The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Methods
- Aim of the analysis:
- -
- Effect of vitamin D supplementation;
- -
- Association with VD serum values (25OHD or 1,25OHD) or Vitamin D intake;
- Health status:
- -
- Healthy subjects;
- -
- Subjects with possible dysbiosis, pregnancies, obesity, diabetes;
- Types of samples for the microbiome:
- -
- Stool;
- -
- Biopsies.
3. Results
3.1. Qualitative Synthesis
3.1.1. Study Designs
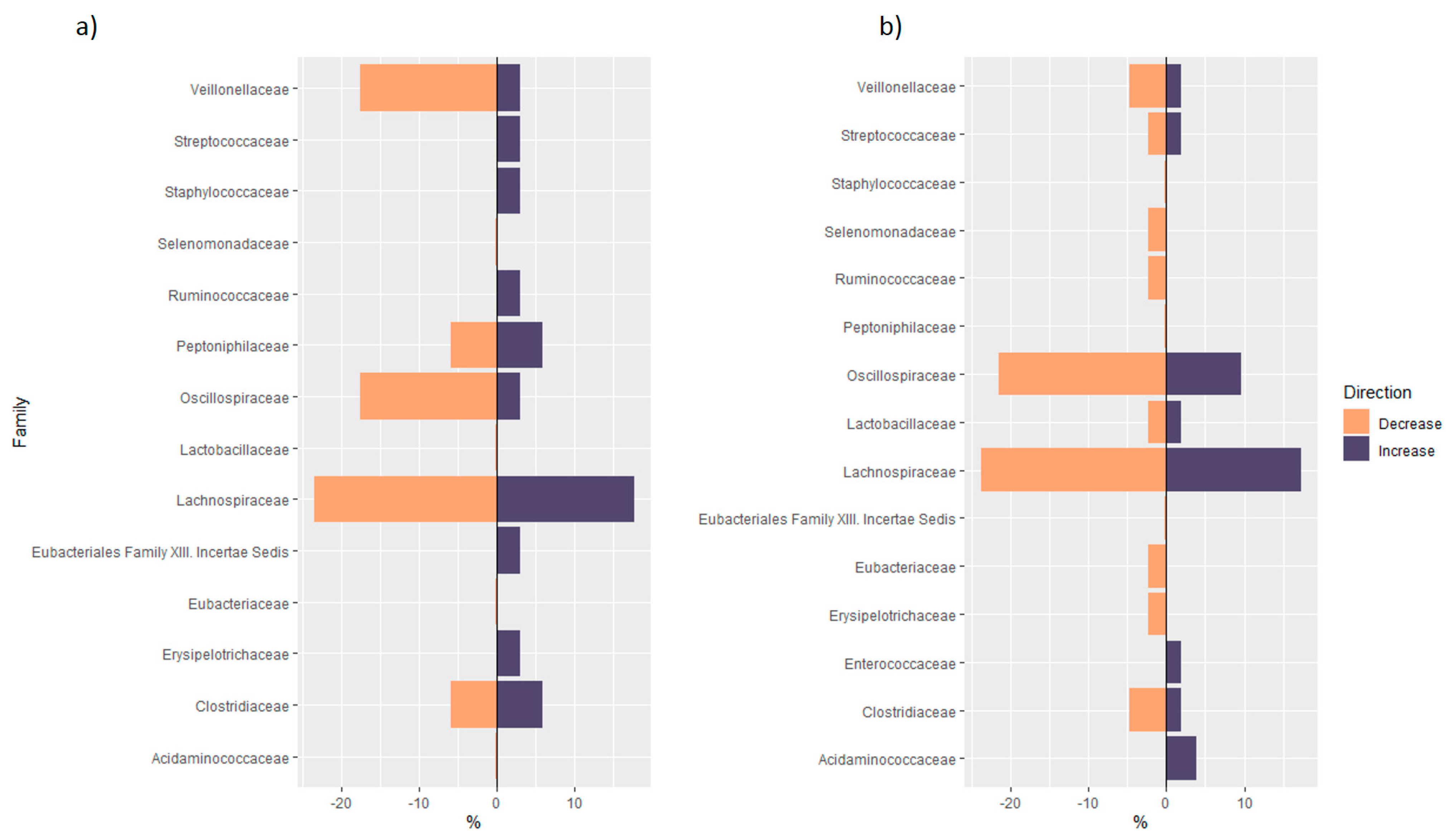
3.1.2. Distribution of Taxa at Phylum Level
3.1.3. Analysis of Phylogenetic Trees of Studies in Healthy Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2015, 96, 365–408. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Parker, J.; Hashmi, O.; Dutton, D.; Mavrodaris, A.; Stranges, S.; Kandala, N.-B.; Clarke, A.; Franco, O.H. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas 2010, 65, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Raimondi, S.; Gnagnarella, P.; Doré, J.-F.; Maisonneuve, P.; Testori, A. Vitamin D and skin cancer: A meta-analysis. Eur. J. Cancer 2009, 45, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.K.; Kim, I.Y.; Hodge, A.M.; English, D.R.; Muller, D.C. Vitamin D status and mortality: A systematic review of observational studies. Int. J. Environ. Res. Public Health 2019, 16, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Al Anouti, F.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef]
- Greenstein, R.J.; Su, L.; Brown, S.T. Vitamins A & D inhibit the growth of mycobacteria in radiometric culture. PLoS ONE 2012, 7, e29631. [Google Scholar] [CrossRef] [Green Version]
- Jahani, R.; Fielding, K.A.; Chen, J.; Villa, C.R.; Castelli, L.M.; Ward, W.E.; Comelli, E.M. Low vitamin D status throughout life results in an inflammatory prone status but does not alter bone mineral or strength in healthy 3-month-old CD-1 male mice. Mol. Nutr. Food Res. 2014, 58, 1491–1501. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis 1—3. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Wu, S.; Zhang, Y.; Lu, R.; Xia, Y.; Dong, H.; Sun, J. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin. Ther. 2015, 37, 996–1009.e7. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: A systematic review of in vivo studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Madden, MS, USA, 2021. [Google Scholar]
- Gominak, S.C. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med. Hypotheses 2016, 94, 103–107. [Google Scholar] [CrossRef]
- Jefferson, K.K.; Parikh, H.I.; Garcia, E.M.; Edwards, D.J.; Serrano, M.G.; Hewison, M.; Shary, J.R.; Powell, A.M.; Hollis, B.W.; Fettweis, J.M.; et al. Relationship between Vitamin D status and the vaginal microbiome during pregnancy. J. Perinatol. 2019, 39, 824–836. [Google Scholar] [CrossRef]
- Tuddenham, S.; Ghanem, K.G.; Caulfield, L.E.; Rovner, A.J.; Robinson, C.; Shivakoti, R.; Miller, R.; Burke, A.; Murphy, C.; Ravel, J.; et al. Associations between dietary micronutrient intake and molecular-Bacterial Vaginosis. Reprod. Health 2019, 16, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Zhu, Q.; Mai, M.; Yang, W.; Du, G. Vitamin B and vitamin D as modulators of gut microbiota in overweight individuals. Int. J. Food Sci. Nutr. 2020, 71, 1001–1009. [Google Scholar] [CrossRef]
- Ciubotaru, I.; Green, S.J.; Kukreja, S.; Barengolts, E. Significant differences in fecal microbiota are associated with various stages of glucose tolerance in African American male veterans. Transl. Res. 2015, 166, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020, 40, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Hjelmsø, M.H.; Shah, S.A.; Thorsen, J.; Rasmussen, M.; Vestergaard, G.; Mortensen, M.S.; Brejnrod, A.; Brix, S.; Chawes, B.; Bønnelykke, K.; et al. Prenatal dietary supplements influence the infant airway microbiota in a randomized factorial clinical trial. Nat. Commun. 2020, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Kanhere, M.; He, J.; Chassaing, B.; Ziegler, T.R.; Alvarez, J.A.; Ivie, E.A.; Hao, L.; Hanfelt, J.; Gewirtz, A.T.; Tangpricha, V. Bolus Weekly Vitamin D3 Supplementation Impacts Gut and Airway Microbiota in Adults with Cystic Fibrosis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2018, 103, 564–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missailidis, C.; Sørensen, N.; Ashenafi, S.; Amogne, W.; Kassa, E.; Bekele, A.; Getachew, M.; Gebreselassie, N.; Aseffa, A.; Aderaye, G.; et al. Vitamin D and Phenylbutyrate Supplementation Does Not Modulate Gut Derived Immune Activation in HIV-1. Nutrients 2019, 11, 1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naderpoor, N.; Mousa, A.; Fernanda Gomez Arango, L.; Barrett, H.L.; Dekker Nitert, M.; de Courten, B. Effect of Vitamin D Supplementation on Faecal Microbiota: A Randomised Clinical Trial. Nutrients 2019, 11, 2888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordillo, J.E.; Zhou, Y.; McGeachie, M.J.; Ziniti, J.; Lange, N.; Laranjo, N.; Savage, J.R.; Carey, V.; O’Connor, G.; Sandel, M.; et al. Factors influencing the infant gut microbiome at age 3-6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J. Allergy Clin. Immunol. 2017, 139, 482–491.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drall, K.M.; Field, C.J.; Haqq, A.M.; de Souza, R.J.; Tun, H.M.; Morales-Lizcano, N.P.; Konya, T.B.; Guttman, D.S.; Azad, M.B.; Becker, A.B.; et al. Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: Implications for viral respiratory infections. Gut Microbes 2020, 12, 1799734. [Google Scholar] [CrossRef] [PubMed]
- Kassem, Z.; Sitarik, A.; Levin, A.M.; Lynch, S.V.; Havstad, S.; Fujimura, K.; Kozyrskyj, A.; Ownby, D.R.; Johnson, C.C.; Yong, G.J.M.; et al. Maternal and cord blood vitamin D level and the infant gut microbiota in a birth cohort study. Matern. Health Neonatol. Perinatol. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Godfrey, K.M.; McDonald, D.; Treuren, W.V.; Bjørnholt, J.V.; Midtvedt, T.; Moen, B.; Rudi, K.; Knight, R.; Brantsæter, A.L.; et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome 2016, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Talsness, C.E.; Penders, J.; Jansen, E.H.J.M.; Damoiseaux, J.; Thijs, C.; Mommers, M. Influence of vitamin D on key bacterial taxa in infant microbiota in the KOALA Birth Cohort Study. PLoS ONE 2017, 12, e0188011. [Google Scholar] [CrossRef]
- Soltys, K.; Stuchlikova, M.; Hlavaty, T.; Gaalova, B.; Budis, J.; Gazdarica, J.; Krajcovicova, A.; Zelinkova, Z.; Szemes, T.; Kuba, D.; et al. Seasonal changes of circulating 25-hydroxyvitamin D correlate with the lower gut microbiome composition in inflammatory bowel disease patients. Sci. Rep. 2020, 10, 6024. [Google Scholar] [CrossRef]
- Jackson, M.A.; Verdi, S.; Maxan, M.-E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef] [Green Version]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.D.; Ferreira, S.R.G. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef]
- Seura, T.; Yoshino, Y.; Fukuwatari, T. The Relationship between Habitual Dietary Intake and Gut Microbiota in Young Japanese Women. J. Nutr. Sci. Vitaminol. 2017, 63, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 5. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.J.; Gan, H.Y.; Li, X.; Huang, Y.; Li, Z.C.; Deng, H.M.; Chen, S.Z.; Zhou, Y.; Wang, L.S.; Han, Y.P.; et al. Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. J. Dig. Dis. 2019, 20, 447–459. [Google Scholar] [CrossRef]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohns Colitis 2018, 12, 963–972. [Google Scholar] [CrossRef]
- Schäffler, H.; Herlemann, D.P.; Klinitzke, P.; Berlin, P.; Kreikemeyer, B.; Jaster, R.; Lamprecht, G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J. Dig. Dis. 2018, 19, 225–234. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut Microbiota in Multiple Sclerosis: Possible Influence of Immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaeizadeh, S.-A.; Fazeli, M.; Meshkat, Z.; Khodashenas, E.; Esmaeili, H.; Mazloum, S.; Ferns, G.A.; Abdizadeh, M.F.; Ghayour-Mobarhan, M. The effects of high doses of vitamin D on the composition of the gut microbiome of adolescent girls. Clin. Nutr. ESPEN 2020, 35, 103–108. [Google Scholar] [CrossRef]
- Bosman, E.S.; Albert, A.Y.; Lui, H.; Dutz, J.P.; Vallance, B.A. Skin Exposure to Narrow Band Ultraviolet (UVB) Light Modulates the Human Intestinal Microbiome. Front. Microbiol. 2019, 10, 2410. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Vitamin D and microbiota: Two sides of the same coin in the immunomodulatory aspects. Int. Immunopharmacol. 2020, 79, 106112. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2019, 10, 3141. [Google Scholar] [CrossRef] [PubMed]
- Bakke, D.; Sun, J. Ancient Nuclear Receptor VDR with New Functions: Microbiome and Inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krutzik, S.R.; Hewison, M.; Liu, P.T.; Robles, J.A.; Stenger, S.; Adams, J.S.; Modlin, R.L. IL-15 Links TLR2/1-Induced Macrophage Differentiation to the Vitamin D-Dependent Antimicrobial Pathway. J. Immunol. 2008, 181, 7115–7120. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Sun, J. Vitamin D, vitamin D receptor and tissue barriers. Tissue Barriers 2013, 1, e23118. [Google Scholar] [CrossRef]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.-I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [Green Version]
- Bharti, R.; Grimm, D.G. Current challenges and best-practice protocols for microbiome analysis. Brief. Bioinform. 2021, 22, 178–193. [Google Scholar] [CrossRef] [Green Version]
- Bora, S.A.; Kennett, M.J.; Smith, P.B.; Patterson, A.D.; Cantorna, M.T. The Gut Microbiota Regulates Endocrine Vitamin D Metabolism through Fibroblast Growth Factor 23. Front. Immunol. 2018, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Gnagnarella, P.; Serrano, D.; Pasquali, E.; Raimondi, S. Vitamin D Receptor Polymorphisms and Cancer. Neurotransm. Interact. Cogn. Funct. 2014, 810, 69–105. [Google Scholar]
- Serrano, D.; Pozzi, C.; Guglietta, S.; Fosso, B.; Suppa, M.; Gnagnarella, P.; Corso, F.; Bellerba, F.; Macis, D.; Aristarco, V.; et al. Microbiome as Mediator of Diet on Colorectal Cancer Risk: The Role of Vitamin D, Markers of Inflammation and Adipokines. Nutrients 2021, 13, 363. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Participants (n°) | Country, Cohort Name | Health status, Inclusion Criteria | Vitamin D Supplementation, Dietary Vitamin D Intakes or 25(OH)D Measure | Microbiota Analysis | Hypervariable Region of 16 sRNA Gene |
|---|---|---|---|---|---|---|
| Double-blind, randomized controlled trials | ||||||
| Ciubotaru, 2015 [27] | 115 | US | Prediabetes, AAM veteran, aged 35–85 years, BMI 28–39, serum 25(OH) D < 29 ng/mL | ARM1: 400 IU/week + placebo; ARM2: 400 IU/week + 50,000 UI/week for 12 weeks | Ion Torrent Personal Genome Machine | V4 |
| Charoenngam, 2020 [28] | 20 | US | Healthy adults, serum 25(OH)D levels < 30 ng/mL | Three different arms: 600, 4000 or 10,000 UI/day for 8 weeks | uBiome Inc. (San Francisco, CA, USA). | NR |
| Hjelmsø, 2020 [29] | 580 | DK, COPSAC2010 cohort | Pregnant women, gestational age 24 weeks | 2800 UI/day from 12 to 16 weeks | Illumina MiSeq | V4 |
| Kanhere, 2018 [30] | 38 | US | Patients with CF, age ≥ 18 year, no contraindication to oral high-dose vitamin D. Serum 25(OH)D level at baseline 37 ± 6 ng/mL | 50,000 UI/week for 12 weeks | Illumina MiSeq | V4 |
| Missailidis, 2019 [31] | 23 | ET | ART-naïve HIV-positive individuals > 18 years, CD4+ T cells counts > 350 cells/mL, and plasma viral loads > 1000 copies/mL | 5000 UI/day (plus phenylbutyrate suppl) for 16 weeks | Illumina MiSeq | V4 |
| Naderpoor, 2018 [32] | 26 | AU | Healthy adults, serum 25(OH)D levels < 20 ng/mL, BMI > 25, stable weight | 100,000 UI at baseline followed by 4000 UI/day for 16 weeks | Illumina MiSeq platform | V6–V8 |
| Sordillo, 2016 [33] | 261 | US | Pregnant women, aged 18–40 years, gestational age 10–18 weeks | Maternal VDS with 400 or 4000 UI/day for 22–30 weeks | Pyrosequencing 16S RNA gene | V3–V5 |
| Non-randomized interventional studies | ||||||
| Bashir, 2016 [49] | 16 | AT | Healthy adults, BMI 20–30, non-smokers | 980 UI/Kg (week 1–4), 490 UI/Kg (week 5–8) | GS FLX | V1–V2 |
| Bosman, 2019 [51] | 21 | CA | Healthy adults, aged 19–40 years, Fitzpatrick skin types I–III | Average 1389 UI/day | Illumina MiSeq | V6–V8 |
| Cantarel, 2015 [47] | 15 | US | Multiple Sclerosis/Healthy women, 25 (OH)D < 30 ng/mL, BMI 18–30 | 5000 UI/day for 90 days | PhyloChip Array | NR |
| Garg, 2018 [45] | 25 | GB | 25(OH)D < 50 ng/mL; For UC patients: partial Mayo index of ≤ 4, and stable therapy | 40,000 UI/week for 8 weeks | Illumina MiSeq | V3–V4 |
| Schäffler, 2018 [46] | 17 | DE | CD/Healthy adults, serum 25(OH)D levels < 30 ng/mL | 20,000 UI/day 1–3 + 20,000 UI every other day for 4 weeks | Illumina MiSeq | V3–V4 |
| Singh, 2020 [48] | 80 | QA | Healthy students, serum 25(OH)D levels < 30 ng/mL | 50,000 UI/week for 12 weeks | Illumina MiSeq. Metagenomic analysis PICRUST | V3–V4 |
| Tabatabaeizadeh, 2020 [50] | 50 | IR | Healthy young girls, no history of diabetes, hypertension, or chronic disease | 50,000 UI/week for 9 weeks | TaqMan assays | NR |
| Observational studies—Cohort | ||||||
| Drall, 2020 [34] | 1157 | CA, CHILD cohort | Pregnant women, gestational age 28 weeks | Maternal and infant VDS of 400 UI/day | Illumina MiSeq platform | V4 |
| Kassem, 2020 [35] | 499 | US, WHEALS cohort | Pregnant women, aged 21–49 years, gestational ages from 25 to 44 weeks | Maternal serum 25(OH)D and cord blood 25(OH)D levels | Illumina MiSeq | V4 |
| Mandal, 2016 [36] | 60 | NO, NoMIC cohort | Pregnant women | Dietary VD intakes during 22 weeks of pregnancy: 3.13 µg/day (median) | Illumina MiSeq platform | V4 |
| Talsness, 2017 [37] | 913 | NL, KOALA cohort | Pregnant women, gestational age 14–18 weeks | Maternal VDS: < or > 400 UI/day for 22–30 weeks. Infant VDS: classified as yes or no | 5′- nuclease technique | NR |
| Observational studies—Cross-sectional | ||||||
| Jackson, 2018 [39] | 1724 | GB, TwinsUK | Healthy adults | Use of VDS | Illumina MiSeq technology | V4 |
| Luthold, 2017 [40] | 150 | BR, NutriHS Study | Healthy students, aged 18–40 years, undergraduate or graduate from nutrition colleges | Dietary VD intakes (I: 1.66–4.95/II: 4.97–7.18/III: 7.56–39.87 µg/day) | Illumina MiSeq technology | V4 |
| Seura, 2017 [41] | 28 | JP | Healthy young women, aged 20–22 years, normal weight | Dietary VD intakes (3.5 ± 2.5 µg/day) | T-RFLP method | NR |
| Soltys, 2020 [38] | 87 | SK | UC and CD | Serum 25(OH)D levels | Illumina MiSeq | V4 |
| Thomas, 2020 [42] | 567 | US | Healthy men (community-dwelling), aged 65 years or older | VDS presents in 424 participants, not quantified. Measure of 25(OH)D; 1,25(OH)2D; 24,25(OH)2D | Illumina bcl2fastq | V4 |
| Wu, 2011 [43] | 98 | US | Healthy volunteers, aged 2 to 50 years | Dietary VD intakes | 454/Roche pyrosequencing. Additional metagenomic analysis with shotgun method | V1–V2 |
| Observational studies—Case-control | ||||||
| Weng, 2019 [44] | 113 | CN | Age >18 years and confirmed diagnosis of IBD (CD); BMI within the normal range and have not taken any antibiotics, probiotics, prebiotics or yogurt within the previous 4 weeks | Dietary VD intakes | Illumina MiSeq System. Additional metagenomic analysis with shotgun method | V4 |
| Author, Year | Comparison | Serum 25(OH) Levels | Sample | Alpha and Beta Diversity |
|---|---|---|---|---|
| Double-blind, randomized controlled trials | ||||
| Ciubotaru, 2015 [27] | Serum 25(OH)D: quintiles | Baseline: 14 ± 6 ng/mL Post: 36 ± 24 ng/mL | Stool | Alpha diversity: NS Beta diversity: significant different bacterial composition found in Q1 vs. Q4 of 25(OH)D at genus and family levels |
| Charoenngam, 2020 [28] | Different doses of VDS | Baseline: 16.9 ± 6.0 ng/mL; 20.3 ± 6.3 ng/mL; 18.5 ± 3.5 ng/mL Post: 20.0 ± 3.4 ng/mL; 39.0 ± 8.7 ng/mL; 67.3 ± 3.1 ng/mL | Stool | Alpha diversity: NS Beta diversity: NR |
| Hjelmsø, 2020 [29] | Different doses of prenatal VDS | Not reported | Infant stool | Alpha diversity: NS Beta diversity: NS |
| Kanhere, 2018 [30] | Supplemented group vs placebo group in vit D insufficient at baseline | Baseline: VD suff: 37 ± 6 ng/mL; VD insuff, Pl.: 22 ± 6; VD insuff, suppl.: 25 ± 5 ng/mL Post: VD insuff, Pl.: 25 ng/mL; VD insuff, suppl.: 45 ng/mL | Stool | Alpha diversity: NS Beta diversity: significantly different composition at follow-up in the supplemented group compared to the placebo |
| Missailidis, 2019 [31] | Supplemented group versus placebo group | Baseline: NR Post: NR | Mucosal gut biopsy | Alpha diversity: NS Beta diversity: NS |
| Naderpoor, 2019 [32] | Supplemented group versus placebo group | Baseline: VD group 31.54 ± 4.4 vs. Pl 31.07 ± 4.1 nmol/L Post: VD group 91.14 ± 25.8 vs. Pl 31.58 ± 14.11 nmol/L | Stool | Alpha diversity: significant reduction in richness at follow-up in the supplemented group Beta diversity: significant difference in composition between groups at follow-up at the genus level |
| Sordillo, 2016 [33] | Maternal VDS Umbilical cordon 25(OH)D levels | Baseline: 22.7 ± 11.9 ng/mL | Stool | Alpha diversity: NS Beta diversity: NR |
| Non-randomized interventional trials | ||||
| Bashir, 2016 [49] | Post- versus pre-supplementation | Baseline: 22.3 ± 13.1 ng/mL Post: 55.2 ± 13.3 ng/mL | Biopsy and stool | Alpha diversity: significant increased richness in GA Beta diversity: significant change in composition only in upper GI tract |
| Bosman, 2019 [51] | Prior VD supplemented group (VDS+) vs prior non-supplemented group (VDS-) before UVB exposure | Baseline: NR Post: NR | Stool | Alpha diversity: VDS- showed significantly lower diversity and richness before UVB exposure than VDS+ Beta diversity: NR |
| Cantarel, 2015 [47] | Post- versus pre-supplementation in healthy controls and in patients with multiple sclerosis | Baseline: 23.2 ± 5.7 ng/mL in the HCs; 25.9 ± 4.4 ng/mL in MS Post: 59.8 ± 11.7 ng/mL in the HCs; 55.6 ± 17.0 ng/mL in MSs | Stool | Alpha diversity:NS Beta diversity: NS |
| Garg, 2018 [45] | Post versus pre-supplementation | Baseline: 34 (range 12–49) nmol/L Post: 111 (range 71–158) nmol/L | Stool | Alpha diversity: NS Beta diversity: NS |
| Schäffler, 2018 [46] | Post versus pre-supplementation in healthy controls and in patients with CD | Baseline: in CD 39.7 ± 23 nmol/L, in HC 29.6 ± 6.3 nmol/L Post: in CD 121.4 ± 43.2 nmol/L, in HC 143.0 ± 25.2 nmol/L | Stool | Alpha diversity: In HC, NS; in CD taxa significantly decreased after VDS. Beta diversity: NS |
| Singh, 2020 [48] | Post- versus pre-supplementation | Baseline: 11.03 ± 0.51 ng/mL Post: 34.37 ± 1.47 ng/mL | Stool | Alpha diversity: Significant increase in observed OTUs and Chao1 indices, no difference in Shannon Index. Beta diversity: significant difference in composition between vs pro supplementation |
| Tabatabaeizadeh, 2020 [50] | Post- versus pre-supplementation | Baseline: 11 ± 9 ng/mL Post: 40 ± 17 ng/mL | Stool | Alpha diversity: NR Beta diversity: NR |
| Observational studies—Cohort | ||||
| Drall, 2020 [34] | Pre vs post maternal VDS and Infant VDS | Baseline: NR Post: NR | Stool | Alpha diversity: NR Beta diversity: NR |
| Talsness, 2017 [37] | Comparisons of 3 levels of maternal VDS; Infant VDS vs non-infant VDS | Baseline: 44.3 ± 18.3 nmol/L Post: NR | Stool | NR |
| Author, Year | Comparison | Serum 25(OH) Levels | Sample | Alfa and Beta Diversity |
|---|---|---|---|---|
| Observational studies—Cohort | ||||
| Kassem, 2020 [35] | Maternal serum 25(OH)D levels Umbilical cord blood 25(OH)D levels | Baseline maternal serum 25(OH)D: 25.04 ± 11.62 ng/mL Baseline umbilical cord blood 25(OH)D levels: 10.88 ± 6.77 ng/mL | Stool | Alpha diversity: Prenatal 25(OH)D level significantly associated with decreased infant richness and diversity at 1 month; cord 25(OH)D level was positively associated with infant gut evenness in White women and negatively associated with infant evenness at 6 months. Beta diversity: both prenatal and cord 25(OH)D were significantly associated with 1-month composition |
| Mandal, 2016 [36] | Dietary maternal VD intakes | Baseline: Not reported Post: Not reported | Alpha diversity: VD intake was significantly and inversely associated to whole tree phylogenetic and Shannon diversity Beta diversity: NS | |
| Observational studies—Cross-sectional | ||||
| Jackson, 2018 [39] | Intake of VD supplements: yes versus no | NR | Stool | Alpha diversity: NS Beta diversity: NS |
| Luthold, 2017 [40] | Dietary VD intakes: high versus low-tertile Serum 25(OH)D leves: high- versus low tertile | Baseline: 23.9 ± 9.7 ng/mL | Stool | Alpha diversity: NR Beta diversity: NR |
| Seura, 2017 [41] | Dietary VD intakes | Baseline: NR | Stool | Alpha diversity: NR Beta diversity: NR |
| Soltys, 2020 [38] | Serum VD levels in patients with UC and CD | Baseline: in winter/spring 25.05 ng/mL and summer/autumn period 37.26 ng/mL | Biopsy and stool | Alpha diversity: NR Beta diversity: NS |
| Thomas, 2020 [42] | VD metabolites | 25(OH)D (34.2 ng/mL), 1,25(OH)2D (56 pg/mL), and 24,25(OH)2D (3.2 ng/mL) | Stool | Alpha diversity: 1,25(OH)2D, active ratio and catabolism ratio are positively and significantly associated with diversity. Beta diversity: 1,25(OH)2D, 24,25(OH)2D, activation ratio, catabolism ratio significantly define clusters of microbial composition |
| Wu, 2011 [43] | Dietary VD intakes | Baseline: NR | Stool | Alpha diversity: NR Beta diversity: NR |
| Observational studies—Case-control | ||||
| Weng, 2019 [44] | Dietary VD intakes in healthy controls and patients with UC and CD | Baseline: NR | Biopsy and stool | Alpha diversity: NR in UC Beta diversity: NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. https://doi.org/10.3390/nu13103378
Bellerba F, Muzio V, Gnagnarella P, Facciotti F, Chiocca S, Bossi P, Cortinovis D, Chiaradonna F, Serrano D, Raimondi S, et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients. 2021; 13(10):3378. https://doi.org/10.3390/nu13103378
Chicago/Turabian StyleBellerba, Federica, Valeria Muzio, Patrizia Gnagnarella, Federica Facciotti, Susanna Chiocca, Paolo Bossi, Diego Cortinovis, Ferdinando Chiaradonna, Davide Serrano, Sara Raimondi, and et al. 2021. "The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies" Nutrients 13, no. 10: 3378. https://doi.org/10.3390/nu13103378
APA StyleBellerba, F., Muzio, V., Gnagnarella, P., Facciotti, F., Chiocca, S., Bossi, P., Cortinovis, D., Chiaradonna, F., Serrano, D., Raimondi, S., Zerbato, B., Palorini, R., Canova, S., Gaeta, A., & Gandini, S. (2021). The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients, 13(10), 3378. https://doi.org/10.3390/nu13103378










