The Association between Branched-Chain Amino Acids (BCAAs) and Cardiometabolic Risk Factors in Middle-Aged Caucasian Women Stratified According to Glycemic Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Blood Sampling & Laboratory Analyses
2.3. Statistical Analysis
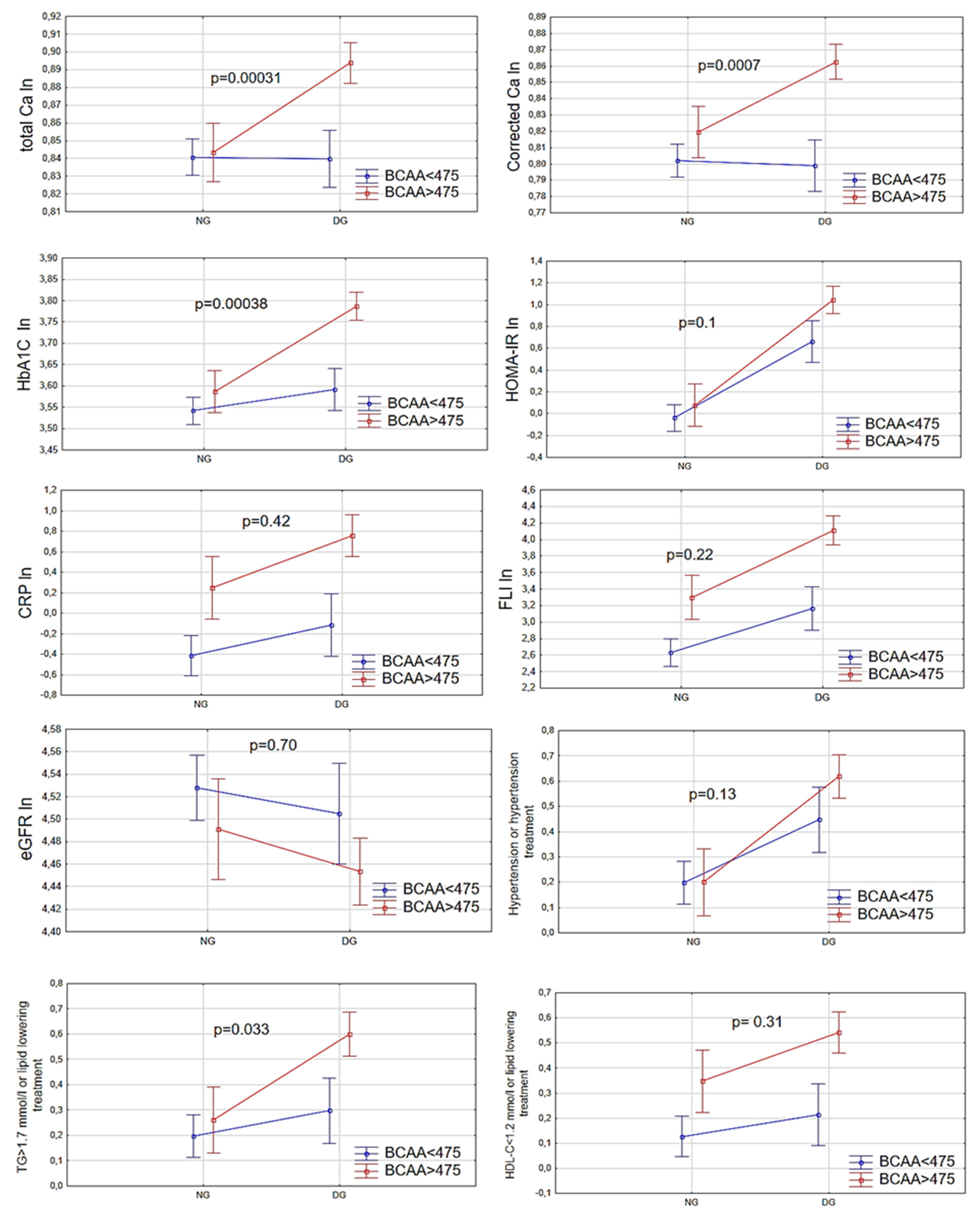
3. Results
4. Discussion
4.1. The Relationship of BCAAs with the Assessments of Glycemic Status
4.2. Potential Pathophysiology Linked BCAAs with Dysglycemia
4.3. The Association between BCAAs and Serum Calcium Concentration in Relation to Glycemic Status
4.4. Potential Pathophysiology Linked BCAAs Metabolism with Circulating Calcium Concentration
4.5. The Relationship of BCAAs with Other Conventional Cardiometabolic Risk Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Joshi, M.; Jeoung, N.H.; Obayashi, M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J. Nutr. 2005, 135, 1527S–1530S. [Google Scholar] [CrossRef] [PubMed]
- Górska-Warsewicz, H.; Laskowski, W.; Kulykovets, O.; Kudlińska-Chylak, A.; Czeczotko, M.; Rejman, K. Food products as sources of protein and amino acids—the case of Poland. Nutrients 2018, 10, 1977. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Yang, R.; Dong, J.; Zhao, H.; Li, H.; Guo, H.; Wang, S.; Zhang, C.; Wang, S.; Wang, M.; Yu, S.; et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS ONE 2014, 9, e99598. [Google Scholar] [CrossRef]
- Yang, P.; Hu, W.; Fu, Z.; Sun, L.; Zhou, Y.; Gong, Y.; Yang, T.; Zhou, H. The positive association of branched-chain amino acids and metabolic dyslipidemia in Chinese Han population. Lipids Health Dis. 2016, 15, 120. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ. Genom. Precis. Med. 2018, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mels, C.M.; Schutte, A.E.; Schütte, R.; Huisman, H.W.; Smith, W.; Fourie, C.M.; Kruger, R.; Van Rooyen, J.M.; Malan, N.T.; Malan, L. The link between vascular deterioration and branched chain amino acids in a population with high glycated haemoglobin: The SABPA study. Amino Acids 2013, 45, 1405–1413. [Google Scholar] [CrossRef]
- Mears, D. Regulation of insulin secretion in islets of langerhans by Ca2+Channels. J. Membr. Biol. 2004, 200, 57–66. [Google Scholar] [CrossRef]
- Conigrave, A.D.; Franks, A.H.; Brown, E.M.; Quinn, S.J. L-amino acid sensing by the calcium-sensing receptor: A general mechanism for coupling protein and calcium metabolism? Eur. J. Clin. Nutr. 2002, 56, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Gamble, G.D.; Bolland, M.J. Circulating calcium concentrations, vascular disease and mortality: A systematic review. J. Intern. Med. 2016, 279, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Osté, M.C.J.; Kieneker, L.M.; Gruppen, E.G.; Wolak-Dinsmore, J.; Otvos, J.D.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Plasma branched-chain amino acids and risk of incident type 2 diabetes: Results from the PREVEND prospective cohort study. J. Clin. Med. 2018, 7, 513. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Soininen, P.; Kangas, A.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2012, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Radikova, Z.; Koska, J.; Huckova, M.; Ksinantova, L.; Imrich, R.; Vigas, M.; Trnovec, T.; Langer, P.; Sebokova, E.; Klimes, I. Insulin sensitivity indices: A proposal of cut-off points for simple identification of insulin-resistant subjects. Exp. Clin. Endocrinol. Diabetes 2006, 114, 249–256. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Monk, R.D. Calcium. Lancet 1998, 352, 306–311. [Google Scholar] [CrossRef]
- Wolak-Dinsmore, J.; Gruppen, E.G.; Shalaurova, I.; Matyus, S.P.; Grant, R.P.; Gegen, R.; Bakker, S.J.; Otvos, J.D.; Connelly, M.A.; Dullaart, R.P. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: Elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin. Biochem. 2018, 54, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Crosslin, D.R.; Haynes, C.S.; Nelson, S.; Turer, C.B.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Laferrere, B.; et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2011, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Batch, B.C.; Shah, S.H.; Newgard, C.B.; Turer, C.B.; Haynes, C.; Bain, J.R.; Muehlbauer, M.; Patel, M.J.; Stevens, R.D.; Appel, L.J.; et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 2013, 62, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, P.; Zhang, X.; Pekkala, S.; Autio, R.; Kong, L.; Yang, Y.; Keinänen-Kiukaanniemi, S.; Alen, M.; Cheng, S. Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Sci. Rep. 2016, 6, 24540. [Google Scholar] [CrossRef]
- Fiehn, O.; Garvey, W.T.; Newman, J.; Lok, K.H.; Hoppel, C.L.; Adams, S. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE 2010, 5, e15234. [Google Scholar] [CrossRef] [PubMed]
- Barceló, A.; Morell-Garcia, D.; Salord, N.; Esquinas, C.; Pérez, G.; Pérez, A.; Monasterio, C.; Gasa, M.; Fortuna, A.M.; Montserrat, J.M.; et al. A randomized controlled trial: Branched-chain amino acid levels and glucose metabolism in patients with obesity and sleep apnea. J. Sleep Res. 2017, 26, 773–781. [Google Scholar] [CrossRef]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef]
- Bendinelli, B.; Palli, D.; Masala, G.; Sharp, S.J.; Schulze, M.B.; Guevara, M.; van der, A.D.; Sera, F.; Amiano, P.; Balkau, B.; et al. Association between dietary meat consumption and incident type 2 diabetes: The EPIC-InterAct study. Diabetologia 2013, 56, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Shah, S.H.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.; Slentz, C.A.; Tanner, C.J.; Kuchibhatla, M.; Houmard, J.A.; Newgard, C.B.; et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009, 32, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Vangipurapu, J.; Stancáková, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 finnish men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Li, C.; Gao, Z.; Stanley, C.A.; Matschinsky, F.M. Glutaminolysis and insulin secretion: From bedside to bench and back. Diabetes 2002, 51, S421–S426. [Google Scholar] [CrossRef]
- Krebs, M.; Brehm, A.; Krssak, M.; Anderwald, C.H.; Bernroider, E.; Nowotny, P.; Roth, E.; Chandramouli, V.; Landau, B.R.; Roden, M. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia 2003, 46, 917–925. [Google Scholar] [CrossRef]
- Tai, E.S.; Tan, M.L.S.; Stevens, R.D.; Low, Y.L.; Muehlbauer, M.J.; Goh, D.L.M.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Lee, J.J.M.; et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010, 53, 757–767. [Google Scholar] [CrossRef]
- Tobias, D.K.; Clish, C.; Mora, S.; Li, J.; Liang, L.; Hu, F.B.; Manson, J.E.; Zhang, C. Dietary intakes and circulating concentrations of branched-chain amino acids in relation to incident type 2 diabetes risk among high-risk women with a history of gestational diabetes mellitus. Clin. Chem. 2018, 64, 1203–1210. [Google Scholar] [CrossRef]
- Zhou, M.; Shao, J.; Wu, C.-Y.; Shu, L.; Dong, W.; Liu, Y.; Chen, M.; Wynn, R.M.; Wang, J.; Wang, J.; et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 2019, 68, 1730–1746. [Google Scholar] [CrossRef]
- Burrage, L.C.; Nagamani, S.C.; Campeau, P.M.; Lee, B.H. Branched-chain amino acid metabolism: From rare Mendelian diseases to more common disorders. Hum. Mol. Genet. 2014, 23, R1–R8. [Google Scholar] [CrossRef]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, Y.; Jonsson, A.; Have, C.T.; Allin, K.H.; Witte, D.R.; Jørgensen, M.E.; Grarup, N.; Pedersen, O.; Kilpeläinen, T.O.; Hansen, T. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia 2017, 60, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Morioka, S.; Kanai, K.; Kako, M.; Nakajima, T.; Yoshimi, T.; Masaka, M.; Sudo, K.; Kanno, T. Effects of branched chain amino acid infusion on glucose metabolism in cirrhotic patients with encephalopathy. Gastroenterol. Jpn. 1983, 18, 553–559. [Google Scholar] [CrossRef]
- Ruiz-Margáin, A.; Macías-Rodríguez, R.; Ríos-Torres, S.; Román-Calleja, B.; Guerrero, O.M.; Rodríguez-Córdova, P.; Torre, A. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Revista Gastroenterología México 2018, 83, 9–15. [Google Scholar] [CrossRef]
- Randolph, A.C.; Markofski, M.M.; Rasmussen, B.B.; Volpi, E. Effect of essential amino acid supplementation and aerobic exercise on insulin sensitivity in healthy older adults: A randomized clinical trial. Clin. Nutr. 2019, 39, 1371–1378. [Google Scholar] [CrossRef]
- Iwasa, M.; Ishihara, T.; Mifuji-Moroka, R.; Fujita, N.; Kobayashi, Y.; Hasegawa, H.; Iwata, K.; Kaito, M.; Takei, Y. Elevation of branched-chain amino acid levels in diabetes and NAFL and changes with antidiabetic drug treatment. Obes. Res. Clin. Pr. 2015, 9, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.K.; Xu, J.; Lawson, E.; DeOliveira, R.; Brandon, A.E.; Kraegen, E.W.; Ruderman, N. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 2010, 59, 2426–2434. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via. mTORC1 activation. Free. Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Zhu, J.; Xun, P.; Bae, J.C.; Kim, J.H.; Kim, D.J.; Yang, K.; He, K. Circulating calcium levels and the risk of type 2 diabetes: A systematic review and meta-analysis. Br. J. Nutr. 2019, 122, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Estruch, R.; Bulló, M.; Casas, R.; Díaz-López, A.; Basora, J.; Fitó, M.; Serra-Majem, L.; Salas-Salvadó, J. Increased serum calcium levels and risk of type 2 diabetes in individuals at high cardiovascular risk. Diabetes Care 2014, 37, 3084–3091. [Google Scholar] [CrossRef]
- Lorenzo, C.; Hanley, A.J.; Rewers, M.J.; Haffner, S.M. Calcium and phosphate concentrations and future development of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetologia 2014, 57, 1366–1374. [Google Scholar] [CrossRef]
- Hagström, E.; Hellman, P.; Lundgren, E.; Lind, L.; Ärnlöv, J. Serum calcium is independently associated with insulin sensitivity measured with euglycaemic–hyperinsulinaemic clamp in a community-based cohort. Diabetologia 2006, 50, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, Q.; Aziz, R.; Hamid, S. To evaluate the levels of glycated hemoglobin, serum calcium, magnesium, phosphate, uric acid and microalbuminuria in patients with newly diagnosed type 2 diabetes mellitus. Int. J. Res. Med Sci. 2014, 2, 1462. [Google Scholar] [CrossRef][Green Version]
- Kanchana, N.; Saikumar, P. Serum calcium levels in type 2 diabetes mellitus. IOSR-JDMS 2014, 13, 109–114. [Google Scholar]
- Akter, S.; Eguchi, M.; Kochi, T.; Kabe, I.; Nanri, A.; Mizoue, T. Association of serum calcium and phosphate concentrations with glucose metabolism markers: The furukawa nutrition and health study. Nutrients 2020, 12, 2344. [Google Scholar] [CrossRef]
- Hassan, S.A.E.; Elsheikh, W.A.R.; Rahman, N.I.A.; Elbagir, N.M. Serum calcium levels in correlation with glycated hemoglobin in type 2 diabetic sudanese patients. Adv. Diabetes Metab. 2016, 4, 59–64. [Google Scholar] [CrossRef]
- Marshnil, R.W.; Mythili, C. Correlation of serum calcium levels with glycated hemoglobin in type 2 diabetes mellitus patient-a comparative study. IJSR 2018, 7, 4–6. [Google Scholar]
- Buege, M.J.; Do, B.; Lee, H.C.; Weber, D.M.; Horowitz, S.B.; Feng, L.; Qing, Y.; Shank, B.R. Corrected calcium versus ionized calcium measurements for identifying hypercalcemia in patients with multiple myeloma. Cancer Treat. Res. Commun. 2019, 21, 100159. [Google Scholar] [CrossRef]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5, S23–S30. [Google Scholar] [CrossRef]
- Sun, G.; Vasdev, S.; Martin, G.; Gadag, V.; Zhang, H. Altered calcium homeostasis is correlated with abnormalities of fasting serum glucose, insulin resistance, and -cell function in the newfoundland population. Diabetes 2005, 54, 3336–3339. [Google Scholar] [CrossRef]
- Rooney, M.R.; Pankow, J.S.; Sibley, S.D.; Selvin, E.; Reis, J.P.; Michos, E.D.; Lutsey, P.L. Serum calcium and incident type 2 diabetes: The atherosclerosis risk in communities (ARIC) study. Am. J. Clin. Nutr. 2016, 104, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Muller, D.; Squires, P.; Asare-Anane, H.; Huang, G.-C.; Amiel, S.; Persaud, S.; Jones, P.M. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of langerhans: Involvement of protein kinases. J. Endocrinol. 2006, 190, 703–710. [Google Scholar] [CrossRef]
- Henquin, J.-C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Curry, D.L.; Bennett, L.L.; Grodsky, G.M. Requirement for calcium ion in insulin secretion by the perfused rat pancreas. Am. J. Physiol. Content 1968, 214, 174–178. [Google Scholar] [CrossRef]
- Draznin, B.; Sussman, K.E.; Eckel, R.H.; Kao, M.; Yost, T.; Sherman, N.A. Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J. Clin. Investig. 1988, 82, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Scheffler, T.L.; Gunawan, A.M.; Shi, H.; Zeng, C.; Hannon, K.M.; Grant, A.L.; Gerrard, D.E. Chronic elevated calcium blocks AMPK-induced GLUT-4 expression in skeletal muscle. Am. J. Physiol. Physiol. 2009, 296, C106–C115. [Google Scholar] [CrossRef] [PubMed]
- Mercan, F.; Lee, H.; Kolli, S.; Bennett, A.M. Novel role for SHP-2 in nutrient-responsive control of s6 kinase 1 signaling. Mol. Cell. Biol. 2012, 33, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wong, R.K.; Park, M.; Wu, J.; Cook, J.R.; York, D.A.; Deng, S.; Markmann, J.; Naji, A.; Wolf, B.A.; et al. Leucine regulation of glucokinase and ATP synthase sensitizes glucose-induced insulin secretion in pancreatic β-cells. Diabetes 2006, 55, 193–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, C.; Najafi, H.; Daikhin, Y.; Nissim, I.B.; Collins, H.W.; Yudkoff, M.; Matschinsky, F.M.; Stanley, C. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J. Biol. Chem. 2003, 278, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Li, D.-Q.; Olofsson, C.; Salehi, A.; Surve, V.V.; Caballero, J.; Ivarsson, R.; Lundquist, I.; Pereverzev, A.; Schneider, T.; et al. CaV2.3 calcium channels control second-phase insulin release. J. Clin. Investig. 2005, 115, 146–154. [Google Scholar] [CrossRef]
- Aoki, K.; Miyagawa, K. Correlation of increased serum calcium with elevated blood pressure and vascular resistance during calcium infusion in normotensive man. J. Hypertens. 1990, 8, 579–583. [Google Scholar] [CrossRef]
- Mook-Kanamori, D.O.; Römisch-Margl, W.; Kastenmüller, G.; Prehn, C.; Petersen, A.K.; Illig, T.; Gieger, C.; Wang-Sattler, R.; Meisinger, C.; Peters, A.; et al. Increased amino acids levels and the risk of developing of hypertriglyceridemia in a 7-year follow-up. J. Endocrinol. Investig. 2014, 37, 369–374. [Google Scholar] [CrossRef]
- Yamakado, M.; Nagao, K.; Imaizumi, A.; Tani, M.; Toda, A.; Tanaka, T.; Jinzu, H.; Miyano, H.; Yamamoto, H.; Daimon, T.; et al. Plasma free amino acid profiles predict four-year risk of developing diabetes, metabolic syndrome, dyslipidemia and hypertension in Japanese population. Sci. Rep. 2015, 5, 11918. [Google Scholar] [CrossRef]
- Fukushima, K.; Harada, S.; Takeuchi, A.; Kurihara, A.; Iida, M.; Fukai, K.; Kuwabara, K.; Kato, S.; Matsumoto, M.; Hirata, A.; et al. Association between dyslipidemia and plasma levels of branched-chain amino acids in the Japanese population without diabetes mellitus. J. Clin. Lipidol. 2019, 13, 932–939.e2. [Google Scholar] [CrossRef] [PubMed]
- Halama, A.; Horsch, M.; Kastenmüller, G.; Möller, G.; Kumar, P.; Prehn, C.; Laumen, H.; Hauner, H.; de Angelis, M.H.; Beckers, J.; et al. Metabolic switch during adipogenesis: From branched chain amino acid catabolism to lipid synthesis. Arch. Biochem. Biophys. 2016, 589, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.A.; Gil-Gómez, G.; Casaroli-Marano, R.P.; Vilaró, S.; Hegardt, F.G.; Haro, D. Transfection of the ketogenic mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase cDNA into Mev-1 cells corrects their auxotrophy for mevalonate. J. Biol. Chem. 1994, 269, 28523–28526. [Google Scholar] [CrossRef]
- Bakan, I.; Laplante, M. Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 2012, 23, 226–234. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell 2018, 9, 145–151. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via. lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.H.V.D.; Flores-Guerrero, J.L.; Gruppen, E.G.; De Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-alcoholic fatty liver disease and risk of incident type 2 diabetes: Role of circulating branched-chain amino acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Bugianesi, E.; Gastaldelli, A.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Kalavalapalli, S.; Bril, F.; Garrett, T.J.; Nautiyal, M.; Mathew, J.T.; Williams, C.M.; Cusi, K. Cross-talk between branched-chain amino acids and hepatic mitochondria is compromised in nonalcoholic fatty liver disease. Am. J. Physiol. Metab. 2015, 309, E311–E319. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Bodoy, S.; Fotiadis, D.; Stoeger, C.; Kanai, Y.; Palacín, M. The small SLC43 family: Facilitator system l amino acid transporters and the orphan EEG1. Mol. Asp. Med. 2013, 34, 638–645. [Google Scholar] [CrossRef]
- Hamaya, R.; Mora, S.; Lawler, P.R.; Cook, N.R.; Ridker, P.M.; Buring, J.E.; Lee, I.-M.; Manson, J.E.; Tobias, D.K. Association of plasma branched-chain amino acid with biomarkers of inflammation and lipid metabolism in women. Circ. Genom. Precis. Med. 2021, 14. [Google Scholar] [CrossRef]
- Sun, L.; Hu, C.; Yang, R.; Lv, Y.; Yuan, H.; Liang, Q.; He, B.; Pang, G.; Jiang, M.; Dong, J.; et al. Association of circulating branched-chain amino acids with cardiometabolic traits differs between adults and the oldest-old. Oncotarget 2017, 8, 88882–88893. [Google Scholar] [CrossRef] [PubMed]
- Beckett, P.R.; Hardin, D.S.; Davis, T.A.; Nguyen, H.V.; Wray-Cahen, D.; Copeland, K.C. Spectrophometric assay for measuring branched-chain amino acid concentrations: Application for measuring the sensitivity of protein metabolism to insulin. Anal. Biochem. 1996, 240, 48–53. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Women with Normoglycemia n = 184 | Women with Dysglycemia n = 165 | p |
|---|---|---|---|
| BCAAs [µmol/L] | 433.0 (407.2–493.8) | 545.0 (468.1–620.9) $ | <0.0001 |
| Age [years] | 48.4 ± 6.0 | 51.2 ± 5.5 | <0.0001 |
| BMI [kg/m2] | 24.1 (21.9–28.4) | 31.2 (25.8–37.7) | <0.0001 |
| WC [cm] | 82.5 (76.5–91.5) | 98.0 (87.0–110.0) | <0.0001 |
| Glucose [mmol/L] | 5.1 (4.8–5.2) | 5.8 (5.4–6.9) | <0.0001 |
| TC [mmol/L] | 5.6 ± 1.0 | 5.5 ± 1.2 | 0.18 |
| LDL-C [mmol/L] | 3.5 ± 0.9 | 3.4 ± 1.0 | 0.06 |
| HDL-C [mmol/L] | 1.6 (1.4–1.9) | 1.4 (1.2–1.7) | <0.0001 |
| TG [mmol/L] | 1.0 (0.8–1.3) | 1.5 (1.1–2.0) | <0.0001 |
| TG/HDL-C | 3.4 (3.0–4.0) | 3.7 (3.3–4.5) | <0.0001 |
| Creatinine [mg/dL] | 0.76 ± 0.11 | 0.78 ± 0.12 | 0.11 |
| eGFR [ml/min/1.73 m2] | 94.1 (84.8–101.7) | 89.6 (78.5–100.4) | 0.008 |
| CRP [mg/L] | 0.82 (0.33–1.76) | 1.70 (0.77–4.23) | <0.0001 |
| HbA1c [mmol/mol] | 36.0 (33.0–37.0) | 39.0 (36.0–44.5) | <0.0001 |
| TSH [mIU/L] | 1.4 (1.1–2.0) | 1.4 (1.0–2.1) | 0.8 |
| Insulin [µIU/mL] | 5.1 (3.7–6.9) | 10.0 (6.3–13.0) | <0.0001 |
| HOMA-IR | 1.11 (0.79–1.56) | 2.6 (1.9–3.6) | <0.0001 |
| TCa [mmol/L] | 2.32 (2.26–2.40) | 2.40 (2.30–2.51) | <0.0001 |
| CCa [mmol/L] | 2.24 (2.18–2.32) | 2.33 (2.22–2.44) | <0.0001 |
| Albumin [g/L] | 44.0 (42.9–45.6) | 43.7 (42.4–45.9) | 0.40 |
| FLI | 16.0 (8.1–37.7) | 64.4 (30.4–93.5) | <0.0001 |
| GGT [U/L] | 17.4 (13.8–21.9) | 22.4 (16.4–33.3) | <0.0001 |
| SBP [mmHg] | 118 (109–130) | 130 (120–140) | <0.0001 |
| DBP [mmHg] | 78 (71–84) | 80 (78–88) | <0.0001 |
| Postmenopausal status [%] | 38 | 56 | 0.0004 |
| Lipid lowering therapy [%] | 11 | 28 | 0.0001 |
| Hypertension treatment [%] | 13 | 45 | <0.0001 |
| Diabetes [%] | 0 | 43 | <0.0001 |
| Obesity [BMI ≥ 30%] | 30 | 70 | <0.0001 |
| Current smoker [%] | 17 | 20 | 0.47 |
| Physically active: never or sporadically [%] | 31 | 32 | 0.84 |
| Parameters | Total Group r0/r0 * | Women with Normoglycemia r1/r1 * | Women with Dysglycemia r2/r2 * | p Unadjusted r1 vs. r2 |
|---|---|---|---|---|
| Age | 0.24/- | 0.17/- | 0.25/- | ns |
| BMI | 0.61/- | 0.49/- | 0.54/- | ns |
| TCa | 0.37/0.17 | 0.05 ns/- | 0.46/0.31 | <0.0001 |
| CCa | 0.47/0.21 | 0.23/0.06 ns | 0.51/0.33 | 0.002 |
| HbA1c | 0.47/0.27 | 0.12 ns | 0.41/0.30 | 0.004 |
| HOMA-IR | 0.41/0.18 | 0.07 ns | 0.27/0.16 | 0.05 |
| Glucose | 0.40/0.32 | 0.10 ns | 0.30/0.26 | 0.05 |
| TC | −0.22/−0.13 | −0.17/−0.18 | −0.22/−0.09 ns | ns |
| LDL-C | −0.20/−0.09 ns | −0.12 ns/- | −0.19/−0.07 ns | ns |
| HDL-C | −0.46/−0.20 | −0.33/−0.17 | −0.46/−0.26 | ns |
| TG | 0.38/0.14 | 0.19/0.07 ns | 0.33/0.14 ns | ns |
| TG/HDL-C | 0.47/0.20 | 0.28/0.12 ns | 0.43/0.21 | ns |
| TC/HDL-C | 0.28/0.10 ns | 0.18/0.04 ns | 0.28/0.15 ns | ns |
| eGFR | −0.29/−011 ns | −0.28/−0.22 | −0.23/−0.02 ns | ns |
| FLI | 0.57/0.10 ns | 0.49/0.008 ns | 0.48/0.10 ns | ns |
| CRP | 0.44/0.08 ns | 0.36/0.17 | 0.35/0.04 ns | ns |
| SBP | 0.18/0.004 ns | 0.06 ns/- | 0.08 ns/- | ns |
| DBP | 0.07 ns/- | −0.02 ns/- | −0.09 ns/- | ns |
| Cardiometabolic Risk Factor | Total Group | NG Group | DG Group | Total Group | NG Group | DG Group |
|---|---|---|---|---|---|---|
| Unadjusted ORs (95%CI) per10 unit increase in BCAAs | Adjusted for age and BMI ORs (95%CI) per10 unit increase in BCAAs | |||||
| CCa > 2.38 [mmol/L] | 1.15 (1.11–1.19) $ | 1.11 (1.04–1.18) & | 1.16 (1.10–1.22) $ | 1.09 (1.05–1.14) $ | 1.03 (0.96–1.11) | 1.13 (1.07–1.19) $ |
| TCa > 2.45 [mmol/L] | 1.11 (1.08–1.15) $ | 1.07 (1.01–1.13) * | 1.12 (1.07–1.17) $ | 1.07 (1.03–1.11) & | 1.01 (0.94–1.07) | 1.10 (1.05–1.15) $ |
| HbA1c > 39 [mmol/mol] | 1.10 (1.07–1.13) $ | 1.03 (0.97–1.10) | 1.11 (1.06–1.15) $ | 1.10 (1.06–1.15) $ | 1.03 (0.95–1.10) | 1.09 (1.04–1.14) & |
| FLI > 60 | 1.17 (1.13–1.21) $ | 1.19 (1.15–1.25) $ | 1.13 (1.08–1.17) $ | 1.04 (0.97–1.09) | 1.06 (0.95–1.15) | 0.98 (0.90–1.04) |
| eGFR < 90 mL/min/1.73 m2 | 1.06 (1.03–1.09) $ | 1.09 (1.04–1.15) & | 1.06 (1.03–1.09) * | 1.04 (1.0–1.07) * | 1.08 (1.02–1.15) * | 1.02 (0.97–1.06) |
| TG > 1.7 [mmol/L] | 1.06 (1.04–1.09) $ | 1.03 (0.98–1.09) | 1.04 (1.07–1.08) * | 1.04 (1.0–1.07) * | 0.98 (0.92–1.04) | 1.03 (0.99–1.07) |
| HDL < 1.2 [mmol/L] | 1.09 (1.06–1.12) $ | 1.09 (1,04–1.16) & | 1.07 (1.03–1.12) * | 1.05 (1.01–1.08) & | 1.04 (0.97–1.12) | 1.05 (1.10–1.09) * |
| CRP > 3.0 [mg/L] | 1.10 (1.07–1.13) $ | 1.12 (1.05–1.20) & | 1.07 (1.03-1.11) & | 1.0 (0.96–1.05) | 0.90 (0.91–1.07) | 0.99 (0.95–1.04) |
| TC/HDL-C > 4.5 | 1.05 (1.02–1.07) & | 1.03 (0.98–1.08) | 1.02 (0.99–1.06) | 1.01 (0.98–1.04) | 0.97 (0.92–1.0) | 1.01 (0.97–1.05) |
| Hypertension or therapy | 1.07 (1.04–1.10) $ | 1.03 (1.0–1.08) | 1.04 (1.0–1.10) * | 1.02 (0.98–1.05) | 0.98 (0.92–1.05) | 0.99 (0.96–1.04) |
| Cardiometabolic Risk Factor | Total Group AUC (95% CI) | NG Group AUC (95% CI) | DG Group AUC (95% CI) | p NG vs. DG |
|---|---|---|---|---|
| CCa > 2.38 mmol/L | 0.81 (0.76–0.85) | 0.67 (0.59–0.74) | 0.83 (0.76–0.89) | 0.043 |
| TCa > 2.45 mmol/L | 0.75 (0.69–0.79) | 0.61 (0.54–0.69) | 0.77 (0.69–0.83) | 0.048 |
| HbA1c > 39 mmol/mol | 0.76 (0.70–0.80) | 0.60 (0.52–0.68) | 0.73 (0.63–0.78) | 0.115 |
| FLI > 60 | 0.84 (0.79–0.88) | 0.85 (0.79–0.91) | 0.75 (0.68–0.82) | 0.111 |
| eGFR < 90 mL/min/1.73 m2 | 0.65 (0.60–0.71) | 0.67 (0.59–0.74) | 0.65 (0.59–0.71) | 0.621 |
| TG > 1.7 mmol/L | 0.69 (0.63–0.74) | 0.57 (0.47–0.63) | 0.63 (0.55–0.77) | 0.273 |
| HDL < 1.2 mmol/L | 0.75 (0.69–0.79) | 0.67 (0.59–0.74) | 0.68 (0.60–0.75) | 0.874 |
| CRP > 3.0 mg/L | 0.77 (0.72–0.81) | 0.79 (0.71–0.84) | 0.68 (0.60–0.76) | 0.191 |
| TC/HDL-C > 4.5 | 0.65 (0.59–0.70) | 0.58 (0.49–0.65) | 0.57 (0.50–0.66) | 0.817 |
| Hypertension or therapy | 0.67 (0.61–0.72) | 0.54 (0.46–0.62) | 0.60 (0.51–0.67) | 0.487 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubacka, J.; Cembrowska, P.; Sypniewska, G.; Stefanska, A. The Association between Branched-Chain Amino Acids (BCAAs) and Cardiometabolic Risk Factors in Middle-Aged Caucasian Women Stratified According to Glycemic Status. Nutrients 2021, 13, 3307. https://doi.org/10.3390/nu13103307
Kubacka J, Cembrowska P, Sypniewska G, Stefanska A. The Association between Branched-Chain Amino Acids (BCAAs) and Cardiometabolic Risk Factors in Middle-Aged Caucasian Women Stratified According to Glycemic Status. Nutrients. 2021; 13(10):3307. https://doi.org/10.3390/nu13103307
Chicago/Turabian StyleKubacka, Justyna, Paulina Cembrowska, Grazyna Sypniewska, and Anna Stefanska. 2021. "The Association between Branched-Chain Amino Acids (BCAAs) and Cardiometabolic Risk Factors in Middle-Aged Caucasian Women Stratified According to Glycemic Status" Nutrients 13, no. 10: 3307. https://doi.org/10.3390/nu13103307
APA StyleKubacka, J., Cembrowska, P., Sypniewska, G., & Stefanska, A. (2021). The Association between Branched-Chain Amino Acids (BCAAs) and Cardiometabolic Risk Factors in Middle-Aged Caucasian Women Stratified According to Glycemic Status. Nutrients, 13(10), 3307. https://doi.org/10.3390/nu13103307






