Diet-Induced Hypothalamic Inflammation, Phoenixin, and Subsequent Precocious Puberty
Abstract
:1. Introduction
2. Physiology of Puberty
3. Precocious Puberty
3.1. Central Precocious Puberty (CPP)
3.2. Peripheral Precocious Puberty (PPP)
4. Potential Mechanisms of Diet-Induced Precocious Pubarche
- (A)
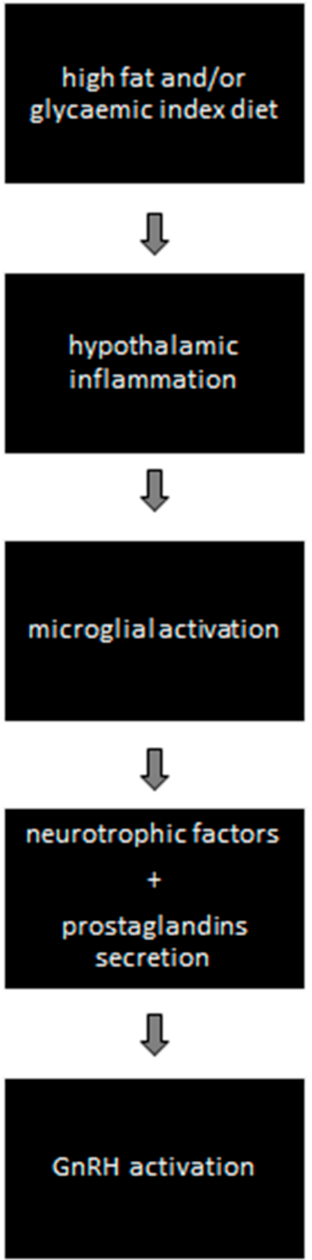
- Activation of GnRH via Hypothalamic Microglial Activation (Figure 1)
- (B)
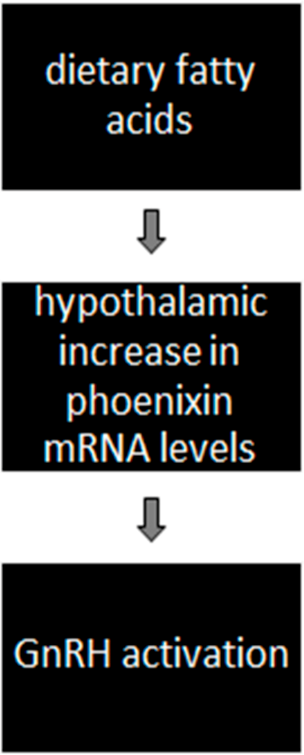
- Activation of GnRH via diet-induced phoenixin action (Figure 2)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cintra, G.; Wajnsztejn, R.; Martins Trevisan, C.; Zaia, V.; Laganà, A.S.; Bianco, B.; Montagna, E. Kisspeptin Levels in Girls with Precocious Puberty: A Systematic Review and Meta-Analysis. Horm. Res. Paediatr. 2020, 93, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Bessa, D.S.; Brito, V.N.; Latronico, A.C. Sexual Precocity—Genetic Bases of Central Precocious Puberty and Autonomous Gonadal Activation. Endocr. Dev. 2016, 29, 50–71. [Google Scholar] [CrossRef]
- Cheuiche, A.V.; da Silveira, L.G.; de Paula, L.C.P.; Lucena, I.R.S.; Silveiro, S.P. Diagnosis and management of precocious sexual maturation: An updated review. Eur. J. Pediatr. 2021, 180, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Teilmann, G.; Pedersen, C.B.; Jensen, T.K.; Skakkebaek, N.E.; Juul, A. Prevalence and incidence of precocious pubertal development in Denmark: An epidemiologic study based on national registries. Pediatrics 2005, 116, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Guillén, L.; Corripio, R.; Labarta, J.I.; Cañete, R.; Castro-Feijóo, L.; Espino, R.; Argente, J. Central Precocious Puberty in Children Living in Spain: Incidence, Prevalence, and Influence of Adoption and Immigration. J. Clin. Endocrinol. Metab. 2010, 95, 4305–4313. [Google Scholar] [CrossRef] [Green Version]
- Aksglaede, L.; Sørensen, K.; Petersen, J.H.; Skakkebaek, N.E.; Juul, A. Recent Decline in Age at Breast Development: The Copenhagen Puberty Study. Pediatrics 2009, 123, e932–e939. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, A.; Pantsiotou, S.; Douros, K.; Papadimitriou, D.T.; Nicolaidou, P.; Fretzayas, A. Timing of Pubertal Onset in Girls: Evidence for Non-Gaussian Distribution. J. Clin. Endocrinol. Metab. 2008, 93, 4422–4425. [Google Scholar] [CrossRef] [Green Version]
- Semiz, S.; Kurt, F.; Kurt, D.T.; Zencir, M.; Sevinc, O. Pubertal development of Turkish children. J. Pediatr. Endocrinol. Metab. 2008, 21, 951–961. [Google Scholar] [CrossRef]
- Elks, C.E.; Perry, J.R.; Sulem, P.; Chasman, D.; Franceschini, N.; He, C.; Lunetta, K.L.; Visser, J.A.; Byrne, E.M.; Cousminer, D.L.; et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 2010, 42, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Calcaterra, V.; Cena, H.; Regalbuto, C.; Vinci, F.; Porri, D.; Verduci, E.; Chiara, M.; Zuccotti, G.V. The Role of Fetal, Infant, and Childhood Nutrition in the Timing of Sexual Maturation. Nutrients 2021, 13, 419. [Google Scholar] [CrossRef]
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; Di Luca, N.M.; Grappasonni, I. Fighting obesity in children from European World Health Organization member states. Epidemiological data, medical-social aspects, and prevention programs. Clin. Ter. 2019, 170, e223–e230. [Google Scholar] [PubMed]
- Schwingshackl, L.; Hobl, L.P.; Hoffmann, G. Effects of low glycaemic index/low glycaemic load vs. high glycaemic index/ high glycaemic load diets on overweight/obesity and associated risk factors in children and adolescents: A systematic review and meta-analysis. Nutr. J. 2015, 14, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Jawaldeh, A.; Taktouk, M.; Nasreddine, L. Food Consumption Patterns and Nutrient Intakes of Children and Adolescents in the Eastern Mediterranean Region: A Call for Policy Action. Nutrients 2020, 12, 3345. [Google Scholar] [CrossRef]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.C.D.; Takahashi, H.K.; et al. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ottaway, N.; Schriever, S.C.; Legutko, B.; García-Cáceres, C.; De La Fuente, E.; Mergen, C.; Bour, S.; Thaler, J.P.; Seeley, R.; et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 2014, 62, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kvist, H.; Sjöström, L.; Tylén, U. Adipose tissue volume determinations in women by computed tomography: Technical considerations. Int. J. Obes. 1986, 10, 53–67. [Google Scholar]
- Thomas, E.L.; Saeed, N.; Hajnal, J.V.; Brynes, A.; Goldstone, A.P.; Frost, G.; Bell, J.D. Magnetic resonance imaging of total body fat. J. Appl. Physiol. 1998, 85, 1778–1785. [Google Scholar] [CrossRef] [Green Version]
- Piche, M.E.; Poirier, P. Obesity, ectopic fat and cardiac metabolism. Expert Rev. Endocrinol. Metab. 2018, 13, 213–221. [Google Scholar] [CrossRef]
- Dionysopoulou, S.; Charmandari, E.; Bargiota, A.; Vlachos, N.; Mastorakos, G.; Valsamakis, G. The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders. Nutrients 2021, 13, 498. [Google Scholar] [CrossRef]
- Sánchez, J.; Cladera, M.M.; Llopis, M.; Palou, A.; Picó, C. The different satiating capacity of CHO and fats can be mediated by different effects on leptin and ghrelin systems. Behav. Brain Res. 2010, 213, 183–188. [Google Scholar] [CrossRef]
- Barlampa, D.; Bompoula, M.; Bargiota, A.; Kalantaridou, S.; Mastorakos, G.; Valsamakis, G. Hypothalamic Inflammation as a Potential Pathophysiologic Basis for the Heterogeneity of Clinical, Hormonal, and Metabolic Presentation in PCOS. Nutrients 2021, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Karakas, S.E.; Surampudi, P. New Biomarkers to Evaluate Hyperandrogenemic Women and Hypogonadal Men. Adv. Clin. Chem. 2018, 86, 71–125. [Google Scholar] [CrossRef] [PubMed]
- Maione, L.; Bouvattier, C.; Kaiser, U.B. Central precocious puberty: Recent advances in understanding the aetiology and in the clinical approach. Clin. Endocrinol. 2021, 95, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Poon, K. Behavioral Feeding Circuit: Dietary Fat-Induced Effects of Inflammatory Mediators in the Hypothalamus. Front. Endocrinol. 2020, 11, 591559. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.V.; Metcalf, G.A.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.P.; Kaiser, U.B. Pubertal development and regulation. Lancet Diab. Endocrinol. 2016, 4, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Waterland, R.A.; Jirtle, R.L. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 2004, 20, 63–68. [Google Scholar] [CrossRef]
- Kiess, W.; Hoppmann, J.; Gesing, J.; Penke, M.; Körner, A.; Kratzsch, J.; Pfaeffle, R. Puberty—Genes, environment and clinical issues. J. Pediatr. Endocrinol. Metab. 2016, 29, 1229–1231. [Google Scholar] [CrossRef]
- Renault, C.H.; Aksglaede, L.; Wøjdemann, D.; Hansen, A.B.; Jensen, R.B.; Juul, A. Mini-puberty of human infancy—A window of opportunity to evaluate hypogonadism and differences of sex development? Ann. Pediatr. Endocrinol. Metab. 2020, 25, 84–91. [Google Scholar] [CrossRef]
- Farello, G.; Altieri, C.; Cutini, M.; Pozzobon, G.; Verrotti, A. Review of the Literature on Current Changes in the Timing of Pubertal Development and the Incomplete Forms of Early Puberty. Front. Pediatr. 2019, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Uenoyama, Y.; Inoue, N.; Nakamura, S.; Tsukamura, H. Central Mechanism Controlling Pubertal Onset in Mammals: A Triggering Role of Kisspeptin. Front. Endocrinol. 2019, 10, 312. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Navarro, V.M.; Simavli, S.; Vong, L.; Carroll, R.S.; Lowell, B.B.; Kaiser, U.B. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J. Neurosci. 2014, 34, 6047–6056. [Google Scholar] [CrossRef] [Green Version]
- Chachlaki, K.; Garthwaite, J.; Prevot, V. The gentle art of saying NO: How nitric oxide gets things done in the hypothalamus. Nat. Rev. Endocrinol. 2017, 13, 521–535. [Google Scholar] [CrossRef]
- Stagi, S.; De Masi, S.; Bencini, E.; Losi, S.; Paci, S.; Parpagnoli, M.; Ricci, F.; Ciofi, D.; Azzari, C. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital. J. Pediatr. 2020, 46, 165. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Mogensen, S.S.; Juul, A. Recent Secular Trends in Pubertal Timing: Implications for Evaluation and Diagnosis of Precocious Puberty. Horm. Res. Paediatr. 2012, 77, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.S.; Ejaz, S. Precocious Puberty; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chang Chen, C.; Chen, Y.; Zhang, Y.; Sun, W.; Jiang, Y.; Song, Y.; Zhu, Q.; Mei, H.; Wang, X.; Liu, S.; et al. Association between Dietary Patterns and Precocious Puberty in Children: A Population-Based Study. Int. J. Endocrinol. 2018, 2018, 4528704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohani, F.; Salehpur, S.; Saffari, F. Etiology of precocious puberty, 10 years study in Endocrine Research Centre (Firouzgar), Tehran. Iran J. Reprod. Med. 2012, 10, 1–6. [Google Scholar] [PubMed]
- Euling, S.Y.; Hermans-Giddens, M.E.; Lee, P.A.; Selevan, S.G.; Juul, A.; Sorensen, T.I.A.; Dunkel, L.; Himes, J.H.; Teilmann, G.; Swn, S.H. Examination of US puberty timing data from 1940 to 1994 for secular trends. Pediatrics 2008, 121, 172–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, N.F.; Kim, Y.B.; Velloso, L.A.; Araújo, E.P. Hypothalamic Microglial Activation in Obesity: A Mini-Review. Front. Neurosci. 2018, 12, 846. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, H.; Kakehashi, C.; Funabashi, T.; Akema, T. Immunohistochemical evidence for the relationship between microglia and GnRH neurons in the preoptic area of ovariectomized rats with and without steroid replacement. Endocr. J. 2013, 60, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Tohyama, Y.; Maeda, S.; Kohsaka, S.; Kurihara, T. Neuronal regulation by which microglia enhance the production of neurotrophic factors for GABAergic, catecholaminergic, and cholinergic neurons. Neurochem. Int. 2007, 50, 807–820. [Google Scholar] [CrossRef]
- Ferrini, F.; De Koninck, Y. Microglia Control Neuronal Network Excitability via BDNF Signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef] [PubMed]
- Monif, M.; Reid, C.A.; Powell, K.L.; Smart, M.L.; Williams, D.A. The P2X7 Receptor Drives Microglial Activation and Proliferation: A Trophic Role for P2X7R Pore. J. Neurosci. 2009, 29, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Cronin, A.S.; Horan, T.L.; Spergel, D.J.; Brooks, A.N.; Hastings, M.H.; Ebling, F.J.P. Neurotrophic effects of BDNF on embryonic gonadotropin-releasing hormone (GnRH) neurons. Eur. J. Neurosci. 2004, 20, 338–344. [Google Scholar] [CrossRef]
- Przybył, B.J.; Szlis, M.; Wójcik-Gładysz, A. Brain-derived neurotrophic factor (BDNF) affects the activity of the gonadotrophic axis in sheep. Horm. Behav. 2021, 131, 104980. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L. The Endocrinology of the Menstrual Cycle. Methods Mol. Biol. 2014, 1154, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, M.; Dardente, H.; Cayla, X.; Caraty, A. Cellular mechanisms and integrative timing of neuroendocrine control of GnRH secretion by kisspeptin. Mol. Cell Endocrinol. 2014, 382, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Gottsch, M.L.; Lee, K.J.; Popa, S.M.; Smith, J.T.; Jakawich, S.K.; Clifton, D.K.; Steiner, R.A.; Herbison, A.E. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 2005, 25, 11349–11356. [Google Scholar] [CrossRef]
- Yosten, G.L.C.; Lyu, R.M.; Hsueh, A.J.W.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Billert, M.; Rak, A.; Nowak, K.W.; Skrzypski, M. Phoenixin: More than Reproductive Peptide. Int. J. Mol. Sci. 2020, 21, 8378. [Google Scholar] [CrossRef]
- Suszka-Świtek, A.; Pałasz, A.; Filipczyk, L.; Menezes, I.C.; Mordecka-Chamera, K.; Angelone, T.; Bogus, K.; Bacopoulou, F.; Worthington, J.J.; Wiaderkiewicz, R. The GnRH analogues affect novel neuropeptide SMIM20/phoenixin and GPR173 receptor expressions in the female rat hypothalamic-pituitary-gonadal (HPG) axis. Clin. Exp. Pharmacol. Physiol. 2019, 46, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Treen, A.K.; Luo, V.; Belsham, D.D. Phoenixin Activates Immortalized GnRH and Kisspeptin Neurons through the Novel Receptor GPR173. Mol. Endocrinol. 2016, 30, 872–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, L.M.; Tullock, C.W.; Mathews, S.K.; Garcia-Galiano, D.; Elias, C.F.; Samson, W.K.; Yosten, G.L.C. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: Importance of the orphan G protein-coupled receptor Gpr173. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R489–R496. [Google Scholar] [CrossRef] [Green Version]
- Dhillo, W.S.; Chaudhri, O.B.; Patterson, M.; Thompson, E.L.; Murphy, K.G.; Badman, M.K.; McGowan, B.M.; Amber, V.; Patel, S.; Ghatei, M.A.; et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. Clin. Endocrinol. Metab. 2005, 90, 6609–6615. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.A.; Dhillo, W.S. Kisspeptin across the human lifespan:evidence from animal studies and beyond. J. Endocrinol. 2016, 229, R83–R98. [Google Scholar] [CrossRef] [Green Version]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef] [Green Version]
- De Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, X.P.; Nakamura, T.; Osuka, S.; Bayasula, B.; Nakanishi, N.; Kasahara, Y.; Muraoka, A.; Hayashi, S.; Nagai, T.; Murase, T.; et al. Effect of the neuropeptide phoenixin and its receptor GPR173 during folliculogenesis. Reproduction 2019, 158, 25–34. [Google Scholar] [CrossRef]
- McIlwraith, E.K.; Loganathan, N.; Belsham, D.D. Phoenixin Expression Is Regulated by the Fatty Acids Palmitate, Docosahexaenoic Acid and Oleate, and the Endocrine Disrupting Chemical Bisphenol A in Immortalized Hypothalamic Neurons. Front. Neurosci. 2018, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, Y.; Ma, S.; Tang, Y.; Li, H. Phoenixin-20 Ameliorates Lipopolysaccharide-Induced Activation of Microglial NLRP3 Inflammasome. Neurotox. Res. 2020, 38, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ren, Q.; Bai, L.; Zhang, L. Phoenixin-20 suppresses lipopolysaccharide-induced inflammation in dental pulp cells. Chem. Biol. Interact. 2020, 318, 108971. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valsamakis, G.; Arapaki, A.; Balafoutas, D.; Charmandari, E.; Vlahos, N.F. Diet-Induced Hypothalamic Inflammation, Phoenixin, and Subsequent Precocious Puberty. Nutrients 2021, 13, 3460. https://doi.org/10.3390/nu13103460
Valsamakis G, Arapaki A, Balafoutas D, Charmandari E, Vlahos NF. Diet-Induced Hypothalamic Inflammation, Phoenixin, and Subsequent Precocious Puberty. Nutrients. 2021; 13(10):3460. https://doi.org/10.3390/nu13103460
Chicago/Turabian StyleValsamakis, Georgios, Angeliki Arapaki, Dimitris Balafoutas, Evangelia Charmandari, and Nikolaos F. Vlahos. 2021. "Diet-Induced Hypothalamic Inflammation, Phoenixin, and Subsequent Precocious Puberty" Nutrients 13, no. 10: 3460. https://doi.org/10.3390/nu13103460
APA StyleValsamakis, G., Arapaki, A., Balafoutas, D., Charmandari, E., & Vlahos, N. F. (2021). Diet-Induced Hypothalamic Inflammation, Phoenixin, and Subsequent Precocious Puberty. Nutrients, 13(10), 3460. https://doi.org/10.3390/nu13103460







