Influence of Tilia tomentosa Moench Extract on Mouse Small Intestine Neuromuscular Contractility
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Solutions
2.2. Plant Material and Extract Preparation of Tilia tomentosa Moench
2.3. Chemical Analysis
2.4. Effects of Tilia tomenstosa Moench on Isolated Mouse Ileum
2.5. Immunohistochemistry on Ileal Whole-Mount Preparations
2.6. Imaging Acquisition and Analysis
2.7. Statistical Analysis
3. Results
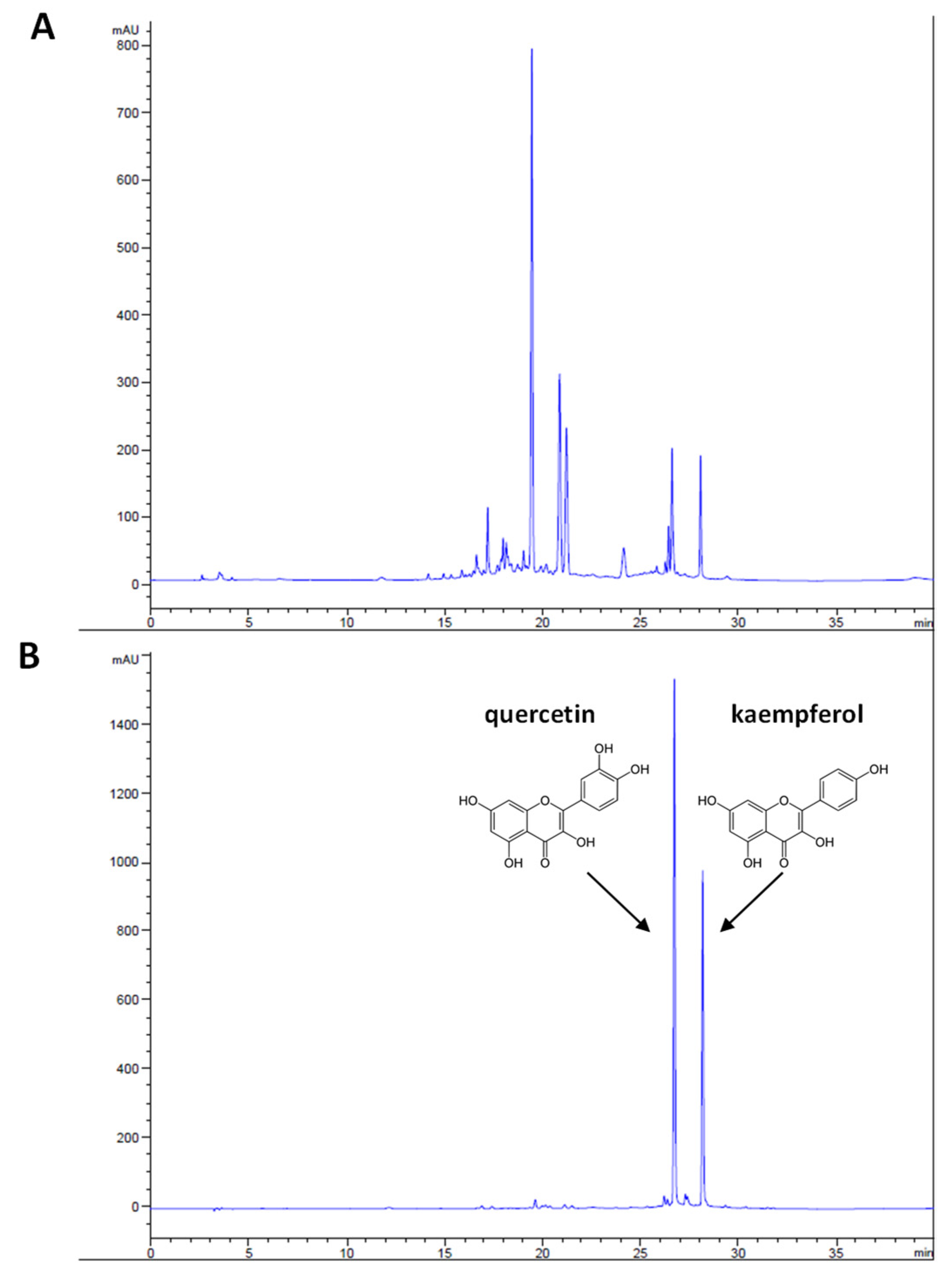
3.1. Phytochemical Analysis
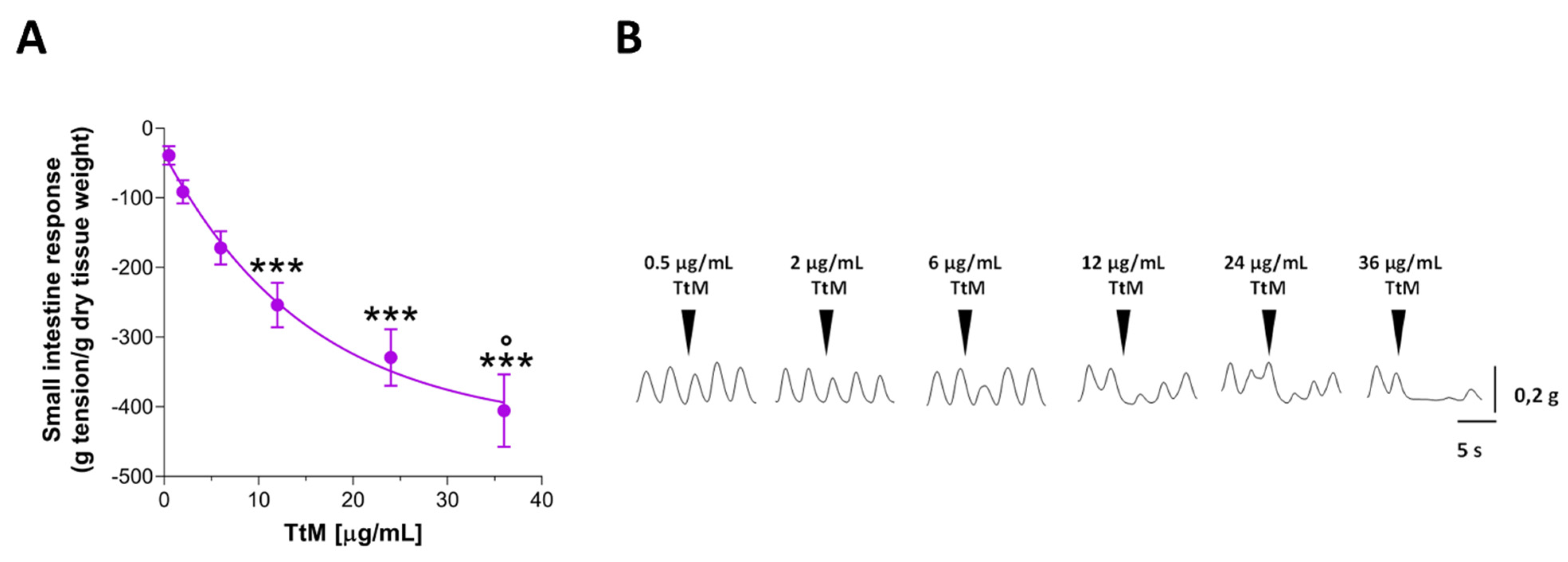
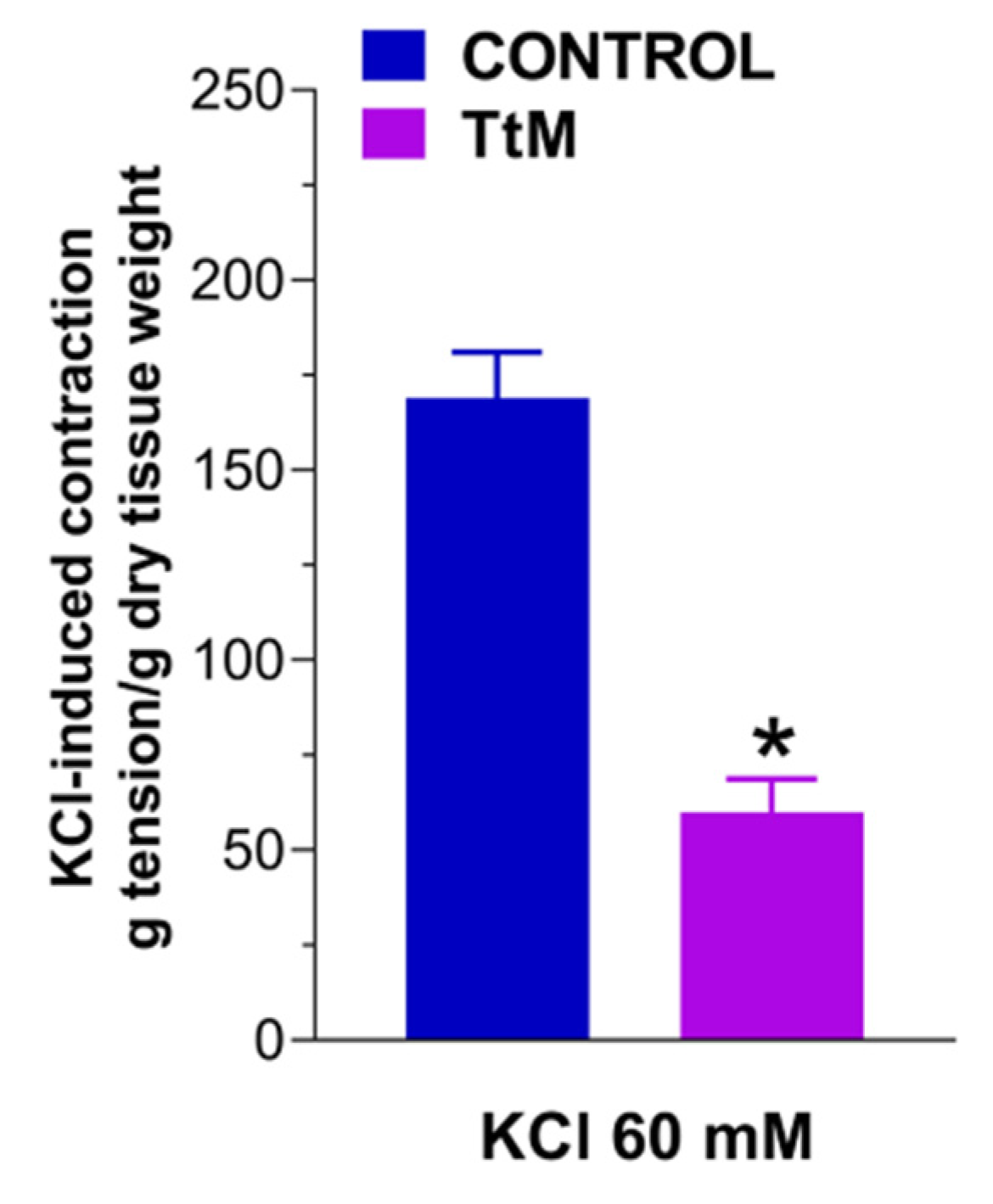
3.2. Tilia Tomentosa Inhibits Basal Contractile Responses of Isolated Small Intestine Preparations
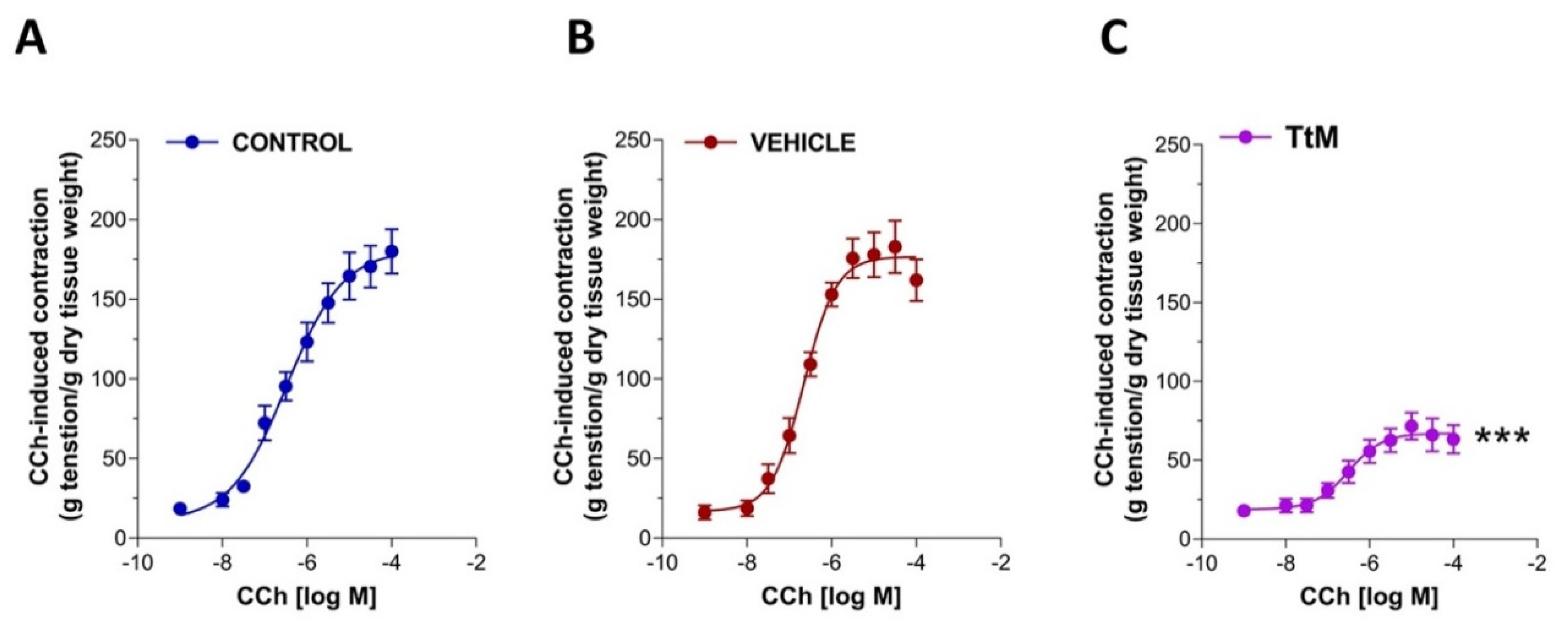
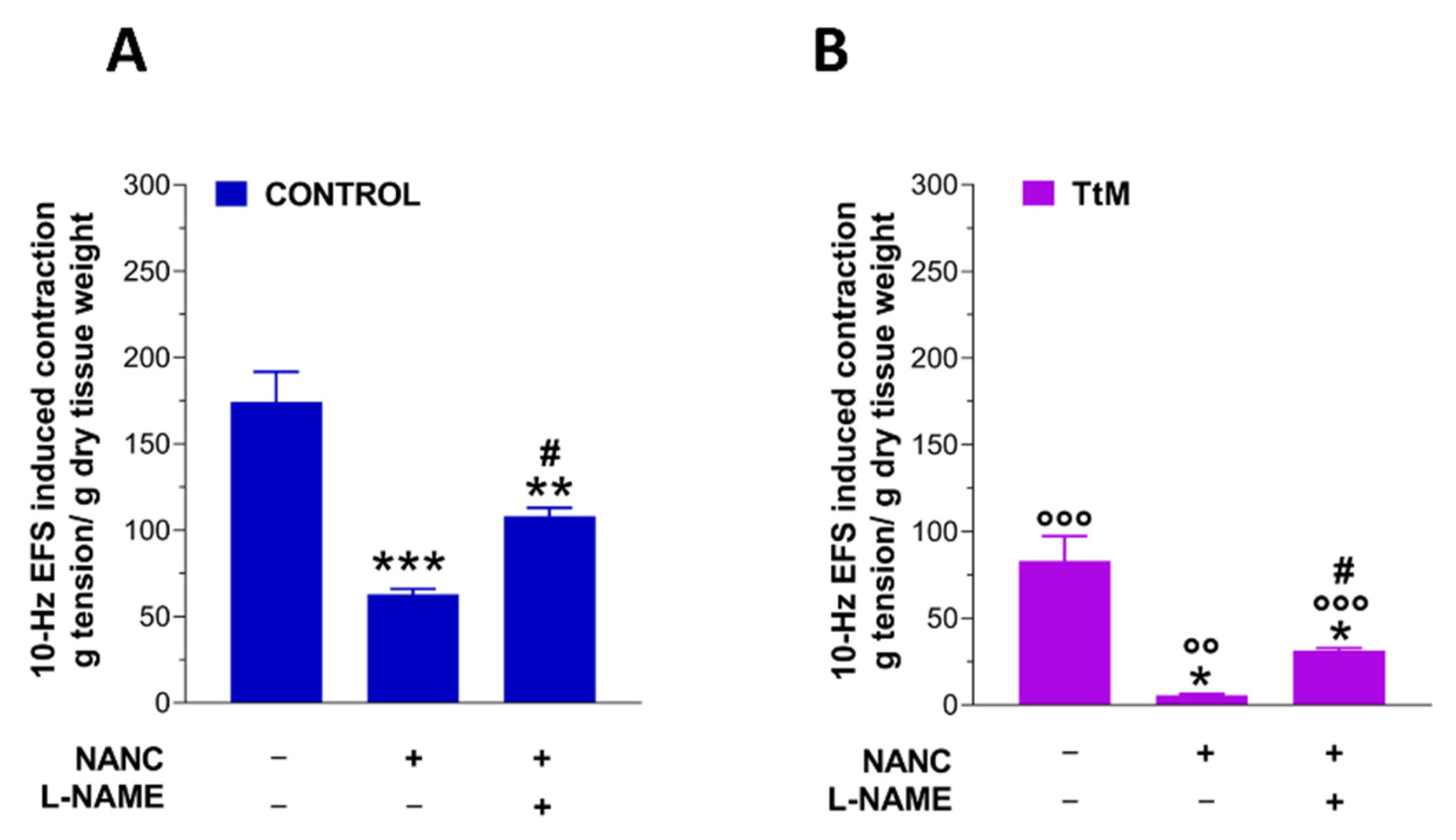
3.3. Tilia Tomentosa Influences Excitatory Neuromuscular Response of Isolated Small Intestine Preparations
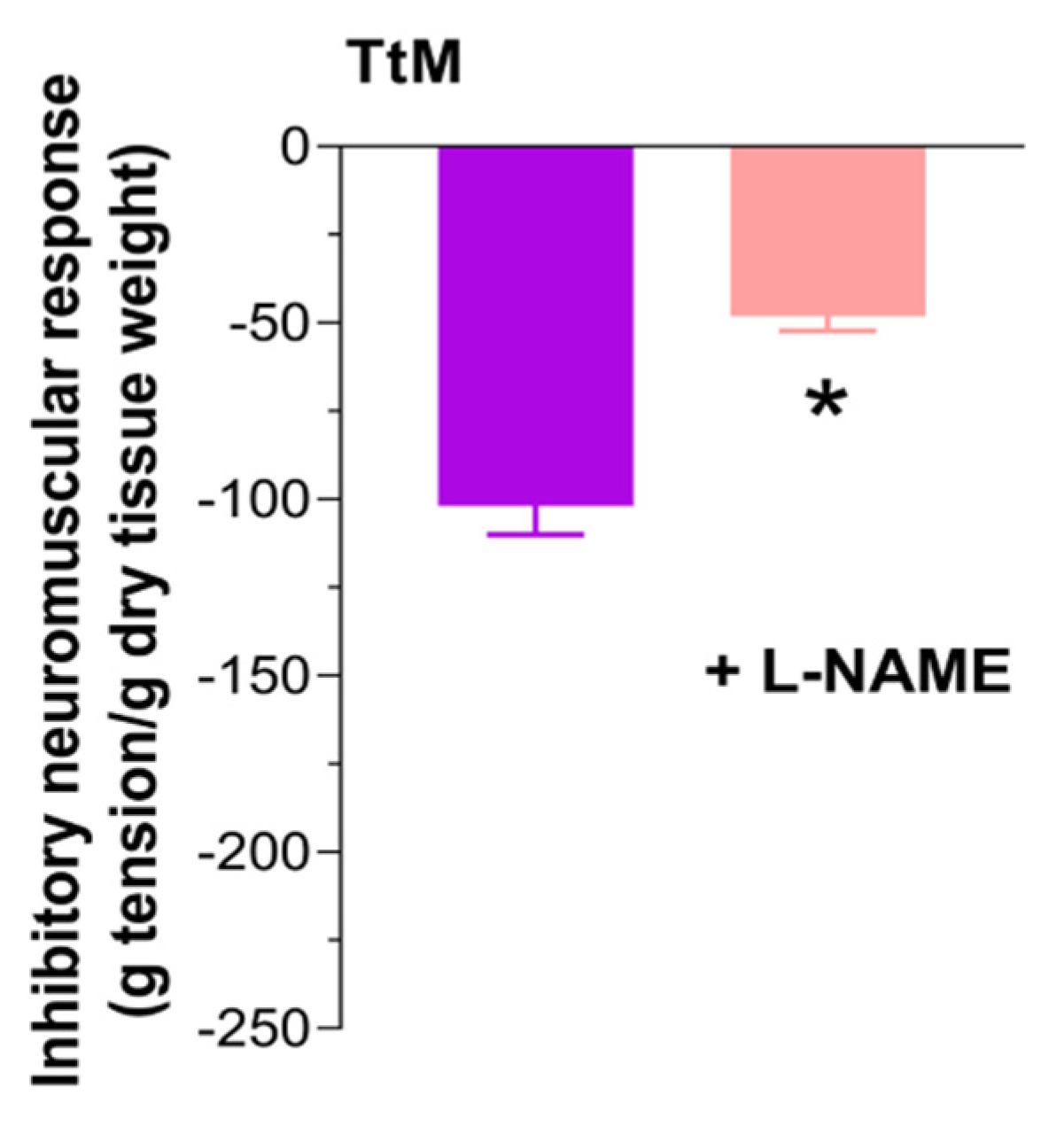
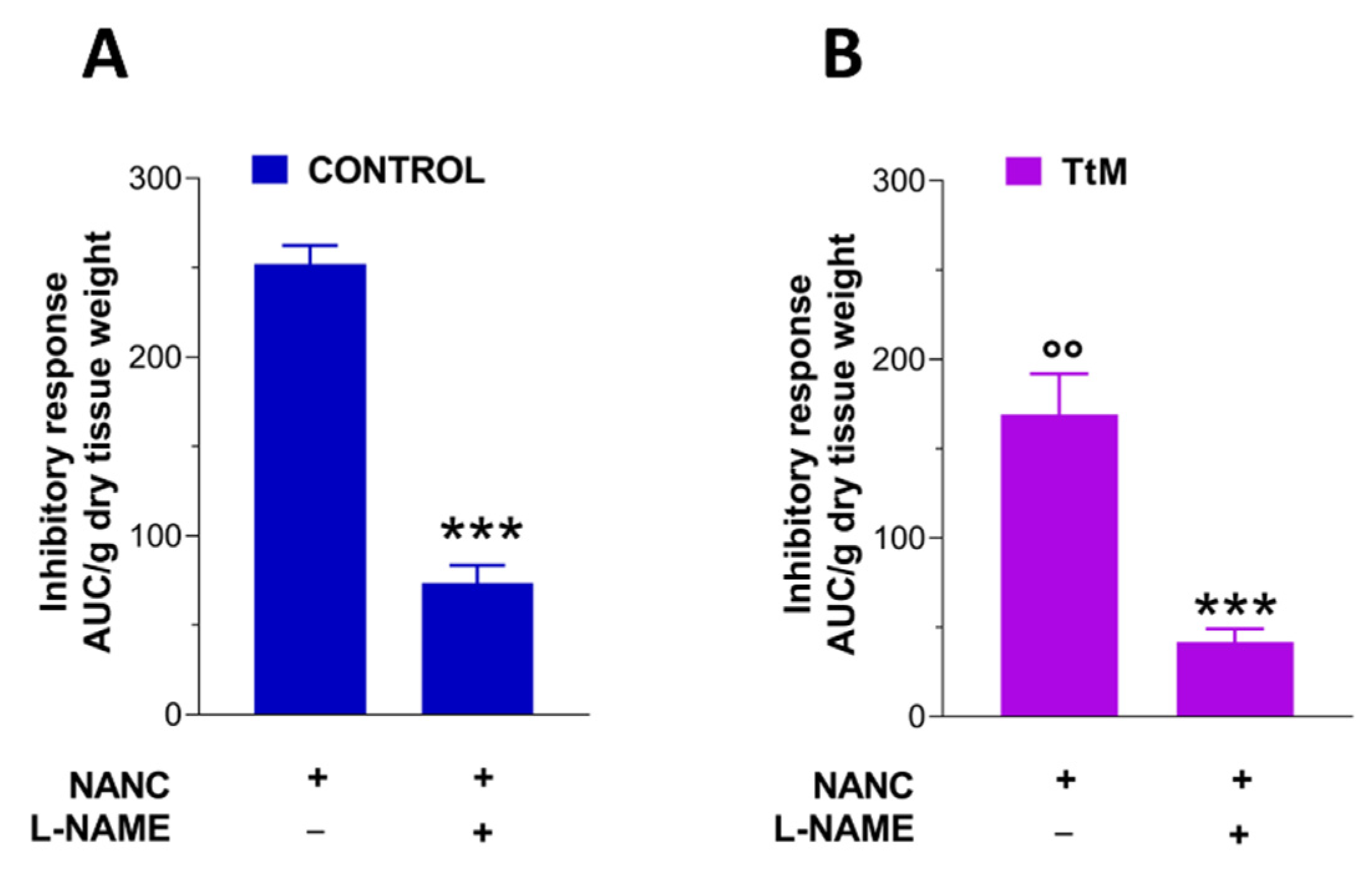
3.4. Tilia Tomentosa Induces Inhibitory Neuromuscular Responses of Isolated Small Intestine Preparations
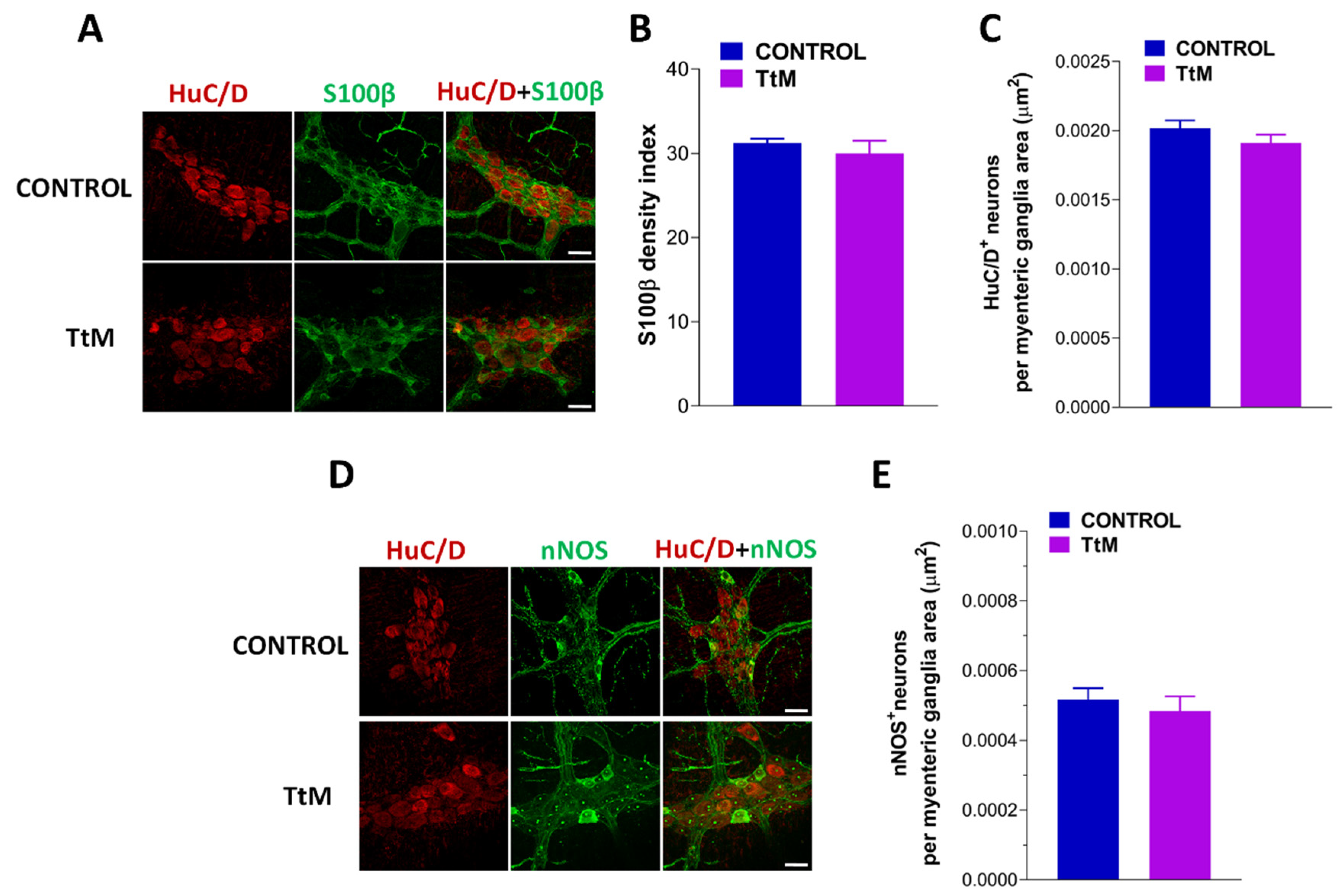
3.5. Tilia Tomentosa Does Not Affect Myenteric Plexus Structure of Isolated Small Intestine Preparations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef]
- Black, C.J.; Yuan, Y.; Selinger, C.P.; Camilleri, M.; Quigley, E.M.M.; Moayyedi, P.; Ford, A.C. Efficacy of soluble fibre, antispasmodic drugs, and gut-brain neuromodulators in irritable bowel syndrome: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 117–131. [Google Scholar] [CrossRef]
- Frezza, C.; De Vita, D.; Spinaci, G.; Sarandrea, M.; Venditti, A.; Bianco, A. Secondary metabolites of Tilia tomentosa Moench inflorescences collected in Central Italy: Chemotaxonomy relevance and phytochemical rationale of traditional use. Nat. Prod. Res. 2019, 34, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Hernández, E.; Martinez, A.L.; González-Trujano, M.; Moreno, J.; Vibrans, H.; Soto-Hernández, M. Pharmacological evaluation of the anxiolytic and sedative effects of Tilia americana L. var. mexicana in mice. J. Ethnopharmacol. 2007, 109, 140–145. [Google Scholar] [CrossRef]
- Sargin, S.A.; Akcicek, E.; Selvi, S. An ethnobotanical study of medicinal plants used by the local people of Alasehir (Manisa) in Turkey. J. Ethnopharmacol. 2013, 150, 860–874. [Google Scholar] [CrossRef]
- Turrini, F.; Vallarino, G.; Cisani, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Boggia, R.; Pittaluga, A.; Grilli, M. Use of an animal model to evaluate anxiolytic effects of dietary supplementation with Tilia tomentosa moench bud extracts. Nutrients 2020, 12, 3328. [Google Scholar] [CrossRef] [PubMed]
- Allio, A.; Calorio, C.; Franchino, C.; Gavello, D.; Carbone, E.; Marcantoni, A. Bud extracts from Tilia tomentosa Moench inhibit hippocampal neuronal firing through GABAA and benzodiazepine receptors activation. J. Ethnopharmacol. 2015, 172, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Rodriguez, N.; Gonzalez-Trujano, M.E.; Aguirre-Hernandez, E.; Ruiz-Garcia, M.; Sampieri, A.; Coballase-Urrutia, E.; Carmona-Aparicio, L. Anticonvulsant and antioxidant effects of Tilia americana var. mexicana and flavonoids constituents in the pentylenetetrazole-induced seizures. Oxid. Med. Cell. Longev. 2014, 2014, 329172. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, Y.N.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.H.; Vasicek, O.; Rise, F.; Denev, P.N.; Lojek, A.; Batsalova, T.G.; et al. Tilia tomentosa pectins exhibit dual mode of action on phagocytes as beta-glucuronic acid monomers are abundant in their rhamnogalacturonans I. Carbohydr. Polym. 2017, 175, 178–191. [Google Scholar] [CrossRef]
- Ieri, F.; Innocenti, M.; Possieri, L.; Gallori, S.; Mulinacci, N. Phenolic composition of “bud extracts” of Ribes nigrum L., Rosa canina L. and Tilia tomentosa M. J. Pharm. Biomed. Anal. 2015, 115, 1–9. [Google Scholar] [CrossRef]
- Viola, H.; Wolfman, C.; de Stein, M.; Wasowski, C.; Peña, C.; Medina, J.; Paladini, A. Isolation of pharmacologically active benzodiazepine receptor ligands from Tilia tomentosa (Tiliaceae). J. Ethnopharmacol. 1994, 44, 47–53. [Google Scholar] [CrossRef]
- Oteiza, P.; Fraga, C.; Mills, D.; Taft, D. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- érez-Cano, F.J.; Massot-Cladera, M.; Rodríguez-Lagunas, M.J.; Castell, M. Flavonoids affect host-microbiota crosstalk through TLR modulation. Antioxidants 2014, 3, 649–670. [Google Scholar] [CrossRef]
- Innocenti, G.; Piovan, A.; Filippini, R.; Caniato, R.; Cappelletti, E.M. Quantitative recovery of furanocoumarins from Psoralea bituminosa. Phytochem. Anal. 1997, 8, 84–86. [Google Scholar] [CrossRef]
- Cerantola, S.; Caputi, V.; Marsilio, I.; Ridolfi, M.; Faggin, S.; Bistoletti, M.; Giaroni, C.; Giron, M.C. Involvement of enteric glia in small intestine neuromuscular dysfunction of toll-like receptor 4-deficient mice. Cells 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Marsilio, I.; Caputi, V.; Latorre, E.; Cerantola, S.; Paquola, A.; Alcalde, A.I.; Mesonero, J.E.; O’Mahony, S.M.; Bertazzo, A.; Giaroni, C.; et al. Oxidized phospholipids affect small intestine neuromuscular transmission and serotonergic pathways in juvenile mice. Neurogastroenterol. Motil. 2020, 33, e14036. [Google Scholar] [CrossRef]
- Caputi, V.; Marsilio, I.; Filpa, V.; Cerantola, S.; Orso, G.; Bistoletti, M.; Paccagnella, N.; DE Martin, S.; Montopoli, M.; Dall’Acqua, S.; et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br. J. Pharmacol. 2017, 174, 3623–3639. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Giron, M.C.; Qesari, M.; Porzionato, A.; Caputi, V.; Zoppellaro, C.; Banzato, S.; Grillo, A.R.; Spagnol, L.; De Caro, R.; et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 2013, 145, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- D’Antongiovanni, V.; Benvenuti, L.; Fornai, M.; Pellegrini, C.; Wijngaard, R.V.D.; Cerantola, S.; Giron, M.C.; Caputi, V.; Colucci, R.; Haskó, G.; et al. Glial A2B adenosine receptors modulate abnormal tachykininergic responses and prevent enteric inflammation associated with high fat diet-induced obesity. Cells 2020, 9, 1245. [Google Scholar] [CrossRef] [PubMed]
- McClain, J.L.; Gulbransen, B.D. The acute inhibition of enteric glial metabolism with fluoroacetate alters calcium signaling, hemichannel function, and the expression of key proteins. J. Neurophysiol. 2017, 117, 365–375. [Google Scholar] [CrossRef]
- Antonioli, L.; D’Antongiovanni, V.; Pellegrini, C.; Fornai, M.; Benvenuti, L.; Di Carlo, A.; Wijngaard, R.V.D.; Caputi, V.; Cerantola, S.; Giron, M.C.; et al. Colonic dysmotility associated with high-fat diet-induced obesity: Role of enteric glia. FASEB J. 2020, 34, 5512–5524. [Google Scholar] [CrossRef] [PubMed]
- Caputi, V.; Marsilio, I.; Cerantola, S.; Roozfarakh, M.; Lante, I.; Galuppini, F.; Rugge, M.; Napoli, E.; Giulivi, C.; Orso, G.; et al. Toll-like receptor 4 modulates small intestine neuromuscular function through nitrergic and purinergic pathways. Front. Pharmacol. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, S.; Caputi, V.; Contarini, G.; Mereu, M.; Bertazzo, A.; Bosi, A.; Banfi, D.; Mantini, D.; Giaroni, C.; Giron, M. Dopamine transporter genetic reduction induces morpho-functional changes in the enteric nervous system. Biomedicines 2021, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Gallego, D.; Mañé, N.; Gil, V.; Martínez-Cutillas, M.; Jiménez, M. Mechanisms responsible for neuromuscular relaxation in the gastrointestinal tract. Rev. Esp. Enferm. Di. 2016, 108, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Knauf, C.; Abot, A.; Wemelle, E.; Cani, P.D. Targeting the enteric nervous system to treat metabolic disorders? “Enterosynes” as therapeutic gut factors. Neuroendocrinology 2019, 110, 139–146. [Google Scholar] [CrossRef]
- Delvalle, N.M.; Fried, D.E.; Rivera-Lopez, G.; Gaudette, L.; Gulbransen, B.D. Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am. J. Physiol. Liver Physiol. 2018, 315, G473–G483. [Google Scholar] [CrossRef]
- Holland, A.M.; Bon-Frauches, A.C.; Keszthelyi, D.; Melotte, V.; Boesmans, W. The enteric nervous system in gastrointestinal disease etiology. Cell. Mol. Life Sci. 2021, 78, 4713–4733. [Google Scholar] [CrossRef] [PubMed]
- Mendel, M.; Chłopecka, M.; Latek, U.; Karlik, W.; Tomczykowa, M.; Strawa, J.; Tomczyk, M. Evaluation of the effects of Bidens tripartita extracts and their main constituents on intestinal motility—An ex vivo study. J. Ethnopharmacol. 2020, 259, 112982. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Chakraborty, S.; Sletten, C.D. Common functional gastroenterological disorders associated with abdominal pain. Mayo Clin. Proc. 2016, 91, 1118–1132. [Google Scholar] [CrossRef]
- Mearin, F.; Malfertheiner, P. Functional gastrointestinal disorders: Complex treatments for complex pathophysiological mechanisms. Dig. Dis. 2017, 35, 1–4. [Google Scholar] [CrossRef]
- Knotek, K.; Verner, V.; Chaloupková, P.; Kokoska, L. Prevalence and use of herbal products in the Czech Republic: Over-the-counter survey among adult pharmacies clients. Complement. Ther. Med. 2012, 20, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Törnqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Öberg, M. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS ONE 2014, 9, e101638. [Google Scholar] [CrossRef]
- Mendel, M.; Chlopecka, M.; Dziekan, N.; Sobczak-Filipiak, M.; Bielecki, W.; Karlik, W. Bovine isolated abomasum specimens—a useful tool in preclinical in vitro studies. Res. Rev. J. Vet. Sci. 2016, 2, 76–83. [Google Scholar]
- Chłopecka, M.; Dziekan, N.; Mendel, M.; Bakała, A.; Małdyk, J.; Wiechetek, M. Evaluation of the time-stability of an alternative research model based on isolated rat gastrointestinal strips. J. Physiol. Pharmacol. Off J. Pol. Physiol. Soc. 2007, 58, 73–86. [Google Scholar]
- Kellow, J.E.; Bennett, E. Functional disorders of the small intestine. Semin. Gastrointest. Dis. 1996, 7, 208–216. [Google Scholar] [PubMed]
- Aguirre-Hernández, E.; González-Trujano, M.E.; Martinez, A.L.; Moreno, J.; Kite, G.; Terrazas, T.; Soto-Hernández, M. HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J. Ethnopharmacol. 2010, 127, 91–97. [Google Scholar] [CrossRef]
- Roudsari, N.M.; Lashgari, N.-A.; Momtaz, S.; Farzaei, M.H.; Marques, A.M.; Abdolghaffari, A.H. Natural polyphenols for the prevention of irritable bowel syndrome: Molecular mechanisms and targets; a comprehensive review. DARU J. Pharm. Sci. 2019, 27, 755–780. [Google Scholar] [CrossRef]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.M.; Fitzpatrick, L.R.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2021, 99, 108840. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in citrobacter rodentium-infected mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Zoppellaro, C.; Bin, A.; Brun, P.; Banzato, S.; Macchi, V.; Castagliuolo, I.; Giron, M.C. Adenosine-mediated enteric neuromuscular function is affected during herpes simplex virus type 1 infection of rat enteric nervous system. PLoS ONE 2013, 8, e72648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez, R.; Ventura-Martinez, R.; Santiago-Mejia, J.; Avila-Costa, M.R.; Fortoul, T. Altered responsiveness of the guinea-pig isolated ileum to smooth muscle stimulants and to electrical stimulation after in situ ischemia. Br. J. Pharmacol. 2006, 147, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Caputi, V.; Fornai, M.; Pellegrini, C.; Gentile, D.; Giron, M.C.; Orso, G.; Bernardini, N.; Segnani, C.; Ippolito, C.; et al. Interplay between colonic inflammation and tachykininergic pathways in the onset of colonic dysmotility in a mouse model of diet-induced obesity. Int. J. Obes. 2018, 43, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Gharzouli, K.; Holzer, P. Inhibition of Guinea pig intestinal peristalsis by the flavonoids quercetin, naringenin, apigenin and genistein. Pharmacology 2003, 70, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.C.; Giaroni, C.; Baj, A. Impact of microbial metabolites on microbiota–gut–brain axis in inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef]
- Antonioli, L.; Pellegrini, C.; Fornai, M.; Tirotta, E.; Gentile, D.; Benvenuti, L.; Giron, M.C.; Caputi, V.; Marsilio, I.; Orso, G.; et al. Colonic motor dysfunctions in a mouse model of high-fat diet-induced obesity: An involvement of A2B adenosine receptors. Purinergic Signal. 2017, 13, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Moon, E.; Cha, J.M.; Lee, S.; Yu, J.S.; Kim, C.S.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Antineuroinflammatory and antiproliferative activities of constituents from Tilia amurensis. Chem. Pharm. Bull. 2015, 63, 837–842. [Google Scholar] [CrossRef]
- Toker, G.; Küpeli, E.; Memisoglu, M.; Yesilada, E. Flavonoids with antinociceptive and anti-inflammatory activities from the leaves of Tilia argentea (silver linden). J. Ethnopharmacol. 2004, 95, 393–397. [Google Scholar] [CrossRef]
- Nigusse, T.; Zhang, L.; Wang, R.; Wang, X.N.; Li, J.; Liu, C. Flavonoids in a crude extract of Catha edulis inhibit rat intestinal contraction via blocking Ca (2+) channels. Neurogastroenterol. Motil 2019, 31, e13602. [Google Scholar] [CrossRef]
- Wani, S.A.; Iqbal, H.; Basir, S.F. Mechanism of flavonoids action in smooth muscle relaxation. WJPPS 2017, 6, 514–550. [Google Scholar]

| Antibody | Host Species | Dilution | Catalog Number | Source |
|---|---|---|---|---|
| Primary Antisera (Clone) | ||||
| HuC/D (16A11) | Mouse biotin-conjugated | 1:100 | A-21272 | Thermo Fisher Scientific (Monza, Italy) |
| nNOS (polyclonal) | Rabbit | 1:100 | 61-700 | Thermo Fisher Scientific |
| S100β (P50114) | Guinea pig | 1:100 | 287 00 | Synaptic Systems, (Göttingen, Germany) |
| Secondary Antisera | ||||
| Goat anti-rabbit IgG Alexa 488-conjugated | - | 1:1000 | A-11008 | Thermo Fisher Scientific |
| Goat anti-guinea pig IgG Alexa Fluor 488-conjugated | - | 1:1000 | AB_2534117 | Thermo Fisher Scientific |
| Streptavidin Alexa 555-conjugated | - | 1:1000 | S21381 | Thermo Fisher Scientific |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerantola, S.; Faggin, S.; Annaloro, G.; Mainente, F.; Filippini, R.; Savarino, E.V.; Piovan, A.; Zoccatelli, G.; Giron, M.C. Influence of Tilia tomentosa Moench Extract on Mouse Small Intestine Neuromuscular Contractility. Nutrients 2021, 13, 3505. https://doi.org/10.3390/nu13103505
Cerantola S, Faggin S, Annaloro G, Mainente F, Filippini R, Savarino EV, Piovan A, Zoccatelli G, Giron MC. Influence of Tilia tomentosa Moench Extract on Mouse Small Intestine Neuromuscular Contractility. Nutrients. 2021; 13(10):3505. https://doi.org/10.3390/nu13103505
Chicago/Turabian StyleCerantola, Silvia, Sofia Faggin, Gabriela Annaloro, Federica Mainente, Raffaella Filippini, Edoardo Vincenzo Savarino, Anna Piovan, Gianni Zoccatelli, and Maria Cecilia Giron. 2021. "Influence of Tilia tomentosa Moench Extract on Mouse Small Intestine Neuromuscular Contractility" Nutrients 13, no. 10: 3505. https://doi.org/10.3390/nu13103505
APA StyleCerantola, S., Faggin, S., Annaloro, G., Mainente, F., Filippini, R., Savarino, E. V., Piovan, A., Zoccatelli, G., & Giron, M. C. (2021). Influence of Tilia tomentosa Moench Extract on Mouse Small Intestine Neuromuscular Contractility. Nutrients, 13(10), 3505. https://doi.org/10.3390/nu13103505







