszewska, B.; Bossowski, A. Overweight, obesity and features of metabolic syndrome in children with diabetes treated with insulin pump therapy. Eur. J. Pediatr. 2011, 170, 891–898. [Google Scholar] [CrossRef]
- Gingras, V.; Leroux, C.; Fortin, A.; Legault, L.; Rabasa-Lhoret, R. Predictors of cardiovascular risk among patients with type 1 diabetes: A critical analysis of the metabolic syndrome and its components. Diabetes Metab. 2017, 43, 217–222. [Google Scholar] [CrossRef]
- Livingstone, S.J.; Looker, H.C.; Hothersall, E.J.; Wild, S.H.; Lindsay, R.; Chalmers, J.; Cleland, S.; Leese, G.P.; McKnight, J.; Morris, A.D.; et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012, 9, e1001321. [Google Scholar] [CrossRef] [PubMed]
- Högel, J.; Grabert, M.; Sorgo, W.; Wudy, S.; Gaus, W.; Heinze, E. Hemoglobin A1c and body mass index in children and adolescents with IDDM. An observational study from 1976–1995. Exp. Clin. Endocrinol. Diabetes 2000, 108, 76–80. [Google Scholar] [CrossRef]
- Conway, B.; Miller, R.G.; Costacou, T.; Fried, L.; Kelsey, S.; Evans, R.W.; Orchard, T.J. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabet. Med. 2010, 27, 398–404. [Google Scholar] [CrossRef]
- Purnell, J.Q.; Dev, R.K.; Steffes, M.W.; Cleary, P.A.; Palmer, J.P.; Hirsch, I.B.; Hokanson, J.E.; Brunzell, J.D. Relationship of family history of type 2 diabetes, hypoglycemia, and autoantibodies to weight gain and lipids with intensive and conventional therapy in the Diabetes Control and Complications Trial. Diabetes 2003, 52, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Guarino, G.; Della Corte, T.; Strollo, F.; Gentile, S.; Nefrocenter Research Study Group. Policaptil Gel Retard in adult subjects with the metabolic syndrome: Efficacy, safety, and tolerability compared to metformin. Diabetes Metab. Syndr. 2021, 15, 901–907. [Google Scholar] [CrossRef] [PubMed]
| Variable | Characteristics | ||
|---|---|---|---|
| Treated | Untreated | p | |
| Subjects, number (M:F) | 16 (8/8) | 11 (6/5) | - |
| Age, years (median and range) | 12.9 (9.5–15.8) | 12.6 (9.4–15.6) | - |
| Prepubertal/pubertal ratio, % | 44.0/56.0 | 45.0/55.0 | - |
| Type 2 diabetes family history, % | 56.2 | 63.6 | - |
| Obesity family history, % | 18.7 | 18.1 | - |
| Ancestry (geographic Italian region), n (%) | |||
| Northern Italy | 2 (12.5) | 1 (9.1) | - |
| Central Italy | 4 (25.0) | 4 (36.4) | <0.05 |
| Southern Italy | 10 (62.5) | 6 (54.5) | - |
| Type 1 diabetes duration, years (median and range) | 6.8 (5.0–9.2) | 6.5 (5.2–9.1) | - |
| Height, SDS | 0.27 ± 0.26 | 0.32 ± 0.21 | - |
| Body Mass Index (BMI), SDS | 2.04 ± 0.12 | 2.00 ± 0.09 | - |
| Waist circumference, SDS | 2.29 ± 0.20 | 2.28 ± 0.27 | - |
| Screen time (computer, TV and video) (%) | |||
| ≤2 h/day | 31.2 | 27.2 | - |
| 2–4 h/day | 37.6 | 36.4 | - |
| ≥4 h/day | 31.2 | 36.4 | - |
| Time weekly spent for exercise (%) | |||
| ≤2 h/week | 25.0 | 27.3 | - |
| 2–4 h/week | 62.5 | 55.4 | - |
| ≥4 h/week | 12.5 | 17.3 | - |
| Dietary glycaemic index | 56.35 ± 2.11 | 50.55 ± 2.05 | - |
| Dietary glycaemic load, units | 132.25 ± 13.15 | 130.00 ± 15.15 | - |
| Treated | Untreated | |||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 3 Months | 6 Months | Baseline | 3 Months | 6 Months |
| Subjects, number (M:F) | 16 (8/8) | 16 (8/8) | 16 (8/8) | 11 (6/5) | 11 (6/5) | 11 (6/5) |
| Age, years (Median and range) | 12.9 (9.5–15.8) | 13.2 (9.8–16.1) | 13.5 (10.0–16.4) | 12.6 (9.4–15.6) | 12.9 (9.7–15.9) | 13.2 (10.0–16.1) |
| Prepubertal/pubertal ratio, % | 44/56 | 37/63 | 31/69 * | 45/55 | 55/45 | 36/64 |
| Height, SDS | 0.27 ± 0.26 | 0.28 ± 0.24 | 0.34 ± 0.29 | 0.32 ± 0.21 | 0.34 ± 0.23 | 0.37 ± 0.25 |
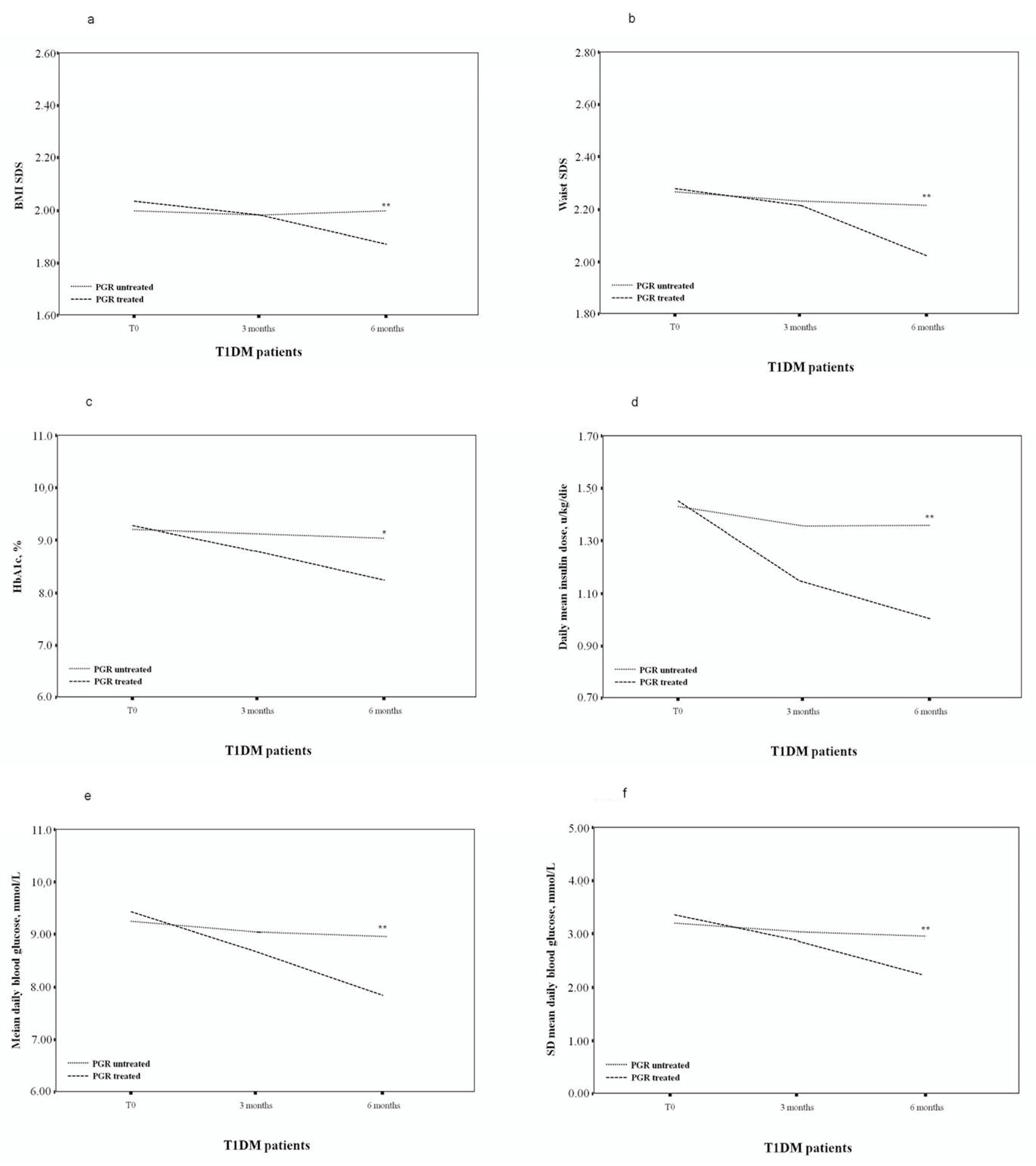
| BMI, SDS | 2.04 ± 0.12 | 1.99 ± 0.17 | 1.88 ± 0.16 ** | 2.00 ± 0.09 | 1.99 ± 0.15 | 2.01 ± 0.15 ^ |
| Waist circumference, SDS | 2.29 ± 0.20 | 2.23 ± 0.23 | 2.04 ± 0.19 ** | 2.28 ± 0.27 | 2.26 ± 0.25 | 2.24 ± 0.27 ^ |
| Screen time (computer, TV and video) (%) | ||||||
| ≤2 h/day | 31.2 | 37.6 | 43.7 * | 27.2 | 36.4 | 36.4 |
| 2–4 h/day | 37.6 | 31.2 | 31.2 | 36.4 | 27.3 | 36.4 |
| ≥4 h/day | 31.2 | 31.2 | 25.1 | 36.4 | 36.4 | 27.3 |
| Time weekly spent for exercise (%) | ||||||
| ≤2 h/week | 25.0 | 31.2 | 37.6 * | 27.3 | 36.3 | 36.3 |
| 2–4 h/week | 62.5 | 62.5 | 56.2 | 55.4 | 45.5 | 45.5 |
| ≥4 h/week | 12.5 | 6.3 | 6.2 * | 17.3 | 18.2 | 18.2 |
| HbA1c, % (range) | 9.30 (7.4–10.2) | 8.85 (7.3–10.0) | 8.20 (7.0–9.5) * | 9.25 (7.6–10.3) | 9.15 (7.5–10.1) | 9.10 (7.4–10.0) ^ |
| Blood glucose measurements per day, n | 8.1 ± 1.2 | 7.7 ± 1.2 | 7.6 ± 1.1 | 8.2 ± 1.2 | 8.0 ± 1.1 | 8.0 ± 1.1 |
| Fasting BG, mmol/L | 8.96 ± 1.31 | 8.03 ± 1.02 * | 7.63 ± 0.88 ** | 8.89 ± 1.27 | 8.66 ± 1.17 | 8.60 ± 1.08 ^ |
| Pre-lunch BG, mmol/L | 11.42 ± 2.48 | 10.86 ± 2.11 | 9.21 ± 1.73 * | 11.56 ± 2.39 | 11.23 ± 2.21 | 11.06 ± 2.00 ^ |
| Pre-dinner BG, mmol/L | 11.63 ± 2.26 | 11.37 ± 2.22 | 10.01 ± 2.07 * | 11.84 ± 2.33 | 11.58 ± 2.28 | 11.76 ± 2.23 ^ |
| Postprandial BG, mmol/L | 12.09 ± 2.21 | 11.64 ± 2.13 | 10.41 ± 2.02 ** | 12.23 ± 2.29 | 12.19 ± 2.16 | 12.14 ± 2.19 ^ |
| Mean insulin dose, u/kg/die | 1.47 ± 0.49 | 1.16 ± 0.34 * | 1.03 ± 0.29 ** | 1.45 ± 0.43 | 1.37 ± 0.36 | 1.38 ± 0.37 ^ |
| Mean daily blood glucose, mmol/L | 9.48 ± 1.57 | 8.78 ± 1.41 | 7.99 ± 1.12 ** | 9.29 ± 1.53 | 9.17 ± 1.48 | 9.13 ± 1.42 ^ |
| SD, mmol/L | 3.45 ± 0.57 | 2.99 ± 0.51 ** | 2.28 ± 0.43 *** | 3.39 ± 0.59 | 3.22 ± 0.56 | 3.03 ± 0.51 ^^ |
| Mean coefficient of variation, % | 36.39% | 34.05% | 28.53% * | 36.49% | 35.11% | 33.18% |
| LBGI | 5.64 ± 2.33 | 4.42 ± 2.11 * | 2.55 ± 1.87 *** | 5.73 ± 2.59 | 5.47 ± 2.43 | 5.13 ± 2.27 ^^ |
| HBGI | 9.87 ± 2.79 | 8.83 ± 2.21 * | 5.46 ± 1.91 *** | 9.94 ± 2.98 | 9.47 ± 2.71 | 9.06 ± 2.56 ^^^ |
| GRADE | ||||||
| Mean score | 15.0 ± 5.2 | 12.9 ± 4.1 | 10.5 ± 3.3 * | 14.8 ± 5.0 | 14.2 ± 4.8 | 14.5 ± 4.5 ^ |
| Hyperglycaemic, % | 66.2 (51.5–81.0) | 59.4 (48.1–73.4) | 54.7 (45.4–66.1) * | 65.6 (51.7–82.3) | 64.4 (52.1–79.4) | 64.2 (51.6–78.4) |
| Euglycemic, % | 23.2 (15.8–33.3) | 31.4 (20.4–47.8) | 38.7 (33.2–53.4) ** | 23.0 (10.0–32.6) | 24.0 (12.1–31.2) | 24.3 (10.8–40.1) ^^ |
| Hypoglycaemic, % | 10.6 (0.8–27.3) | 9.2 (0.6–25.4) | 6.6 (0.5–22.3) * | 11.4 (0.9–25.4) | 11.6 (0.8–26.1) | 11.5 (0.8–25.7) ^ |
| J-index | 54.16 ± 17.22 | 44.88 ± 13.63 | 34.17 ± 9.11 *** | 52.09 ± 16.88 | 49.73 ± 14.54 | 47.90 ± 13.99 ^^ |
| MODD, mmol/L | 6.4 ± 2.6 | 5.2 ± 2.1 | 4.7 ± 1.6 * | 6.4 ± 2.5 | 6.3 ± 2.4 | 6.2 ± 2.4 ^ |
| ADRR ° | 44.91 ± 14.29 | 40.28 ± 12.99 * | 32.75 ± 10.08 * | 44.83 ± 13.76 | 43.99 ± 14.11 | 43.94 ± 13.53 ^ |
| MAGE | 7.49 ± 1.76 | 6.33 ± 1.02 | 5.57 ± 1.13 *** | 7.41 ± 1.70 | 6.99 ± 1.62 | 6.93 ± 1.63 ^ |
| Hyperglycaemia index | 1.9 ± 1.0 | 1.6 ± 0.9 | 1.2 ± 0.8 * | 1.9 ± 1.2 | 1.8 ± 1.0 | 1.8 ± 0.9 |
| Hypoglycaemia index | 1.8 ± 1.0 | 1.2 ± 0.8 | 0.9 ± 0.7 * | 1.8 ± 1.1 | 1.9 ± 1.1 | 1.7 ± 0.9 ^ |
| Index of Glycaemic Control | 3.7 ± 1.0 | 2.8 ± 0.9 | 2.1 ± 0.8 ** | 3.6 ± 1.2 | 3.3 ± 1.1 | 3.4 ± 0.9 ^^^ |
| Patients experienced morning hypoglycaemia, % | 12.5 | 6.3 * | 6.3 * | 9.1 | 9.1 | 9.1 |
| Patients experienced nocturnal hypoglycaemia, % | 18.7 | 12.5 | 12.5 | 27.3 | 27.3 | 27.3 ^^^ |
| Morning hypoglycaemia episodes per month per patient, n | 3.9 ± 1.1 | 3.3 ± 1.0 | 2.9 ± 1.0 * | 4.0 ± 1.2 | 3.9 ± 1.1 | 3.8 ± 1.2 ^ |
| Nocturnal hypoglycaemia episodes per month per patient, n | 6.7 ± 3.0 | 5.5 ± 2.7 | 4.1 ± 2.5 * | 6.6 ± 3.3 | 6.3 ± 3.2 | 6.1 ± 3.0 ^ |
| Triglycerides, mmol/L | 1.81 ± 0.39 | 1.65 ± 0.34 | 1.51 ± 0.31 * | 1.83 ± 0.41 | 1.80 ± 0.38 | 1.74 ± 0.37 |
| Total cholesterol, mmol/L | 5.41 ± 0.64 | 5.29 ± 0.47 | 4.81 ± 0.41 ** | 5.48 ± 0.69 | 5.37 ± 0.61 | 5.33 ± 0.60 ^ |
| HDL-cholesterol, mmol/L | 0.86 ± 0.13 | 0.93 ± 0.14 | 1.01 ± 0.13 ** | 0.92 ± 0.14 | 0.94 ± 0.15 | 0.90 ± 0.14 ^ |
| LDL-cholesterol, mmol/L | 3.73 ± 0.52 | 3.61 ± 0.50 * | 3.12 ± 0.37 *** | 3.73 ± 0.53 | 3.62 ± 0.51 | 3.64 ± 0.52 ^^ |
| Systolic BP, SDS | 2.11 ± 0.56 | 1.83 ± 0.62 | 1.63 ± 0.60 * | 2.14 ± 0.63 | 2.07 ± 0.56 | 2.11 ± 0.57 ^ |
| Diastolic BP, SDS | 2.17 ± 0.59 | 1.99 ± 0.60 | 1.71 ± 0.58 * | 2.09 ± 0.57 | 2.11 ± 0.59 | 2.08 ± 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stagi, S.; Papacciuoli, V.; Ciofi, D.; Piccini, B.; Farello, G.; Toni, S.; Ferrari, M.; Chiarelli, F. Retrospective Evaluation on the Use of a New Polysaccharide Complex in Managing Paediatric Type 1 Diabetes with Metabolic Syndrome (MetS). Nutrients 2021, 13, 3517. https://doi.org/10.3390/nu13103517
Stagi S, Papacciuoli V, Ciofi D, Piccini B, Farello G, Toni S, Ferrari M, Chiarelli F. Retrospective Evaluation on the Use of a New Polysaccharide Complex in Managing Paediatric Type 1 Diabetes with Metabolic Syndrome (MetS). Nutrients. 2021; 13(10):3517. https://doi.org/10.3390/nu13103517
Chicago/Turabian StyleStagi, Stefano, Valeria Papacciuoli, Daniele Ciofi, Barbara Piccini, Giovanni Farello, Sonia Toni, Marta Ferrari, and Francesco Chiarelli. 2021. "Retrospective Evaluation on the Use of a New Polysaccharide Complex in Managing Paediatric Type 1 Diabetes with Metabolic Syndrome (MetS)" Nutrients 13, no. 10: 3517. https://doi.org/10.3390/nu13103517
APA StyleStagi, S., Papacciuoli, V., Ciofi, D., Piccini, B., Farello, G., Toni, S., Ferrari, M., & Chiarelli, F. (2021). Retrospective Evaluation on the Use of a New Polysaccharide Complex in Managing Paediatric Type 1 Diabetes with Metabolic Syndrome (MetS). Nutrients, 13(10), 3517. https://doi.org/10.3390/nu13103517








