Carrot Consumption Frequency Associated with Reduced BMI and Obesity through the SNP Intermediary rs4445711
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Frequency of Vegetable Intake Measurement and Definition of BMI
2.3. DNA Sampling, Genotyping and Quality Control
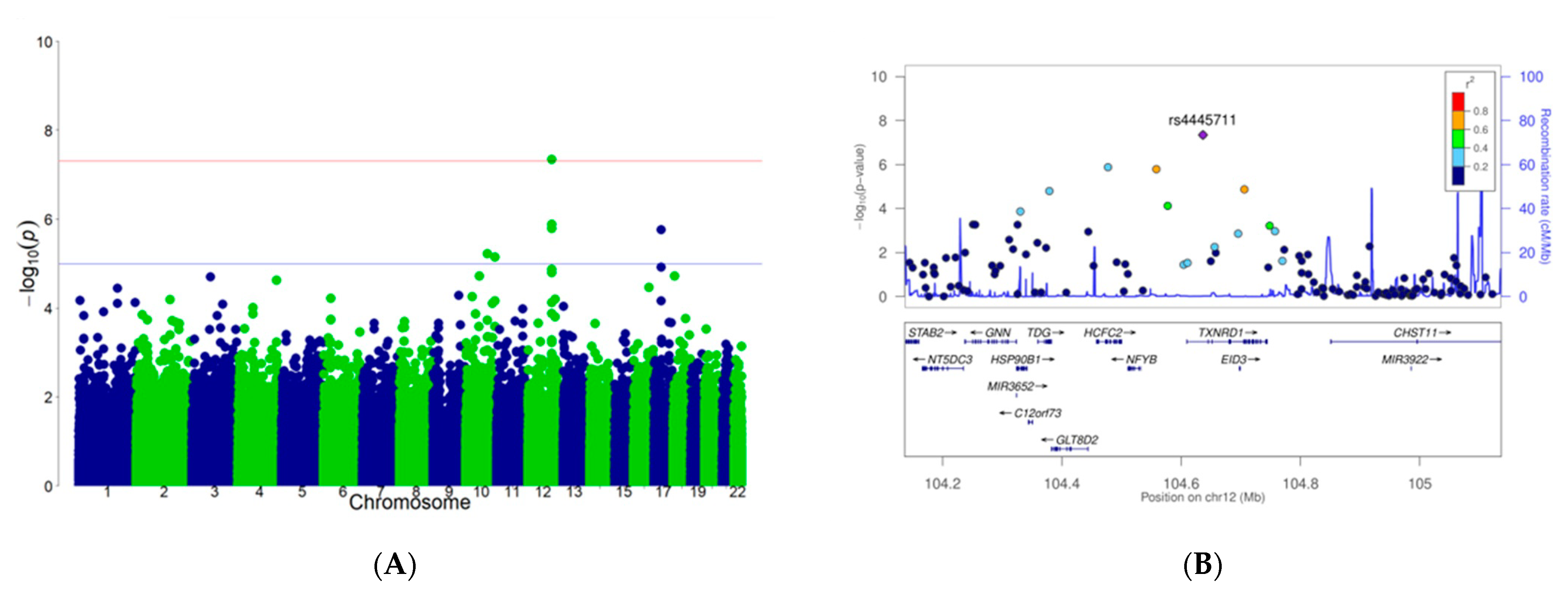
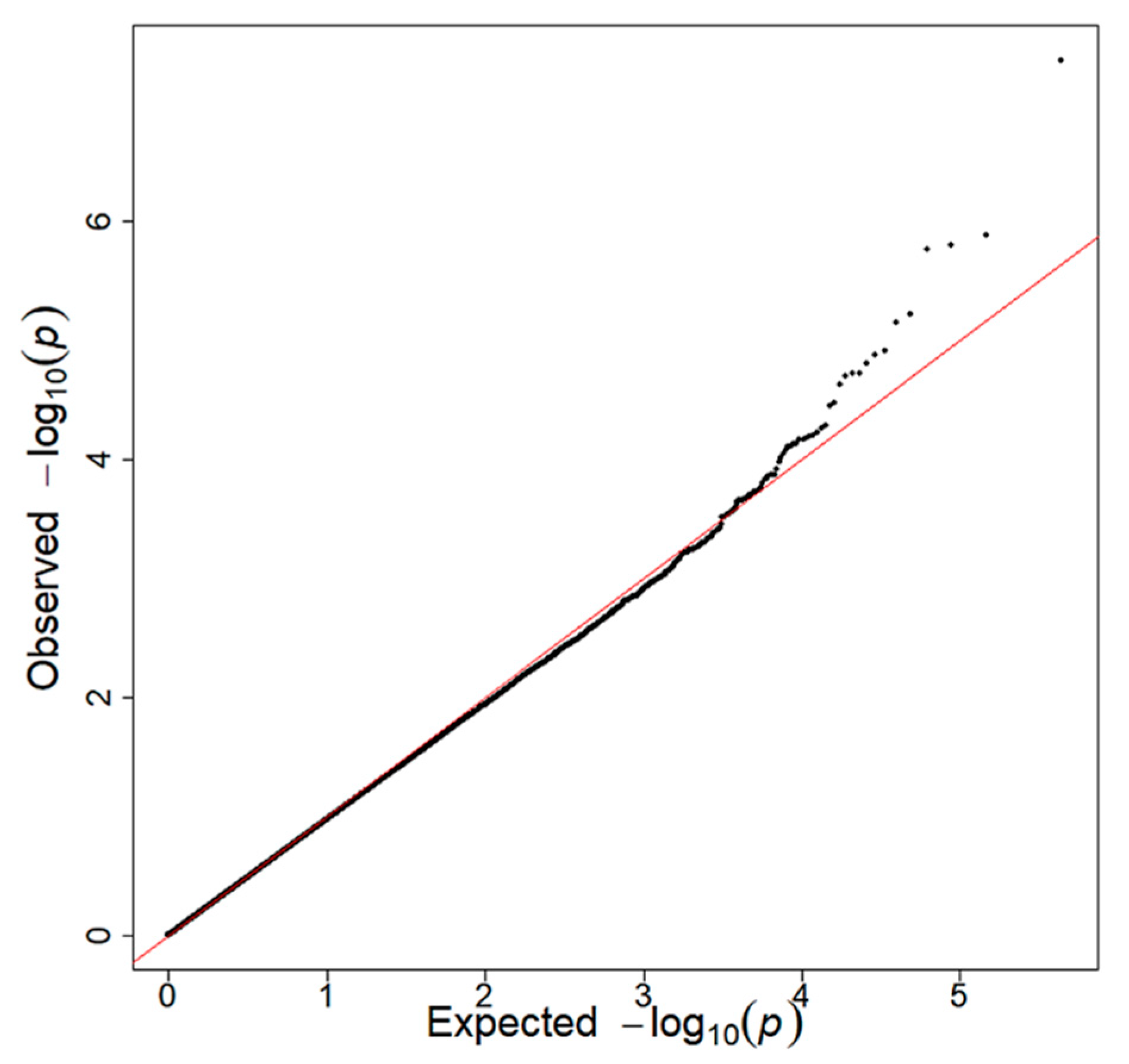
2.4. Genome-Wide Association Study (GWAS)
3. Results
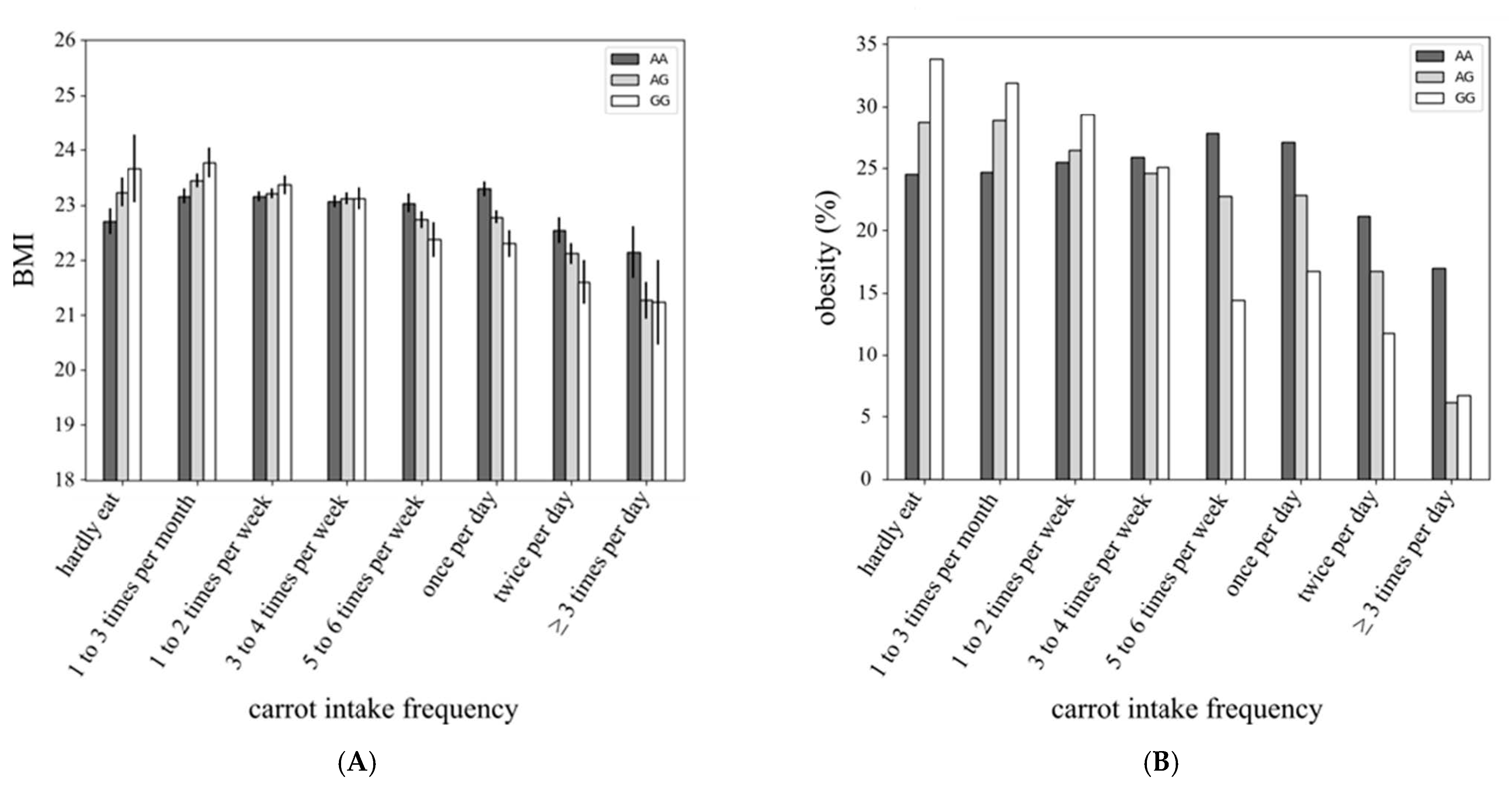
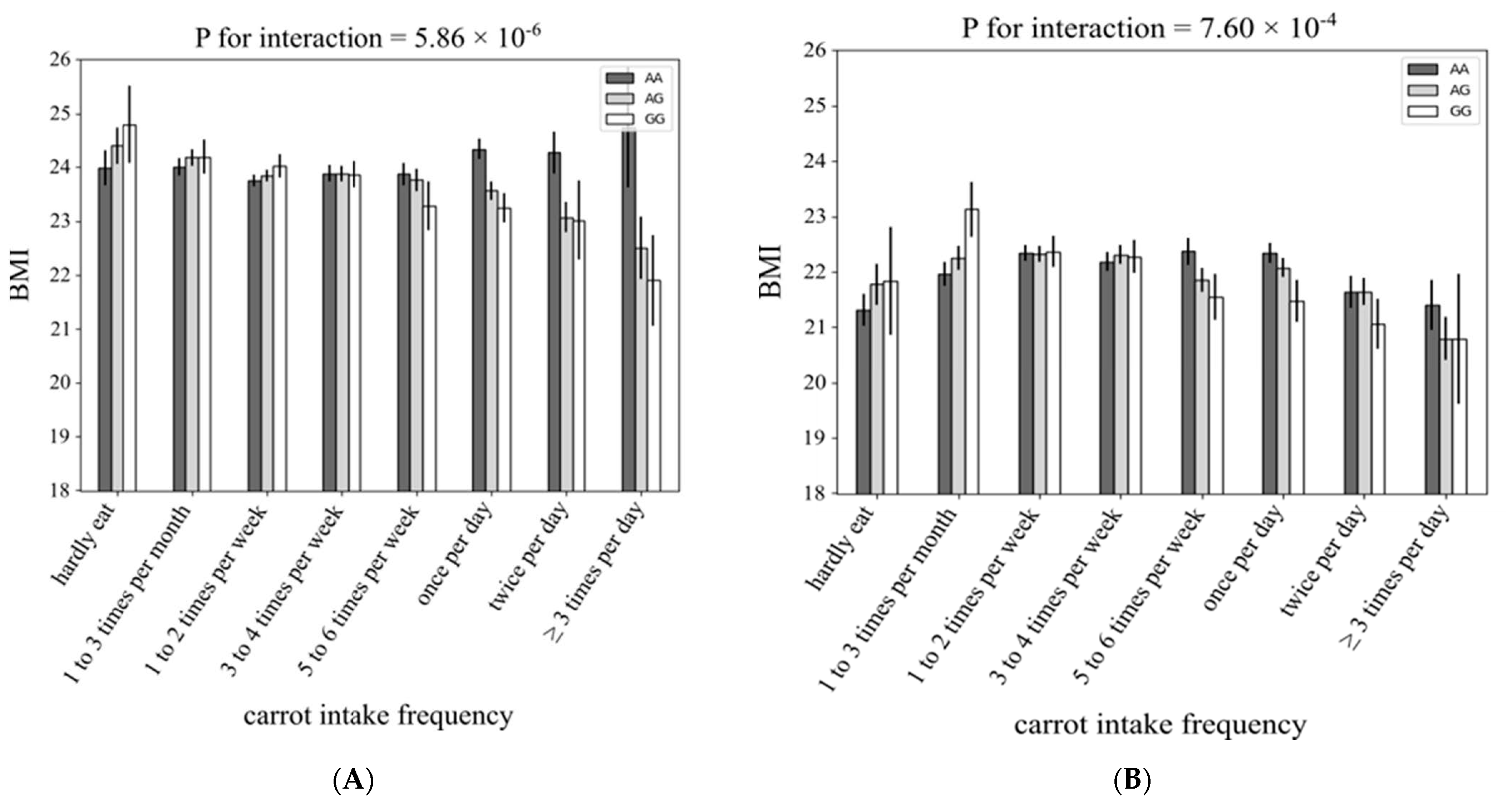
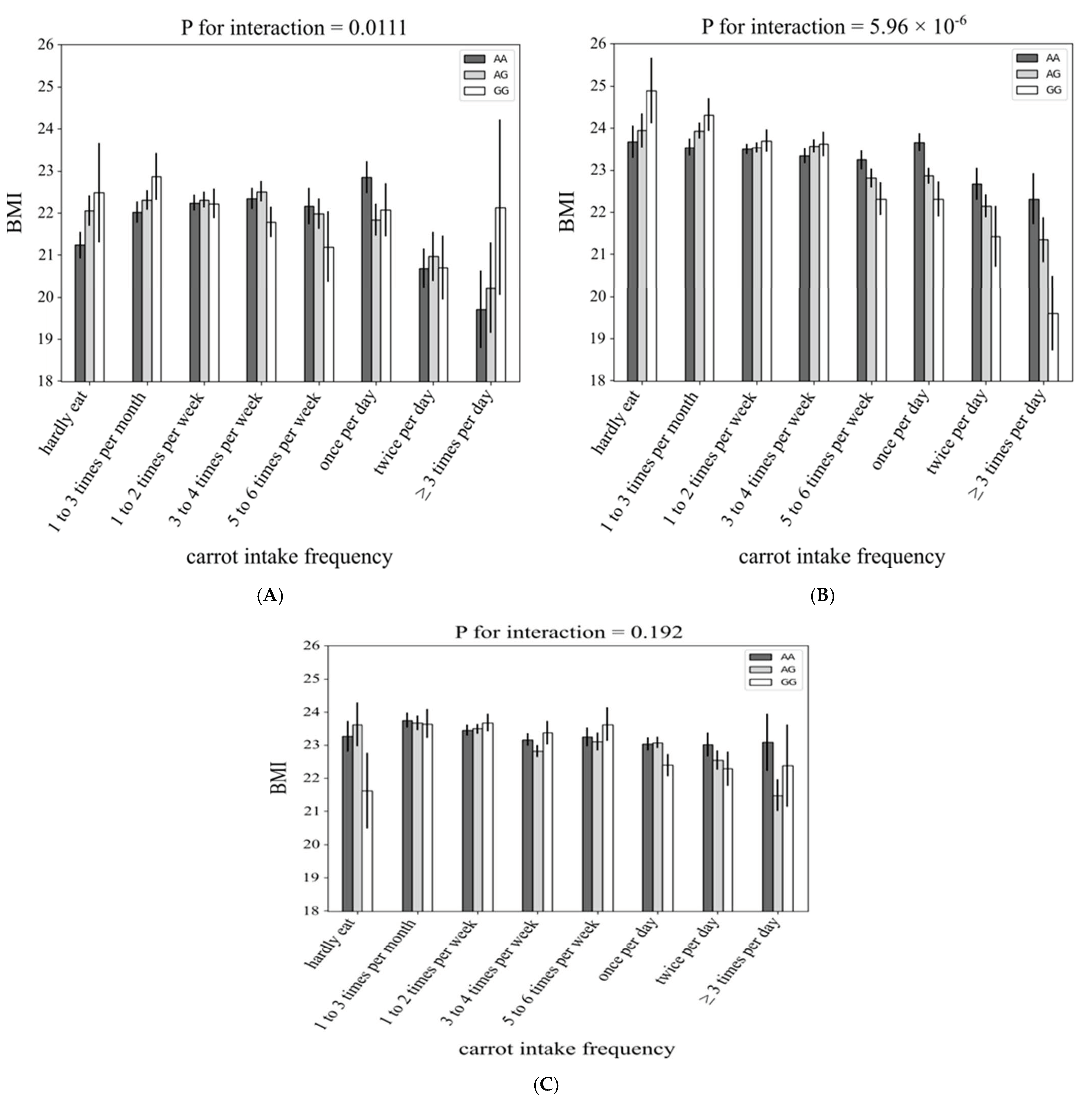
3.1. Interaction between rs4445711 and Frequency of Carrot Intake on BMI
3.2. Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
Informed Consent
Abbreviations
| BMI | body mass index |
| EID | E1A-like inhibitor of differentiation |
| GWAS | genome wide association study |
| SNPs | single nucleotide polymorphisms |
| TXNRD | thioredoxin reductase |
References
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- Katz, D.A.; McHorney, C.A.; Atkinson, R.L. Impact of obesity on health-related quality of life in patients with chronic illness. J. Gen. Intern. Med. 2000, 15, 789–796. [Google Scholar] [CrossRef]
- Taylor, V.H.; Forhan, M.; Vigod, S.; McIntyre, R.S.; Morrison, K. The impact of obesity on quality of life. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef]
- Nour, M.; Lutze, S.A.; Grech, A.; Allman-Farinelli, M. The Relationship between Vegetable Intake and Weight Outcomes: A Systematic Review of Cohort Studies. Nutrients 2018, 10, 1626. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Bertoia, M.L.; Mukamal, K.J.; Cahill, L.E.; Hou, T.; Ludwig, D.S.; Mozaffarian, D.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLoS Med. 2015, 12, e1001878. [Google Scholar] [CrossRef]
- Charlton, K.; Kowal, P.; Soriano, M.M.; Williams, S.; Banks, E.; Vo, K.; Byles, J. Fruit and Vegetable Intake and Body Mass Index in a Large Sample of Middle-Aged Australian Men and Women. Nutrients 2014, 6, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.R.; Stewart, A.W.; Hancox, R.J.; Murphy, R.; Braithwaite, I.; Beasley, R.; Mitchell, E.A.; ISAAC Phase Three Study Group. Association between Frequency of Consumption of Fruit, Vegetables, Nuts and Pulses and BMI: Analyses of the International Study of Asthma and Allergies in Childhood (ISAAC). Nutrients 2018, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Field, A.E.; Gillman, M.W.; Rosner, B.; Rockett, H.R.; Colditz, G. Association between fruit and vegetable intake and change in body mass index among a large sample of children and adolescents in the United States. Int. J. Obes. 2003, 27, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Wang, L.; Lee, I.M.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Higher Intake of Fruit, but Not Vegetables or Fiber, at Baseline Is Associated with Lower Risk of Becoming Overweight or Obese in Middle-Aged and Older Women of Normal BMI at Baseline. J. Nutr. 2015, 145, 960–968. [Google Scholar] [CrossRef]
- Yuan, S.; Yu, H.J.; Liu, M.W.; Huang, Y.; Yang, X.H.; Tang, B.W.; Song, Y.; Cao, Z.K.; Wu, H.J.; He, Q.Q.; et al. The association of fruit and vegetable consumption with changes in weight and body mass index in Chinese adults: A cohort study. Public Health 2018, 157, 121–126. [Google Scholar] [CrossRef]
- Matoba, N.; Akiyama, M.; Ishigaki, K.; Kanai, M.; Takahashi, A.; Momozawa, Y.; Ikegawa, S.; Ikeda, M.; Iwata, N.; Hirata, M.; et al. GWAS of 165,084 Japanese individuals identified nine loci associated with dietary habits. Nat. Hum. Behav. 2020, 4, 308–316. [Google Scholar] [CrossRef]
- Calancie, L.; Keyserling, T.C.; Taillie, L.S.; Robasky, K.; Patterson, C.; Ammerman, A.S.; Schisler, J.C. TAS2R38 Predisposition to Bitter Taste Associated with Differential Changes in Vegetable Intake in Response to a Community-Based Dietary Intervention. G3 Genes Genomes Genet. 2018, 8, 2107–2119. [Google Scholar] [CrossRef]
- Mikołajczyk-Stecyna, J.; Malinowska, A.; Chmurzynska, A. TAS2R38 and CA6 genetic polymorphisms, frequency of bitter food intake, and blood biomarkers among elderly woman. Appetite 2017, 116, 57–64. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Examination Committee of Criteria for ’Obesity Disease’ in Japan. New criteria for ‘obesity disease’ in Japan. Circ. J. 2002, 66, 987–992. [Google Scholar] [CrossRef]
- Yamaguchi-Kabata, Y.; Nakazono, K.; Takahashi, A.; Saito, S.; Hosono, N.; Kubo, M.; Nakamura, Y.; Kamatani, N. Japanese Population Structure, Based on SNP Genotypes from 7003 Individuals Compared to Other Ethnic Groups: Effects on Population-Based Association Studies. Am. J. Hum. Genet. 2008, 83, 445–456. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.D.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.D. qqman: An R package for visualizing GWAS results using QQ and manhattan plots. BioRxiv 2014, 1, 005165. [Google Scholar]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.; Willer, C.J. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Will, J.C.; Bowman, B.A.; Narayan, K.M.V. Diabetes Mellitus and Serum Carotenoids: Findings from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1999, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; Dobson, A.; McClintock, C.; Dunn, S.; Leonard, D.; Shaw, J. Diabetes mellitus and serum carotenoids: Findings of a population-based study in Queensland, Australia. Am. J. Clin. Nutr. 2005, 82, 685–693. [Google Scholar] [CrossRef]
- Zheng, J.S.; Sharp, S.J.; Imamura, F.; Chowdhury, R.; Gundersen, T.E.; Steur, M.; Sluijs, I.; van der Schouw, Y.T.; Agudo, A.; Aune, D.; et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ 2020, 370, m2194. [Google Scholar] [CrossRef] [PubMed]
- Albanes, D. Beta-carotene and lung cancer: A case study. Am. J. Clin. Nutr. 1999, 69, 1345S–1350S. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Serum Beta Carotene and Overall and Cause-Specific Mortality. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Erlinger, T.P.; Guallar, E.; Miller, E.R., 3rd; Stolzenberg-Solomon, R.; Appel, L.J. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch. Intern. Med. 2001, 161, 1903–1908. [Google Scholar] [CrossRef]
- Higuchi, K.; Saito, I.; Maruyama, K.; Eguchi, E.; Mori, H.; Tanno, S.; Sakurai, S.; Kishida, T.; Nishida, W.; Osawa, H.; et al. Associations of serum β-carotene and retinol concentrations with insulin resistance: The Toon Health Study. Nutrition 2015, 31, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Hirose, A.; Terauchi, M.; Hirano, M.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Higher intake of cryptoxanthin is related to low body mass index and body fat in Japanese middle-aged women. Maturitas 2016, 96, 89–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andersen, L.F.; Jacobs, D.R.; Gross, M.D.; Schreiner, P.J.; Williams, O.D.; Lee, D.-H. Longitudinal associations between body mass index and serum carotenoids: The CARDIA study. Br. J. Nutr. 2006, 95, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Iwata, A.; Matsubara, S.; Miyazaki, K. Beneficial effects of a beta-cryptoxanthin-containing beverage on body mass index and visceral fat in pre-obese men: Double-blind, placebo-controlled parallel trials. J. Funct. Foods 2018, 41, 250–257. [Google Scholar] [CrossRef]
- Crosley, L.; Méplan, C.; Nicol, F.; Rundlöf, A.; Arnér, E.; Hesketh, J.; Arthur, J. Differential regulation of expression of cytosolic and mitochondrial thioredoxin reductase in rat liver and kidney. Arch. Biochem. Biophys. 2007, 459, 178–188. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Comuzzie, A.G.; Cole, S.A.; Laston, S.L.; Voruganti, V.S.; Haack, K.; Gibbs, R.A.; Butte, N.F. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PLoS ONE 2012, 7, e51954. [Google Scholar] [CrossRef]
- Rundlöf, A.-K.; Arnér, E. Regulation of the Mammalian Selenoprotein Thioredoxin Reductase 1 in Relation to Cellular Phenotype, Growth, and Signaling Events. Antioxid. Redox Signal. 2004, 6, 41–52. [Google Scholar] [CrossRef]
- Peng, X.; Giménez-Cassina, A.; Petrus, P.; Conrad, M.; Rydén, M.; Arnér, E.S. Thioredoxin reductase 1 suppresses adipocyte differentiation and insulin responsiveness. Sci. Rep. 2016, 6, 28080. [Google Scholar] [CrossRef] [PubMed]
- GTEx Portal. Available online: https://gtexportal.org/home/faq#citePortal (accessed on 1 August 2021).
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Pursey, K.; Burrows, T.L.; Stanwell, P.; Collins, C.E. How accurate is web-based self-reported height, weight, and body mass index in young adults? J. Med. Internet Res. 2014, 16, e4. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Men | Women |
|---|---|---|
| Number of participants (n) | 6495 | 5730 |
| Age (year) | 51 ± 13 | 50 ± 13 |
| Body mass index (kg/m2) | 23.9 ± 3.4 | 21.1 ± 3.8 |
| Obesity (n, %) | 2097 (33) | 994 (17) |
| Carrot (n, %) | ||
| hardly eat | 321 (5) | 267 (5) |
| 1 to 3 times per month | 1204 (19) | 790 (14) |
| 1 to 2 times per week | 2216 (34) | 1569 (27) |
| 3 to 4 times per week | 1257 (19) | 1172 (20) |
| 5 to 6 times per week | 500 (8) | 602 (11) |
| once per day | 777 (12) | 858 (15) |
| twice per day | 183 (3) | 370 (6) |
| ≥3 times per day | 37 (1) | 102 (2) |
| Broccoli (n, %) | ||
| hardly eat | 1140 (18) | 761 (13) |
| 1 to 3 times per month | 2491 (38) | 2295 (40) |
| 1 to 2 times per week | 1992 (31) | 1777 (31) |
| 3 to 4 times per week | 556 (9) | 565 (10) |
| 5 to 6 times per week | 153 (2) | 160 (3) |
| once per day | 147 (2) | 147 (3) |
| twice per day | 11 (0) | 18 (0) |
| ≥3 times per day | 5 (0) | 7 (0) |
| Spinach (n, %) | ||
| hardly eat | 321 (5) | 267 (5) |
| 1 to 3 times per month | 1204 (19) | 790 (14) |
| 1 to 2 times per week | 2216 (34) | 1569 (27) |
| 3 to 4 times per week | 1257 (19) | 1172 (20) |
| 5 to 6 times per week | 500 (8) | 602 (11) |
| once per day | 777 (12) | 858 (35) |
| twice per day | 183 (3) | 370 (6) |
| ≥3 times per day | 37 (1) | 102 (2) |
| Other green vegetables (green pepper and green bean) (n, %) | ||
| hardly eat | 629 (10) | 368 (6) |
| 1 to 3 times per month | 2118 (33) | 1542 (27) |
| 1 to 2 times per week | 2555 (39) | 2273 (40) |
| 3 to 4 times per week | 807 (12) | 982 (17) |
| 5 to 6 times per week | 192 (3) | 275 (5) |
| once per day | 164 (3) | 246 (4) |
| twice per day | 24 (0) | 34 (1) |
| ≥3 times per day | 6 (0) | 10 (0) |
| Pumpkin (n, %) | ||
| hardly eat | 1405 (22) | 1020 (18) |
| 1 to 3 times per month | 2809 (43) | 2587 (45) |
| 1 to 2 times per week | 1703 (26) | 1454 (25) |
| 3 to 4 times per week | 349 (5) | 388 (7) |
| 5 to 6 times per week | 108 (2) | 124 (2) |
| once per day | 100 (2) | 129 (2) |
| twice per day | 18 (0) | 22 (0) |
| ≥3 times per day | 3 (0) | 6 (0) |
| Cabbage (n, %) | ||
| hardly eat | 148 (2) | 118 (2) |
| 1 to 3 times per month | 1037 (16) | 976 (17) |
| 1 to 2 times per week | 2733 (42) | 2272 (40) |
| 3 to 4 times per week | 1640 (25) | 1441 (25) |
| 5 to 6 times per week | 446 (7) | 483 (8) |
| once per day | 419 (6) | 356 (6) |
| twice per day | 57 (1) | 68 (1) |
| ≥3 times per day | 15 (0) | 16 (0) |
| CHR | SNP | Position | EA | NEA | BETA | SE | p |
|---|---|---|---|---|---|---|---|
| Carrot | |||||||
| 12 | rs4445711 | 104636601 | G | A | −0.1682 | 0.03073 | 4.53 × 10−5 |
| 17 | rs223154 | 29928083 | T | G | −0.1445 | 0.0302 | 1.73 × 10−6 |
| 10 | rs4919491 | 95515515 | G | A | 0.2113 | 0.0467 | 6.09 × 10−6 |
| 10 | rs2483855 | 128933992 | A | G | 0.1761 | 0.03924 | 7.27 × 10−6 |
| Broccoli | |||||||
| 4 | rs993775 | 94731641 | G | T | −0.1944 | 0.04095 | 2.07 × 10−6 |
| 5 | rs13185886 | 79108476 | C | T | 0.216 | 0.04792 | 6.63 × 10−6 |
| Spinach | |||||||
| None | |||||||
| Other green vegetables (green pepper and green bean) | |||||||
| 2 | rs11692441 | 156950640 | G | T | 0.2319 | 0.05183 | 7.73 × 10−6 |
| 2 | rs13429725 | 147292648 | G | A | 0.186 | 0.04198 | 9.44 × 10−6 |
| Pumpkin | |||||||
| 3 | rs902192 | 193111865 | A | G | −0.3404 | 0.07406 | 4.34 × 10−6 |
| 16 | rs9932117 | 54906895 | C | A | 0.2347 | 0.05141 | 5.01 × 10−6 |
| Cabbage | |||||||
| 3 | rs12490455 | 176910577 | T | C | −0.2017 | 0.04464 | 6.32 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujihara, K.; Nogawa, S.; Saito, K.; Horikawa, C.; Takeda, Y.; Cho, K.; Ishiguro, H.; Kodama, S.; Nakagawa, Y.; Matsuzaka, T.; et al. Carrot Consumption Frequency Associated with Reduced BMI and Obesity through the SNP Intermediary rs4445711. Nutrients 2021, 13, 3478. https://doi.org/10.3390/nu13103478
Fujihara K, Nogawa S, Saito K, Horikawa C, Takeda Y, Cho K, Ishiguro H, Kodama S, Nakagawa Y, Matsuzaka T, et al. Carrot Consumption Frequency Associated with Reduced BMI and Obesity through the SNP Intermediary rs4445711. Nutrients. 2021; 13(10):3478. https://doi.org/10.3390/nu13103478
Chicago/Turabian StyleFujihara, Kazuya, Shun Nogawa, Kenji Saito, Chika Horikawa, Yasunaga Takeda, Kaori Cho, Hajime Ishiguro, Satoru Kodama, Yoshimi Nakagawa, Takashi Matsuzaka, and et al. 2021. "Carrot Consumption Frequency Associated with Reduced BMI and Obesity through the SNP Intermediary rs4445711" Nutrients 13, no. 10: 3478. https://doi.org/10.3390/nu13103478
APA StyleFujihara, K., Nogawa, S., Saito, K., Horikawa, C., Takeda, Y., Cho, K., Ishiguro, H., Kodama, S., Nakagawa, Y., Matsuzaka, T., Shimano, H., & Sone, H. (2021). Carrot Consumption Frequency Associated with Reduced BMI and Obesity through the SNP Intermediary rs4445711. Nutrients, 13(10), 3478. https://doi.org/10.3390/nu13103478








