Relationship between Dietary Fatty Acid Intake with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in People with HIV
Abstract
:1. Introduction
2. Materials and Methods
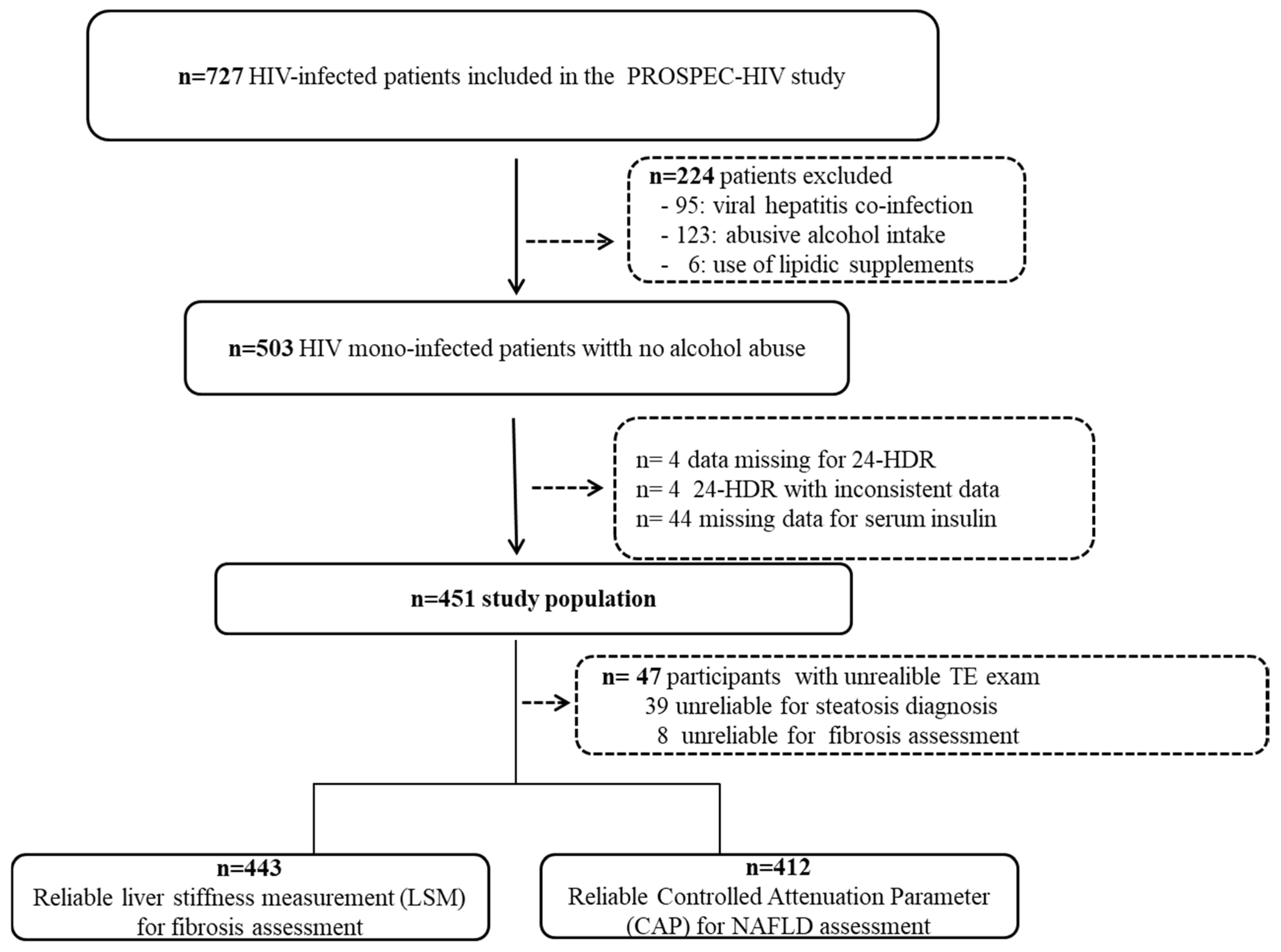
2.1. Study Design and Participants
2.2. Clinical Assessment and HIV Infection History
2.3. Laboratory Tests and Transient Elastography
2.4. Dietary Data
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Relationship between Dietary Intake and NAFLD or Liver Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- UNAIDS 2020 Global AIDS Update. Available online: https://www.unaids.org/en/resources/documents/2020/global-aids-report (accessed on 14 November 2020).
- Grinsztejn, B.; Luz, P.M.; Pacheco, A.G.; Santos, D.V.G.; Velasque, L.; Moreira, R.I.; Guimarães, M.R.C.; Nunes, E.P.; Lemos, A.S.; Ribeiro, S.R.; et al. Changing Mortality Profile among HIV-Infected Patients in Rio de Janeiro, Brazil: Shifting from AIDS to Non-AIDS Related Conditions in the HAART Era. PLoS ONE 2013, 8, e59768. [Google Scholar] [CrossRef] [PubMed]
- Castilho, J.L.; Escuder, M.M.; Veloso, V.; Gomes, J.O.; Jayathilake, K.; Ribeiro, S.; Souza, R.A.; Ikeda, M.L.; de Alencastro, P.R.; Tupinanbas, U.; et al. Trends and Predictors of Non-communicable Disease Multimorbidity among Adults Living with HIV and Receiving Antiretroviral Therapy in Brazil. J. Int. AIDS Soc. 2019, 22, e25233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased Risk of Mortality by Fibrosis Stage in Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Hepatology 2017, 65, 1557. [Google Scholar] [CrossRef] [PubMed]
- Vuille-Lessard, É.; Lebouché, B.; Lennox, L.; Routy, J.; Costiniuk, C.T.; Pexos, C.; Giannakis, A.; Szabo, J.; Klein, M.B.; Sebastiani, G. Nonalcoholic Fatty Liver Disease Diagnosed by Transient Elastography with Controlled Attenuation Parameter in Unselected HIV Monoinfected Patients. Available online: https://pubmed.ncbi.nlm.nih.gov/27603289/ (accessed on 7 December 2020).
- Pembroke, T.; Deschenes, M.; Lebouché, B.; Benmassaoud, A.; Sewitch, M.; Ghali, P.; Wong, P.; Halme, A.; Vuille-Lessard, E.; Pexos, C.; et al. Hepatic Steatosis Progresses Faster in HIV Mono-Infected than HIV/HCV Co-Infected Patients and Is Associated with Liver Fibrosis. J. Hepatol. 2017, 67, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Aepfelbacher, J.A.; Balmaceda, J.; Purdy, J.; Mattingly, A.; Zambell, K.; Hawkins, K.; Chairez, C.; Curl, K.A.; Dee, N.; Hadigan, C. Increased Prevalence of Hepatic Steatosis in Young Adults With Lifelong HIV. J. Infect. Dis. 2019, 220, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Phillips, C.; Shivappa, N.; Hébert, J.; Perry, I.; Phillips, C.M.; Shivappa, N.; Hébert, J.R.; Perry, I.J. Dietary Inflammatory Index and Biomarkers of Lipoprotein Metabolism, Inflammation and Glucose Homeostasis in Adults. Nutrients 2018, 10, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantero, I.; Abete, I.; Babio, N.; Arós, F.; Corella, D.; Estruch, R.; Fitó, M.; Hebert, J.R.; Martínez-González, M.Á.; Pintó, X.; et al. Dietary Inflammatory Index and Liver Status in Subjects with Different Adiposity Levels within the PREDIMED Trial. Clin. Nutr. 2018, 37, 1736–1743. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean Diet Improves Hepatic Steatosis and Insulin Sensitivity in Individuals with Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.A.; Hodson, L. Influence of Dietary Macronutrients on Liver Fat Accumulation and Metabolism. J. Investig. Med. 2017, 65, 1102–1115. [Google Scholar] [CrossRef]
- Vergani, L. Fatty Acids and Effects on In Vitro and In Vivo Models of Liver Steatosis. Curr. Med. Chem. 2019, 26, 3439–3456. [Google Scholar] [CrossRef]
- Jurado-Ruiz, E.; Varela, L.M.; Luque, A.; Berná, G.; Cahuana, G.; Martinez-Force, E.; Gallego-Durán, R.; Soria, B.; de Roos, B.; Romero Gómez, M.; et al. An Extra Virgin Olive Oil Rich Diet Intervention Ameliorates the Nonalcoholic Steatohepatitis Induced by a High-Fat “Western-Type” Diet in Mice. Mol. Nutr. Food Res. 2017, 61, 1600549. [Google Scholar] [CrossRef]
- Ferolla, S.; Ferrari, T.; Lima, M.; Reis, T.; Tavares, W., Jr.; Couto, O.; Vidigal, P.; Fausto, M.; Couto, C. Dietary Patterns in Brazilian Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. Clinics 2013, 68, 11–17. [Google Scholar] [CrossRef]
- Deresz, L.F.; de Brito, C.; Schneider, C.D.; Rabito, E.I.; Ikeda, M.L.R.; Lago, P.D. Dietary Intake and Cardiovascular Risk among People Living with HIV/AIDS. Ciênc. Amp Saúde Coletiva 2018, 23, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, H.; Cardoso, S.W.; Yanavich, C.; Nunes, E.P.; Morata, M.; Gorni, N.; da Silva, P.S.; Cardoso, C.; Almeida, C.; Luz, P.; et al. Predictive Factors Associated with Liver Fibrosis and Steatosis by Transient Elastography in Patients with HIV Mono-Infection under Long-Term Combined Antiretroviral Therapy. J. Int. AIDS Soc. 2018, 21, e25201. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; De La Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef]
- Travassos, C.; Laguardia, J.; Marques, P.M.; Mota, J.C.; Szwarcwald, C.L. Comparison between Two Race/Skin Color Classifications in Relation to Health-Related Outcomes in Brazil. Int. J. Equity Health 2011, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome—A New World-wide Definition. A Consensus Statement from the International Diabetes Federation. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1464-5491.2006.01858.x (accessed on 18 August 2019).
- WHO. Obesity: Preventing and Managing the Global Epidemic. Available online: http://www.who.int/entity/nutrition/publications/obesity/WHO_TRS_894/en/index.html (accessed on 18 May 2018).
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. Edinb. Scotl. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Boyko, E.J.; Jensen, C.C. Do We Know What Homeostasis Model Assessment Measures?: If Not, Does It Matter? Diabetes Care 2007, 30, 2725–2728. [Google Scholar] [CrossRef] [Green Version]
- Boursier, J.; Vergniol, J.; Guillet, A.; Hiriart, J.-B.; Lannes, A.; Bail, B.L.; Michalak, S.; Chermak, F.; Bertrais, S.; Foucher, J.; et al. Diagnostic Accuracy and Prognostic Significance of Blood Fibrosis Tests and Liver Stiffness Measurement by FibroScan in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 65, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Vergniol, J.; Wong, G.L.-H.; Foucher, J.; Chan, A.W.-H.; Chermak, F.; Choi, P.C.-L.; Merrouche, W.; Chu, S.H.-T.; Pesque, S.; et al. Liver Stiffness Measurement Using XL Probe in Patients with Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2012, 107, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Kirkpatrick, S.I.; Subar, A.F.; Reedy, J.; Schap, T.E.; Wilson, M.M.; Krebs-Smith, S.M. The National Cancer Institute’s Dietary Assessment Primer: A Resource for Diet Research. J. Acad. Nutr. Diet. 2015, 115, 1986–1995. [Google Scholar] [CrossRef] [Green Version]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method Reduces Bias in the Collection of Energy Intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Verly, E., Jr.; Oliveira, D.C.R.S.; Fisberg, R.M.; Marchioni, D.M.L. Performance of Statistical Methods to Correct Food Intake Distribution: Comparison between Observed and Estimated Usual Intake. Br. J. Nutr. 2016, 116, 897–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, L.S.; Schatzkin, A.; Midthune, D.; Kipnis, V. Dealing with Dietary Measurement Error in Nutritional Cohort Studies. J. Natl. Cancer Inst. 2011, 103, 1086–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilar, L.; Oliveira, C.P.M.S.; Faintuch, J.; Mello, E.S.; Nogueira, M.A.; Santos, T.E.; Alves, V.A.F.; Carrilho, F.J. High-Fat Diet: A Trigger of Non-Alcoholic Steatohepatitis? Preliminary Findings in Obese Subjects. Nutrition 2008, 24, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Campo, L.; Eiseler, S.; Apfel, T.; Pyrsopoulos, N. Fatty Liver Disease and Gut Microbiota: A Comprehensive Update. J. Clin. Transl. Hepatol. 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, Z.; Gibson, D.L.; Hekmatdoost, A. Nonalcoholic Fatty Liver Disease, the Gut Microbiome, and Diet. Adv. Nutr. 2017, 8, 240. [Google Scholar] [CrossRef]
- Shim, P.; Choi, D.; Park, Y. Association of Blood Fatty Acid Composition and Dietary Pattern with the Risk of Non-Alcoholic Fatty Liver Disease in Patients Who Underwent Cholecystectomy. Ann. Nutr. Metab. 2017, 70, 303–311. [Google Scholar] [CrossRef]
- Rietman, A.; Sluik, D.; Feskens, E.J.M.; Kok, F.J.; Mensink, M. Associations between Dietary Factors and Markers of NAFLD in a General Dutch Adult Population. Eur. J. Clin. Nutr. 2018, 72, 117–123. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Review: Lauric Acid-Rich Medium-Chain Triglycerides Can Substitute for Other Oils in Cooking Applications and May Have Limited Pathogenicity. Open Heart 2016, 3, e000467. [Google Scholar] [CrossRef] [Green Version]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.A.; Desouza, C. Lauric Acid versus Palmitic Acid: Effects on Adipose Tissue Inflammation, Insulin Resistance, and Non-Alcoholic Fatty Liver Disease in Obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef]
- Yoo, H.J.; Jung, K.J.; Kim, M.; Kim, M.; Kang, M.; Jee, S.H.; Choi, Y.; Lee, J.H. Liver Cirrhosis Patients Who Had Normal Liver Function Before Liver Cirrhosis Development Have the Altered Metabolic Profiles Before the Disease Occurrence Compared to Healthy Controls. Front. Physiol. 2019, 10, 1421. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Arab, A.; Paknahad, Z. The Effect of a Mediterranean Diet on Metabolic Parameters in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review of Randomized Controlled Trials. Clin. Nutr. ESPEN 2020, 35, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean Dietary Pattern as the Diet of Choice for NAFLD.; Evidence and Plausible Mechanisms. Liver Int. Off. J. Int. Assoc. Study Liver 2017, 37, 936–949. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic Acid Protects Saturated Fatty Acid Mediated Lipotoxicity in Hepatocytes and Rat of Non-Alcoholic Steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Errazuriz, I.; Dube, S.; Slama, M.; Visentin, R.; Nayar, S.; O’Connor, H.; Cobelli, C.; Das, S.K.; Basu, A.; Kremers, W.K.; et al. Randomized Controlled Trial of a MUFA or Fiber-Rich Diet on Hepatic Fat in Prediabetes. J. Clin. Endocrinol. Metab. 2017, 102, 1765–1774. [Google Scholar] [CrossRef] [Green Version]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef]
- Cui, J.; Li, L.; Ren, L.; Sun, J.; Zhao, H.; Sun, Y. Dietary N-3 and n-6 Fatty Acid Intakes and NAFLD: A Cross-Sectional Study in the United States. Asia Pac. J. Clin. Nutr. 2021, 30, 87–98. [Google Scholar] [CrossRef]
- Juárez-Hernández, E.; Chávez-Tapia, N.C.; Uribe, M.; Barbero-Becerra, V.J. Role of Bioactive Fatty Acids in Nonalcoholic Fatty Liver Disease. Nutr. J. 2016, 15, 72. [Google Scholar] [CrossRef] [Green Version]
- Machado, M.V.; Cortez-Pinto, H. Non-Alcoholic Fatty Liver Disease: What the Clinician Needs to Know. World J. Gastroenterol. WJG 2014, 20, 12956–12980. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and Sugar: A Major Mediator of Nonalcoholic Fatty Liver Disease. J. Hepatol. 2018, 68, 1063. [Google Scholar] [CrossRef] [Green Version]
- Zolfaghari, H.; Askari, G.; Siassi, F.; Feizi, A.; Sotoudeh, G. Intake of Nutrients, Fiber, and Sugar in Patients with Nonalcoholic Fatty Liver Disease in Comparison to Healthy Individuals. Int. J. Prev. Med. 2016, 7, 98. [Google Scholar] [CrossRef]
- Souza, A.d.M.; Pereira, R.A.; Yokoo, E.M.; Levy, R.B.; Sichieri, R. Alimentos mais consumidos no Brasil: Inquérito Nacional de Alimentação 2008–2009. Rev. Saúde Pública 2013, 47, 190s–199s. [Google Scholar] [CrossRef]
- Krawczyk, M.; Maciejewska, D.; Ryterska, K.; Czerwińka-Rogowska, M.; Jamioł-Milc, D.; Skonieczna-Żydecka, K.; Milkiewicz, P.; Raszeja-Wyszomirska, J.; Stachowska, E. Gut Permeability Might Be Improved by Dietary Fiber in Individuals with Nonalcoholic Fatty Liver Disease (NAFLD) Undergoing Weight Reduction. Nutrients 2018, 10, 1793. [Google Scholar] [CrossRef] [Green Version]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C.; et al. Individual Patient Data Meta-Analysis of Controlled Attenuation Parameter (CAP) Technology for Assessing Steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Herkner, H.; Sporea, I.; Salzl, P.; Sirli, R.; Neghina, A.M.; Peck-Radosavljevic, M. Meta-analysis: ARFI Elastography versus Transient Elastography for the Evaluation of Liver Fibrosis. Liver Int. 2013, 33, 1138–1147. [Google Scholar] [CrossRef]
- Myers Robert, P.; Aaron, P.; Richard, K.; Gilles, P.; Melanie, B.; Mark, L.; Andres, D.; David, W.; Pam, C.; Magdy, E. Controlled Attenuation Parameter (CAP): A Noninvasive Method for the Detection of Hepatic Steatosis Based on Transient Elastography. Liver Int. 2012, 32, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Dodd, K.W.; Kipnis, V.; Thompson, F.E.; Potischman, N.; Schoeller, D.A.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H.; et al. Comparison of Self-Reported Dietary Intakes from the Automated Self-Administered 24-h Recall, 4-d Food Records, and Food-Frequency Questionnaires against Recovery Biomarkers. Am. J. Clin. Nutr. 2018, 107, 80. [Google Scholar] [CrossRef] [Green Version]
- Carmen, P.R.; Juan, M.F.L.; Pilar, R.S.; Javier, A.B. Screeners and Brief Assessment Methods. Nutr. Hosp. 2015, 31 (Suppl. 3), 91–97. [Google Scholar] [CrossRef]
- Weiss, J.J.; Sanchez, L.; Hubbard, J.; Lo, J.; Grinspoon, S.K.; Fitch, K.V. Diet Quality Is Low and Differs by Sex in People with HIV. J. Nutr. 2019, 149, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Giudici, K.V.; Duran, A.C.F.L.; Jaime, P.C. Inadequate Food Intake among Adults Living with HIV. Sao Paulo Med. J. 2013, 131, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Monographs in epidemiology and biostatistics; Oxford University Press: Oxford, UK; New York, NY, USA, 2013; ISBN 978-0-19-975403-8. [Google Scholar]
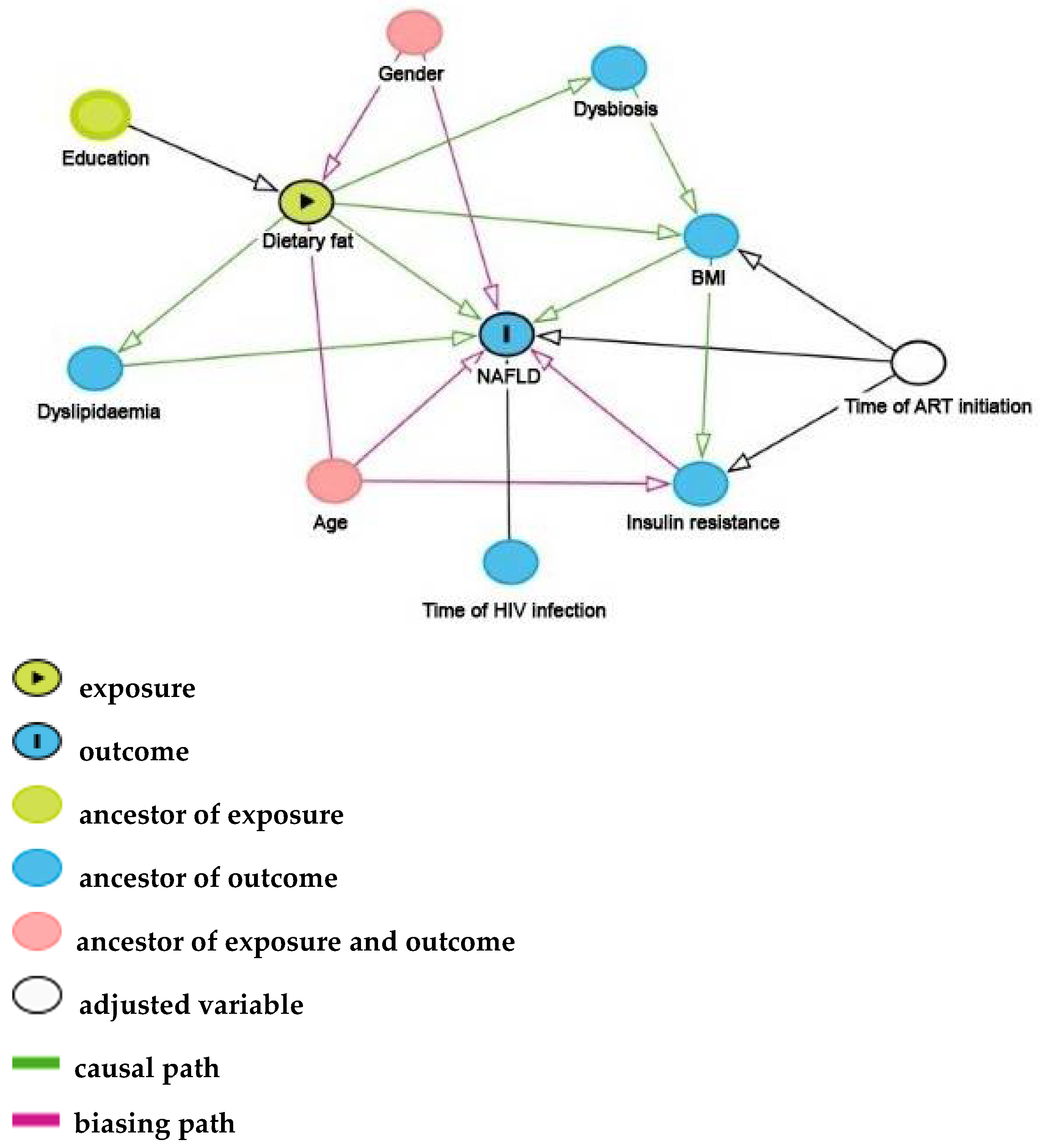
| Variables | All (n = 451) |
|---|---|
| Social and demographic | |
| Female sex a | 272 (60.3) |
| Age, years b | 45 (36–53) |
| Self-reported skin color a | |
| White | 214 (47.5) |
| Brown | 139 (30.8) |
| Black | 94 (20.8) |
| Others | 4 (0.9) |
| Education a < 8 years of study | 209 (46.4) |
| Comorbidities | |
| Diabetes mellitus a | 46 (10.2) |
| Hypertension a | 100 (22.2) |
| Dyslipidemia a | 78 (17.3) |
| Metabolic syndrome a | 150 (33.9) |
| Biochemistry | |
| ALT, IU/L b | 29 (23–43) |
| AST, IU/L b | 25 (20–33) |
| Alkaline phosphatase, IU/L b | 89 (70–111) |
| GGT, IU/L b | 45 (32–70) |
| Total cholesterol, mg/dL b | 185 (158–219) |
| LDL—cholesterol, mg/dL b | 112 (90–138) |
| HDL—cholesterol, mg/dL b | 43 (35–54) |
| Triglycerides, mg/dL b | 124 (84–171) |
| Fasting glucose, mg/dL b | 93 (88–100) |
| Insulin, um/L | 11 (8–16) |
| HOMA-IR | 3 (2–4) |
| Nutritional Status | |
| Body mass index, (kg/m2) b | 25 (23–29) |
| Lean [<25 Kg/m2] a | 207 (45.9) |
| Overweight [25–29.99 Kg/m2] a | 153 (33.9) |
| Obesity [≥30 Kg/m2] a | 91 (20.2) |
| Body fat, (%), by bioimpedance b | 30 (24–35) |
| Waist circumference, (cm) b | 87 (79–95) |
| HIV history and characteristics | |
| Duration of HIV infection, years b | 10 (5–17) |
| CD4+ T-lymphocyte count (cells/m3) b | 665 (421–881) |
| Detectable HIV RNA viral load (>40 cópias/mm3) a | 74 (16.1) |
| Current c-ART use a | 436 (96.7) |
| Duration of c-ART, years b | 7 (4–14) |
| NAFLD | ||
|---|---|---|
| Variables | Univariate Model | Multivariate Models |
| OR (95% CI) | aOR (95% CI) | |
| Energy; kcal | ||
| Q1 < 1587.76 | Reference | Reference |
| Q2 (1587.76–1952.72) | 0.80 (0.46–1.39) | 1.00 (0.50–2.00) |
| Q3 (1952.72–2299.45) | 0.73 (0.42–1.28) | 1.07 (0.43–2.68) |
| Q4 > 2299.45 | 0.68 (0.39–1.21) | 1.43 (0.36–5.69) |
| Carbohydrate; % kcal | ||
| Q1 < 49.07 | Reference | Reference |
| Q2 (49.07–53.16) | 0.62 (0.35–1.07) | 0.56 (0.31–1.01) |
| Q3 (53.16–56.79) | 0.56 (0.32–0.99) | 0.56 (0.31–1.01) |
| Q4 > 56.79 | 0.48 (0.27–0.85) | 0.44 (0.24–0.80) |
| Protein; % kcal | ||
| Q1 < 14.49 | Reference | Reference |
| Q2 (14.49–16.12) | 1.19 (0.67–2.12) | 0.99 (0.54–1.82) |
| Q3 (16.12–17.93) | 1.37 (0.77–2.45) | 1.18 (0.64–2.18) |
| Q4 > 17.93 | 1.71 (0.97–3.04) | 1.41 (0.75–2.65) |
| Total fat; % kcal | ||
| Q1 < 28.56 | Reference | Reference |
| Q2 (28.56–31) | 0.95 (0.53–1.68) | 0.97 (0.53–1.76) |
| Q3 (31–34.38) | 0.70 (0.39–1.26) | 0.65 (0.35–1.21) |
| Q4 > 34.38 | 1.81 (1.04–3.17) | 1.91 (1.06–3.44) |
| Fiber, density; g/1000 kcal | ||
| Q1 < 8.47 | Reference | Reference |
| Q2 (8.47–10.13) | 0.87 (0.49–1.52) | 0.71 (0.39–1.29) |
| Q3 (10.13–11.80) | 0.68 (0.38–1.21) | 0.51 (0.27–0.96) |
| Q4 > 11.80 | 1.13 (0.65–1.98) | 0.88 (0.48–1.60) |
| Saturate fat; % kcal | ||
| Q1 < 8.36 | Reference | Reference |
| Q2 (8.36–9.59) | 0.91 (0.51–1.61) | 0.89 (0.49–1.62) |
| Q3 (9.59–10.77) | 0.80 (0.45–1.41) | 0.85 (0.46–1.55) |
| Q4 > 10.77 | 1.45 (0.83–2.54) | 1.62 (0.9–2.92) |
| PUFA fat; % kcal | ||
| Q1 < 5.87 | Reference | Reference |
| Q2 (5.87–7.23) | 0.63 (0.36–1.11) | 0.57 (0.31–1.02) |
| Q3 (7.23–8.28) | 0.68 (0.38–1.19) | 0.60 (0.33–1.08) |
| Q4 > 8.28 | 0.77 (0.44–1.35) | 0.70 (0.39–1.26) |
| MUFA fat; % kcal | ||
| Q1 < 7.47 | Reference | Reference |
| Q2 (7.47–8.38) | 1.04 (0.59–1.83) | 0.78 (0.43–1.43) |
| Q3 (8.38–9.48) | 0.74 (0.42–1.34) | 0.58 (0.31–1.07) |
| Q4 > 9.48 | 1.32 (0.75–2.31) | 0.93 (0.49–1.76) |
| Trans FA; % kcal | ||
| Q1 < 0.28 | Reference | Reference |
| Q2 (0.28–0.37) | 0.77 (0.44–1.37) | 0.80 (0.44–1.46) |
| Q3 (0.37–0.46) | 1.34 (0.77–2.35) | 1.28 (0.71–2.31) |
| Q4 > 0.46 | 0.81 (0.46–1.43) | 0.68 (0.37–1.25) |
| Cholesterol; % kcal | ||
| Q1 < 0.1 | Reference | Reference |
| Q2 (0.1–0.12) | 1.33 (0.80–2.23) | 1.25 (0.73–2.15) |
| Q3 (0.12–0.14) | 0.99 (0.55–1.79) | 0.84 (0.44–1.6) |
| Q4 > 0.14 | 1.14 (0.65–1.98) | 0.96 (0.51–1.79) |
| n-6 PUFA; % kcal | ||
| Q1 < 3.47 | Reference | Reference |
| Q2 (3.47–4.34) | 0.57 (0.32–1.01) | 0.54 (0.30–0.98) |
| Q3 (4.34–5.28) | 0.73 (0.41–1.27) | 0.66 (0.37–1.20) |
| Q4 > 5.28 | 0.64 (0.36–1.13) | 0.59 (0.32–1.08) |
| n-3 PUFA; % kcal | ||
| Q1 < 0.41 | Reference | Reference |
| Q2 (0.41–0.53) | 0.75 (0.42–1.31) | 0.71 (0.40–1.29) |
| Q3 (0.53–0.67) | 0.72 (0.41–1.28) | 0.65 (0.36–1.19) |
| Q4 > 0.67 | 0.89 (0.51–1.56) | 0.76 (0.42–1.38) |
| Lauric FA (12:00); % kcal | ||
| Q1 < 0.08 | Reference | Reference |
| Q2 (0.08–0.13) | 0.69 (0.40–1.19) | 0.80 (0.45–1.40) |
| Q3 (0.13–0.18) | 0.42 (0.23–0.77) | 0.42 (0.22–0.78) |
| Q4 > 0.18 | 0.74 (0.44–1.27) | 0.75 (0.43–1.32) |
| Myristic FA (14:00); % kcal | ||
| Q1 < 2.36 | Reference | Reference |
| Q2 (2.36–3.27) | 1.23 (0.70–2.16) | 1.25 (0.69–2.24) |
| Q3 (3.27–5.14) | 1.00 (0.56–1.79) | 1.09 (0.59–2.00) |
| Q4 > 5.14 | 1.34 (0.76–2.36) | 1.26 (0.70–2.29) |
| Palmitic FA (16:00); % kcal | ||
| Q1 < 2.58 | Reference | Reference |
| Q2 (2.58–3.09) | 1.23 (0.70–2.15) | 1.30 (0.73–2.33) |
| Q3 (3.09–3.63) | 1.30 (0.74–2.27) | 1.20 (0.67–2.15) |
| Q4 > 3.63 | 0.89 (0.50–1.58) | 0.82 (0.45–1.50) |
| Stearic FA (18:00); % kcal | ||
| Q1 < 1.06 | Reference | Reference |
| Q2 (1.06–1.3) | 1.13 (0.65–1.99) | 1.28 (0.72–2.30) |
| Q3 (1.3–1.57) | 1.27 (0.73–2.22) | 1.19 (0.66–2.14) |
| Q4 > 1.57 | 0.87 (0.49–1.55) | 0.81 (0.44–1.49) |
| Arachidic FA (20:00); % kcal | ||
| Q1 < 0.035 | Reference | Reference |
| Q2 (0.035–0.04) | 0.89 (0.53–1.51) | 0.76 (0.44–1.33) |
| Q3 (0.04–0.05) | 1.06 (0.61–1.83) | 0.87 (0.49–1.55) |
| Q4 > 0.05 | 1.37 (0.66–2.83) | 1.02 (0.47–2.21) |
| Myristoleic FA (14:1); % kcal | ||
| Q1 < 0.02 | Reference | Reference |
| Q2 (0.02–0.03) | 0.78 (0.46–1.31) | 0.72 (0.42–1.25) |
| Q3 (0.03–0.04) | 0.53 (0.29–0.99) | 0.63 (0.33–1.21) |
| Q4 > 0.04 | 0.70 (0.41–1.19) | 0.56 (0.32–0.99) |
| Palmitoleic FA (16:1); % kcal | ||
| Q1 < 0.17 | Reference | Reference |
| Q2 (0.17–0.21) | 0.80 (0.45–1.43) | 0.80 (0.44–1.45) |
| Q3 (0.21–0.26) | 1.27 (0.75–2.16) | 1.06 (0.61–1.84) |
| Q4 > 0.26 | 1.08 (0.61–1.91) | 0.87 (0.47–1.60) |
| Oleic FA (18:1); % kcal | ||
| Q1 < 4.29 | Reference | Reference |
| Q2 (4.29–5.09) | 0.97 (0.55–1.71) | 0.97 (0.54–1.75) |
| Q3 (5.09–5.94) | 0.86 (0.49–1.52) | 0.71 (0.39–1.29) |
| Q4 > 5.94 | 1.08 (0.61–1.89) | 0.87 (0.47–1.58) |
| Linoleic FA (18:2-n6); % kcal | ||
| Q1 < 3.45 | Reference | Reference |
| Q2 (3.45–4.33) | 0.61 (0.35–1.08) | 0.59 (0.33–1.08) |
| Q3 (4.33–5.26) | 0.75 (0.43–1.32) | 0.70 (0.39–1.26) |
| Q4 > 5.26 | 0.66 (0.38–1.17) | 0.63 (0.34–1.14) |
| Linolenic FA (18:3-n3); % kcal | ||
| Q1 < 0.4 | Reference | Reference |
| Q2 (0.4–0.51) | 0.71 (0.40–1.26) | 0.68 (0.38–1.24) |
| Q3 (0.51–0.63) | 0.77 (0.44–1.34) | 0.67 (0.37–1.21) |
| Q4 > 0.63 | 0.88 (0.50–1.55) | 0.77 (0.43–1.40) |
| n6/n3 PUFA ratio; g | ||
| Q1 < 7.45 | Reference | Reference |
| Q2 (7.45–8.21) | 0.61 (0.35–1.07) | 0.59 (0.33–1.06) |
| Q3 (8.21–9.18) | 0.66 (0.37–1.16) | 0.69 (0.38–1.25) |
| Q4 > 9.18 | 0.84 (0.48–1.46) | 0.96 (0.54–1.71) |
| Fibrosis | ||
|---|---|---|
| Variables | Univariate Model | Multivariate Models |
| OR [95%IC] | aOR [95%IC] | |
| Energy; kcal | ||
| Q1 < 1587.76 | Reference | Reference |
| Q2 (1587.76–1952.72) | 0.97 (0.49–1.89) | 0.97 (0.43–2.20) |
| Q3 (1952.72–2299.45) | 0.82 (0.41–1.64) | 0.80 (0.26–2.46) |
| Q4 > 2299.45 | 0.50 (0.23–1.08) | 0.48 (0.08–2.79) |
| Carbohydrate; % kcal | ||
| Q1 < 49.07 | Reference | Reference |
| Q2 (49.07–53.16) | 1.33 (0.65–2.70) | 1.37 (0.67–2.82) |
| Q3 (53.16–56.79) | 1.25 (0.61–2.59) | 1.33 (0.64–2.76) |
| Q4 > 56.79 | 0.99 (0.47–2.09) | 0.97 (0.46–2.07) |
| Protein; % kcal | ||
| Q1 < 14.49 | Reference | Reference |
| Q2 (14.49–16.12) | 1.94 (0.91–4.18) | 1.75 (0.81–3.82) |
| Q3 (16.12–17.93) | 1.11 (0.48–2.54) | 0.97 (0.41–2.28) |
| Q4 > 17.93 | 2.61 (1.24–5.49) | 2.13 (0.96–4.70) |
| Total fat; % kcal | ||
| Q1 < 28.56 | Reference | Reference |
| Q2 (28.56–31) | 1.45 (0.75–2.80) | 1.55 (0.79–3.05) |
| Q3 (31–34.38) | 0.69 (0.33–1.46) | 0.70 (0.33–1.49) |
| Q4 > 34.38 | 0.64 (0.30–1.37) | 0.64 (0.30–1.39) |
| Fiber; g/1000 kcal | ||
| Q1 < 8.47 | Reference | Reference |
| Q2 (8.47–10.13) | 0.77 (0.37–1.61) | 0.65 (0.30–1.38) |
| Q3 (10.13–11.80) | 0.83 (0.40–1.72) | 0.69 (0.33–1.46) |
| Q4 > 11.80 | 1.21 (0.61–2.39) | 0.95 (0.47–1.95) |
| Saturated fat; % kcal | ||
| Q1 < 8.36 | Reference | Reference |
| Q2 (8.36–9.59) | 1.03 (0.52–2.02) | 1.04 (0.53–2.08) |
| Q3 (9.59–10.77) | 0.88 (0.44–1.77) | 0.96 (0.47–1.96) |
| Q4 > 10.77 | 0.60 (0.28–1.28) | 0.63 (0.29–1.36) |
| PUFA fat; % kcal | ||
| Q1 < 5.87 | Reference | Reference |
| Q2 (5.87–7.23) | 1.67 (0.84–3.33) | 1.61 (0.80–3.25) |
| Q3 (7.23–8.28) | 0.94 (0.44–2.00) | 0.89 (0.41–1.91) |
| Q4 > 8.28 | 1.03 (0.49–2.19) | 0.96 (0.45–2.06) |
| MUFA fat; % kcal | ||
| Q1 < 7.47 | Reference | Reference |
| Q2 (7.47–8.38) | 0.68 (0.33–1.41) | 0.54 (0.26–1.14) |
| Q3 (8.38–9.48) | 0.85 (0.42–1.70) | 0.67 (0.33–1.38) |
| Q4 > 9.48 | 0.84 (0.42–1.68) | 0.54 (0.25–1.17) |
| Trans FA; % kcal | ||
| Q1 < 0.28 | Reference | Reference |
| Q2 (0.28–0.37) | 0.87 (0.40–1.90) | 0.89 (0.41–1.96) |
| Q3 (0.37–0.46) | 1.37 (0.65–2.88) | 1.24 (0.58–2.65) |
| Q4 > 0.46 | 1.90 (0.94–3.84) | 1.68 (0.82–3.45) |
| Cholesterol; % kcal | ||
| Q1 < 0.1 | Reference | Reference |
| Q2 (0.1–0.12) | 1.20 (0.63–2.32) | 1.10 (0.57–2.15) |
| Q3 (0.12–0.14) | 1.05 (0.49–2.24) | 0.87 (0.40–1.92) |
| Q4 > 0.14 | 1.29 (0.65–2.57) | 1.03 (0.49–2.18) |
| n-6 PUFA; % kcal | ||
| Q1 < 3.47 | Reference | Reference |
| Q2 (3.47–4.34) | 2.10 (0.96–4.59) | 2.10 (0.95–4.63) |
| Q3 (4.34–5.28) | 2.57 (1.19–5.54) | 2.45 (1.12–5.32) |
| Q4 > 5.28 | 1.53 (0.68–3.47) | 1.40 (0.61–3.21) |
| n-3 PUFA; % kcal | ||
| Q1 < 0.41 | Reference | Reference |
| Q2 (0.41–0.53) | 0.66 (0.31–1.42) | 0.64 (0.30–1.39) |
| Q3 (0.53–0.66) | 1.34 (0.67–2.66) | 1.27 (0.63–2.54) |
| Q4 > 0.655 | 1.08 (0.53–2.19) | 0.94 (0.46–1.93) |
| Lauric FA (12:00); % kcal | ||
| Q1 < 0.08 | Reference | Reference |
| Q2 (0.08–0.13) | 0.34 (0.16–0.72) | 0.38 (0.18–0.80) |
| Q3 (0.13–0.18) | 0.47 (0.23–0.98) | 0.49 (0.24–1.02) |
| Q4 > 0.18 | 0.60 (0.31–1.16) | 0.63 (0.32–1.22) |
| Myristic FA (14:00); % kcal | ||
| Q1 < 2.36 | Reference | Reference |
| Q2 (2.36–3.27) | 0.97 (0.50–1.87) | 0.98 (0.50–1.91) |
| Q3 (3.27–5.135) | 0.36 (0.16–0.83) | 0.38 (0.17–0.89) |
| Q4 > 5.135 | 0.85 (0.43–1.69) | 0.80 (0.40–1.60) |
| Palmitic FA (16:00); % kcal | ||
| Q1 < 2.58 | Reference | Reference |
| Q2 (2.58–3.09) | 0.71 (0.36–1.41) | 0.73 (0.36–1.46) |
| Q3 (3.09–3.625) | 0.66 (0.32–1.34) | 0.62 (0.30–1.28) |
| Q4 > 3.625 | 0.71 (0.36–1.41) | 0.64 (0.32–1.30) |
| Stearic FA (18:00); % kcal | ||
| Q1 < 1.06 | Reference | Reference |
| Q2 (1.06–1.3) | 0.76 (0.38–1.51) | 0.82 (0.41–1.64) |
| Q3 (1.3–1.57) | 0.50 (0.23–1.07) | 0.48 (0.22–1.04) |
| Q4 > 1.57 | 0.95 (0.48–1.87) | 0.91 (0.46–1.80) |
| Arachidic FA (20:00); % kcal | ||
| Q1 < 0.035 | Reference | Reference |
| Q2 (0.035–0.04) | 0.93 (0.48–1.81) | 0.81 (0.41–1.6) |
| Q3 (0.04–0.05) | 1.00 (0.50–1.98) | 0.83 (0.41–1.69) |
| Q4 > 0.05 | 1.29 (0.53–3.14) | 0.98 (0.39–2.46) |
| Myristoleic FA (14:1); % kcal | ||
| Q1 < 0.02 | Reference | Reference |
| Q2 (0.02–0.03) | 0.65 (0.34–1.27) | 0.63 (0.32–1.24) |
| Q3 (0.03–0.04) | 0.51 (0.23–1.14) | 0.59 (0.26–1.34) |
| Q4 > 0.04 | 0.71 (0.37–1.38) | 0.65 (0.33–1.27) |
| Palmitoleic FA (16:1); % kcal | ||
| Q1 < 0.17 | Reference | Reference |
| Q2 (0.17–0.21) | 0.78 (0.40–1.52) | 0.80 (0.40–1.58) |
| Q3 (0.21–0.26) | 0.46 (0.23–0.95) | 0.40 (0.19–0.82) |
| Q4 > 0.26 | 0.68 (0.34–1.35) | 0.52 (0.25–1.09) |
| Oleic FA (18:1); % kcal | ||
| Q1 < 4.29 | Reference | Reference |
| Q2 (4.29–5.09) | 0.74 (0.37–1.46) | 0.71 (0.36–1.43) |
| Q3 (5.09–5.94) | 0.40 (0.18–0.88) | 0.35 (0.16–0.79) |
| Q4 > 5.94 | 0.89 (0.46–1.73) | 0.70 (0.35–1.41) |
| Linoleic FA (18:2-n6); % kcal | ||
| Q1 < 3.45 | Reference | Reference |
| Q2 (3.45–4.33) | 1.61 (0.75–3.44) | 1.63 (0.75–3.51) |
| Q3 (4.33–5.23) | 2.17 (1.04–4.53) | 2.08 (0.99–4.38) |
| Q4 > 5.23 | 1.30 (0.59–2.84) | 1.19 (0.54–2.64) |
| Linolenic FA (18:3-n3); % kcal | ||
| Q1 < 0.4 | Reference | Reference |
| Q2 (0.4–0.51) | 0.68 (0.31–1.46) | 0.67 (0.31–1.45) |
| Q3 (0.51–0.63) | 1.32 (0.67–2.61) | 1.25 (0.63–2.49) |
| Q4 > 0.63 | 1.04 (0.51–2.14) | 0.92 (0.44–1.91) |
| n6/n3 PUFA ratio, g | ||
| Q1 < 7.445 | Reference | Reference |
| Q2 (7.445–8.21) | 1.69 (0.82–3.51) | 1.69 (0.81–3.52) |
| Q3 (8.21–9.18) | 1.57 (0.75–3.30) | 1.62 (0.77–3.44) |
| Q4 > 9.18 | 1.20 (0.56–2.60) | 1.27 (0.58–2.78) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, C.F.; da Silva, P.S.; Cardoso, C.S.d.A.; Moreira, N.G.; Antunes, J.C.; de Andrade, M.M.; Silva, J.; Araujo, M.C.; Peres, W.A.F.; do Brasil, P.E.A.A.; et al. Relationship between Dietary Fatty Acid Intake with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in People with HIV. Nutrients 2021, 13, 3462. https://doi.org/10.3390/nu13103462
de Almeida CF, da Silva PS, Cardoso CSdA, Moreira NG, Antunes JC, de Andrade MM, Silva J, Araujo MC, Peres WAF, do Brasil PEAA, et al. Relationship between Dietary Fatty Acid Intake with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in People with HIV. Nutrients. 2021; 13(10):3462. https://doi.org/10.3390/nu13103462
Chicago/Turabian Stylede Almeida, Cristiane Fonseca, Paula Simplicio da Silva, Claudia Santos de Aguiar Cardoso, Nathalia Gorni Moreira, Julliana Cormack Antunes, Michelle Morata de Andrade, Julio Silva, Marina Campos Araujo, Wilza Arantes Ferreira Peres, Pedro Emmanuel Alvarenga Americano do Brasil, and et al. 2021. "Relationship between Dietary Fatty Acid Intake with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in People with HIV" Nutrients 13, no. 10: 3462. https://doi.org/10.3390/nu13103462
APA Stylede Almeida, C. F., da Silva, P. S., Cardoso, C. S. d. A., Moreira, N. G., Antunes, J. C., de Andrade, M. M., Silva, J., Araujo, M. C., Peres, W. A. F., do Brasil, P. E. A. A., Moreira, R. I., Cardoso, S. W., Veloso, V. G., Grinsztejn, B., de Brito, P. D., & Perazzo, H. (2021). Relationship between Dietary Fatty Acid Intake with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in People with HIV. Nutrients, 13(10), 3462. https://doi.org/10.3390/nu13103462







