Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease
Abstract
1. Introduction
2. Parkinson’s Disease (PD) as Brain–Gut Disorder
3. Gut Microbiome
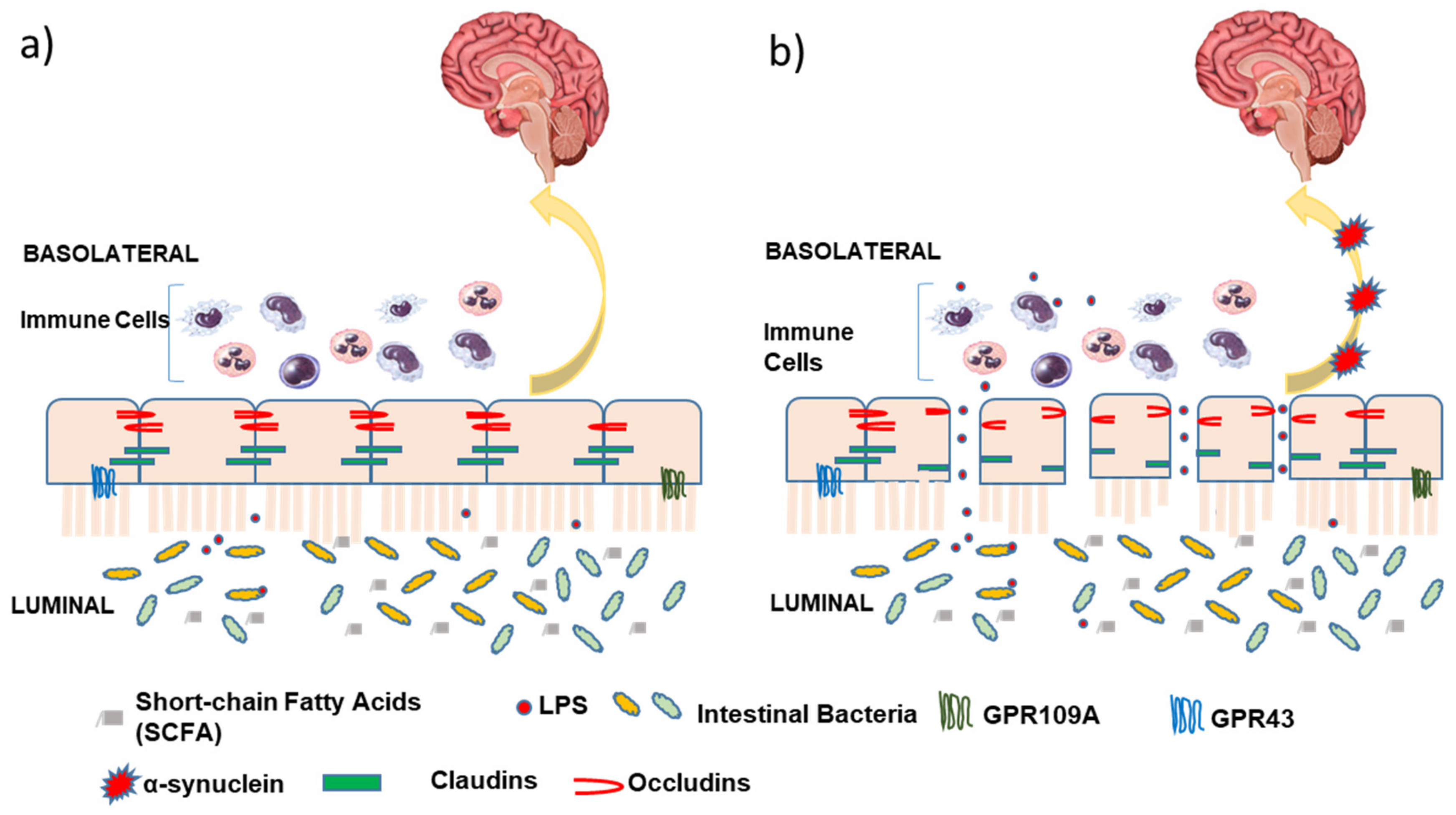
4. Intestinal Barrier
5. GPR109A
6. Butyrate
7. Niacin
8. Reactive Oxygen Species (ROS)
9. Other Nutrients
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary bile acids and short chain fatty acids in the colon: A focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Danese, S. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J. Gastroenterol. 2011, 17, 557. [Google Scholar] [CrossRef] [PubMed]
- Saurman, V.; Margolis, K.G.; Luna, R.A. Autism Spectrum Disorder as a Brain-Gut-Microbiome Axis Disorder. Dig. Dis. Sci. 2020, 65, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Vazquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef]
- Dong, T.S.; Gupta, A.; Jacobs, J.P.; Lagishetty, V.; Gallagher, E.; Bhatt, R.R.; Vora, P.; Osadchiy, V.; Stains, J.; Balioukova, A.; et al. Improvement in Uncontrolled Eating Behavior after Laparoscopic Sleeve Gastrectomy Is Associated with Alterations in the Brain-Gut-Microbiome Axis in Obese Women. Nutrients 2020, 12, 2924. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef]
- Luna, R.A.; Oezguen, N.; Balderas, M.; Venkatachalam, A.; Runge, J.K.; Versalovic, J.; Veenstra-VanderWeele, J.; Anderson, G.M.; Savidge, T.; Williams, K.C. Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 218–230. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Chen, G.; Huang, B.; Fu, S.; Li, B.; Ran, X.; He, D.; Jiang, L.; Li, Y.; Liu, B.; Xie, L.; et al. G Protein-Coupled Receptor 109A and Host Microbiota Modulate Intestinal Epithelial Integrity During Sepsis. Front. Immunol. 2018, 9, 2079. [Google Scholar] [CrossRef]
- D’Souza, W.N.; Douangpanya, J.; Mu, S.; Jaeckel, P.; Zhang, M.; Maxwell, J.R.; Rottman, J.B.; Labitzke, K.; Willee, A.; Beckmann, H.; et al. Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PLoS ONE 2017, 12, e0180190. [Google Scholar] [CrossRef]
- Feng, W.; Wu, Y.; Chen, G.; Fu, S.; Li, B.; Huang, B.; Wang, D.; Wang, W.; Liu, J. Sodium Butyrate Attenuates Diarrhea in Weaned Piglets and Promotes Tight Junction Protein Expression in Colon in a GPR109A-Dependent Manner. Cell Physiol. Biochem. 2018, 47, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C. GPR109a: The missing link between microbiome and good health? Immunity 2014, 40, 8–10. [Google Scholar] [CrossRef][Green Version]
- Giri, B.; Belanger, K.; Seamon, M.; Bradley, E.; Purohit, S.; Chong, R.; Morgan, J.C.; Baban, B.; Wakade, C. Niacin Ameliorates Neuro-Inflammation in Parkinson’s Disease via GPR109A. Int. J. Mol. Sci. 2019, 20, 4559. [Google Scholar] [CrossRef]
- Felice, V.D.; Quigley, E.M.; Sullivan, A.M.; O’Keeffe, G.W.; O’Mahony, S.M. Microbiota-gut-brain signalling in Parkinson’s disease: Implications for non-motor symptoms. Parkinsonism Relat. Disord. 2016, 27, 1–8. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Autonomic Dysfunction in Parkinson’s Disease. Neurotherapeutics 2020, 134, 104700. [Google Scholar] [CrossRef]
- Lubomski, M.; Davis, R.L.; Sue, C.M. Gastrointestinal dysfunction in Parkinson’s disease. J. Neurol. 2020, 267, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Curr. Treat. Options Neurol. 2018, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lin, J.-W.; Liu, Y.-C.; Chang, C.-H.; Wu, R.-M. Risk of Parkinson’s disease following severe constipation: A nationwide population-based cohort study. Parkinsonism Relat. Disord. 2014, 20, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kurek, J.; Morgan, J.C.; Wakade, C.; Rao, S.S.C. Constipation in Parkinson’s Disease: A Nuisance or Nuanced Answer to the Pathophysiological Puzzle? Curr. Gastroenterol. Rep. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Gandhy, R.; Barlow, C.; Triadafilopoulos, G. Utility of high-resolution anorectal manometry and wireless motility capsule in the evaluation of patients with Parkinson’s disease and chronic constipation. BMJ Open Gastroenterol. 2016, 3, e000118. [Google Scholar] [CrossRef]
- Sharma, A.; Yan, Y.; Karunaratne, T.; Herekar, A.A.; Kurek, J.; Morgan, J.; Rao, S.S. S0500 Intestinal Methanogen Overgrowth (IMO) in Parkinson’s Disease: High Prevalence and Correlation with Constipation. Off. J. Am. Coll. Gastroenterol. 2020, 115, S249–S250. [Google Scholar] [CrossRef]
- Sharma, A.; Yan, Y.; Xiang, X.; Fiedler, S.; Parr, R.; Herekar, A.; Jimenez, E.; Kurek, J.A.; Morgan, J.C.; Rao, S.S.C. 353 Parkinson’s disease: An efferent brain-gut disorder with severe anorectal hyposensitivity. Gastroenterology 2020, 158. [Google Scholar] [CrossRef]
- Hilton, D.; Stephens, M.; Kirk, L.; Edwards, P.; Potter, R.; Zajicek, J.; Broughton, E.; Hagan, H.; Carroll, C. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014, 127, 235–241. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef]
- Stolzenberg, E.; Berry, D.; Yang, D.; Lee, E.Y.; Kroemer, A.; Kaufman, S.; Wong, G.C.; Oppenheim, J.J.; Sen, S.; Fishbein, T. A role for neuronal alpha-synuclein in gastrointestinal immunity. J. Innate Immun. 2017, 9, 456–463. [Google Scholar] [CrossRef]
- Wang, S.; Chu, C.-H.; Stewart, T.; Ginghina, C.; Wang, Y.; Nie, H.; Guo, M.; Wilson, B.; Hong, J.-S.; Zhang, J. α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E1926–E1935. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.R.; Lee, B.D. Function and dysfunction of leucine-rich repeat kinase 2 (LRRK2): Parkinson’s disease and beyond. BMB Rep. 2015, 48, 243. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-L.; Lin, C.-H. Altered gut microbiome and intestinal pathology in Parkinson’s disease. J. Mov. Disord. 2019, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K. Where and how alpha-synuclein pathology spreads in Parkinson’s disease. Neuropathology 2020. [Google Scholar] [CrossRef]
- Shen, L. Gut, oral and nasal microbiota and Parkinson’s disease. Microb. Cell Factories 2020, 19, 1–7. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.-H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Boka, G.; Anglade, P.; Wallach, D.; Javoy-Agid, F.; Agid, Y.; Hirsch, E. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 1994, 172, 151–154. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009, 23, 55–63. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F.; Qin, B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.-D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Bessac, A.; Cani, P.D.; Meunier, E.; Dietrich, G.; Knauf, C. Inflammation and gut-brain axis during type 2 diabetes: Focus on the crosstalk between intestinal immune cells and enteric nervous system. Front. Neurosci. 2018, 12, 725. [Google Scholar] [CrossRef]
- Chalazonitis, A.; Rao, M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res. 2018, 1693, 207–213. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Wallen, Z.D.; Appah, M.; Dean, M.N.; Sesler, C.L.; Factor, S.A.; Molho, E.; Zabetian, C.P.; Standaert, D.G.; Payami, H. Characterizing dysbiosis of gut microbiome in PD: Evidence for overabundance of opportunistic pathogens. NPJ Parkinson’s Dis. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson’s Dis. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Wakade, C.; Chong, R.; Bradley, E.; Thomas, B.; Morgan, J. Upregulation of GPR109A in Parkinson’s disease. PLoS ONE 2014, 9, e109818. [Google Scholar] [CrossRef]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Lankelma, J.M.; van Vught, L.A.; Belzer, C.; Schultz, M.J.; van der Poll, T.; de Vos, W.M.; Wiersinga, W.J. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: A pilot study. Intensive Care Med. 2017, 43, 59–68. [Google Scholar] [CrossRef]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef]
- Bender, D.A.; Earl, C.J.; Lees, A.J. Niacin depletion in Parkinsonian patients treated with L-dopa, benserazide and carbidopa. Clin. Sci. (London) 1979, 56, 89–93. [Google Scholar] [CrossRef]
- Tunaru, S.; Lattig, J.; Kero, J.; Krause, G.; Offermanns, S. Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G). Mol. Pharm. 2005, 68, 1271–1280. [Google Scholar] [CrossRef]
- Wakade, C.; Giri, B.; Malik, A.; Khodadadi, H.; Morgan, J.C.; Chong, R.K.; Baban, B. Niacin modulates macrophage polarization in Parkinson’s disease. J. Neuroimmunol. 2018, 320, 76–79. [Google Scholar] [CrossRef]
- Alisky, J.M. Niacin improved rigidity and bradykinesia in a Parkinson’s disease patient but also caused unacceptable nightmares and skin rash—A case report. Nutr. Neurosci. 2005, 8, 327–329. [Google Scholar] [CrossRef]
- Wakade, C.; Chong, R.; Bradley, E.; Morgan, J.C. Low-dose niacin supplementation modulates GPR109A, niacin index and ameliorates Parkinson’s disease symptoms without side effects. Clin. Case Rep. 2015, 3, 635–637. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc Child Neurol. Soc. 2003, 53, S26–S38. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef]
- Fu, S.-P.; Wang, J.-F.; Xue, W.-J.; Liu, H.-M.; Liu, B.-r.; Zeng, Y.-L.; Li, S.-N.; Huang, B.-X.; Lv, Q.-K.; Wang, W. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 1–14. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Sun, J.; Gong, Q.; Ma, H.; Kan, X.; Cao, Y.; Wang, J.; Fu, S. Butyrate alleviates oxidative stress by regulating NRF2 nuclear accumulation and H3K9/14 acetylation via GPR109A in bovine mammary epithelial cells and mammary glands. Free Radic. Biol. Med. 2020, 152, 728–742. [Google Scholar] [CrossRef]
- Van den Bos, F.; Speelman, A.D.; van Nimwegen, M.; van der Schouw, Y.T.; Backx, F.J.; Bloem, B.R.; Munneke, M.; Verhaar, H.J. Bone mineral density and vitamin D status in Parkinson’s disease patients. J. Neurol. 2013, 260, 754–760. [Google Scholar] [CrossRef]
- Lin, A.M.; Fan, S.F.; Yang, D.M.; Hsu, L.L.; Yang, C.H. Zinc-induced apoptosis in substantia nigra of rat brain: Neuroprotection by vitamin D3. Free Radic. Biol. Med. 2003, 34, 1416–1425. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wu, J.N.; Cherng, T.L.; Hoffer, B.J.; Chen, H.H.; Borlongan, C.V.; Wang, Y. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001, 904, 67–75. [Google Scholar] [CrossRef]
- Hiller, A.L.; Murchison, C.F.; Lobb, B.M.; O’Connor, S.; O’Connor, M.; Quinn, J.F. A randomized, controlled pilot study of the effects of vitamin D supplementation on balance in Parkinson’s disease: Does age matter? PLoS ONE 2018, 13, e0203637. [Google Scholar] [CrossRef]
- Suzuki, M.; Yoshioka, M.; Hashimoto, M.; Murakami, M.; Noya, M.; Takahashi, D.; Urashima, M. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am. J. Clin. Nutr. 2013, 97, 1004–1013. [Google Scholar] [CrossRef]
- Anderson, C.; Checkoway, H.; Franklin, G.M.; Beresford, S.; Smith-Weller, T.; Swanson, P.D. Dietary factors in Parkinson’s disease: The role of food groups and specific foods. Mov. Disord. 1999, 14, 21–27. [Google Scholar] [CrossRef]
- Cui, X.; Pelekanos, M.; Liu, P.Y.; Burne, T.H.; McGrath, J.J.; Eyles, D.W. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience 2013, 236, 77–87. [Google Scholar] [CrossRef]
- Covarrubias-Pinto, A.; Acuna, A.I.; Beltran, F.A.; Torres-Diaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef]
- Hellenbrand, W.; Boeing, H.; Robra, B.P.; Seidler, A.; Vieregge, P.; Nischan, P.; Joerg, J.; Oertel, W.H.; Schneider, E.; Ulm, G. Diet and Parkinson’s disease. II: A possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology 1996, 47, 644–650. [Google Scholar] [CrossRef]
- Fahn, S. A pilot trial of high-dose alpha-tocopherol and ascorbate in early Parkinson’s disease. Ann. Neurol. 1992, 32, S128–132. [Google Scholar] [CrossRef]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Di Lazzaro, G.; Franco, D.; Colona, V.L.; Alwardat, M.; Sinibaldi Salimei, P.; Mercuri, N.B.; Pierantozzi, M.; et al. Dietary Vitamin E as a Protective Factor for Parkinson’s Disease: Clinical and Experimental Evidence. Front. Neurol. 2019, 10, 148. [Google Scholar] [CrossRef]
- Storch, A.; Kaftan, A.; Burkhardt, K.; Schwarz, J. 6-Hydroxydopamine toxicity towards human SH-SY5Y dopaminergic neuroblastoma cells: Independent of mitochondrial energy metabolism. J. Neural. Transm. (Vienna) 2000, 107, 281–293. [Google Scholar] [CrossRef]
- Seamon, M.; Purohit, S.; Giri, B.; Baban, B.; Morgan, J.; Chong, R.; Wakade, C. Niacin for Parkinson’s disease. Clin. Exp. Neuroimmunol. 2020, 11, 47–56. [Google Scholar] [CrossRef]
- Pinnen, F.; Cacciatore, I.; Cornacchia, C.; Sozio, P.; Cerasa, L.S.; Iannitelli, A.; Nasuti, C.; Cantalamessa, F.; Sekar, D.; Gabbianelli, R. Codrugs linking L-dopa and sulfur-containing antioxidants: New pharmacological tools against Parkinson’s disease. J. Med. Chem. 2009, 52, 559–563. [Google Scholar] [CrossRef]
- Di Stefano, A.; Marinelli, L.; Eusepi, P.; Ciulla, M.; Fulle, S.; Di Filippo, E.S.; Magliulo, L.; Di Biase, G.; Cacciatore, I. Synthesis and Biological Evaluation of Novel Selenyl and Sulfur-l-Dopa Derivatives as Potential Anti-Parkinson’s Disease Agents. Biomolecules 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.-W.; Wintering, N.A.; Cai, J.; Wei, X.; Bazzan, A.J.; Zhong, L.; Bowen, B. N-acetyl cysteine may support dopamine neurons in Parkinson’s disease: Preliminary clinical and cell line data. PLoS ONE 2016, 11, e0157602. [Google Scholar] [CrossRef] [PubMed]
- Investigators, N.N.-P. A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin. Neuropharmacol. 2008, 31, 141. [Google Scholar]
- Storch, A.; Jost, W.H.; Vieregge, P.; Spiegel, J.; Greulich, W.; Durner, J.; Müller, T.; Kupsch, A.; Henningsen, H.; Oertel, W.H. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q10 in Parkinson disease. Arch. Neurol. 2007, 64, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Ávila, M.; Vega, A.F.; De La Mata, M.; Pavón, A.D.; De Miguel, M.; Calero, C.P.; Paz, M.V.; Cotán, D. Coenzyme q10 therapy. Mol. Syndromol. 2014, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; He, Q.; Yu, J.-z.; Liu, C.-y.; Feng, L.; Chai, Z.; Wang, Q.; Zhang, H.-z.; Zhang, G.-X.; Xiao, B.-g. Lipoic acid protects dopaminergic neurons in LPS-induced Parkinson’s disease model. Metab. Brain Dis. 2015, 30, 1217–1226. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Kakhaki, R.D.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Shoulson, I.; Group, P.S. DATATOP: A decade of neuroprotective inquiry. Ann. Neurol. 1998, 44, S160–S166. [Google Scholar] [CrossRef]
- Steering, D. DATATOP: A multicenter controlled clinical trial in early Parkinson’s disease. Arch. Neurol. 1989, 46, 1052–1060. [Google Scholar]
- Evatt, M.L.; DeLong, M.R.; Khazai, N.; Rosen, A.; Triche, S.; Tangpricha, V. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch. Neurol. 2008, 65, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid. Med. Cell Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Bidel, S.; Jousilahti, P.; Antikainen, R.; Tuomilehto, J. Coffee and tea consumption and the risk of Parkinson’s disease. Mov. Disord. 2007, 22, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.; Marinelli, L.; Cacciatore, I.; Stefano, A.D. Role of Dietary Supplements in the Management of Parkinson’s Disease. Biomolecules 2019, 9, 271. [Google Scholar] [CrossRef]

| Levels Compared to Controls | Bacteria Phylum/Family/Genus | References |
|---|---|---|
| Decreased | Bacterioidetes, Enterobacteriaceae, Enterococcaceae, Faecalibacterium, Lactobacillaceae, Lachnospiracea, Prevotellaceae, Ruminococcus, Sediminibacterium | [38,41,42,43] |
| Increased | Actinobacteria, Akkermansia, Anaerotruncus, Aquabacterium, Bifidobacterium, Butryociococcus, Clostridium, Holdemania, Proteobacteria, Sphingomonas | [38,41,42,43] |
| Nutrient | Dose | n * | Function | Outcome | Ref. |
|---|---|---|---|---|---|
| Niacin | 250 mg | 46 | support and stabilize mitochondrial function | improvement in Unified Parkinson’s Disease Rating Scale (UPDRS) III scores, improvement in cognition | [62] |
| 100 mg, 250 mg | 46 | changes in macrophage polarization, improved Quality of Life (QoL, reduction in rigidity and bradykinesia | [17,60,61] | ||
| Glutathione | 1400 mg | 21 | Antioxidant, neuroprotection | UPDRS—Activities of Daily Living (ADL) + motor scores did not significantly change. Possibility of a mild symptomatic effect. The high-dose group showed improvement in total UPDRS and motor subscore over baseline | [80] |
| 300, 600 mg | 30 | ||||
| 100, 200 mg | 41 | ||||
| N-acetyl Cysteine | 150 mg/Kg | 6 | Antioxidant, neuroprotection | Significantly increased dopamine transporter (DAT) binding in the caudate and putamen. UPDRS scores were significantly improved. Peripheral anti-oxidant measures increased significantly, but indicators of oxidative damage were unchanged | [81,82,83] |
| 6000 mg | 8 | ||||
| Hydrogen water | 1000 mL | 17 | Significant improvement in total UPDRS scores. This study will confirm whether H2 water can improve PD symptoms | [80] | |
| 1000 mL | 178 | ||||
| Creatinine minocycline | 10 g/d 200 mg/d | 67 66 | support and stabilize mitochondrial function Antioxidant | creatinine group showed improvement in UPDRS3 scores after 18 months | [84] |
| Co-enzyme Q10 | 300 mg/d | Antioxidant, neuroprotection | no benefits at mid-stage PD | [85,86] | |
| Lipoic Acid | Antioxidant, Anti-inflammatory, neuroprotection | [87] | |||
| Vitamin E and Omega-3 fatty acids | 400 IU and 1000 mg flaxseed oil | 30 treated 30 placebo | Antioxidant Antioxidant, Anti-inflammatory, neuroprotection | [88] | |
| dephrenyl/ Vitamin E | 10 mg/d 2000 IU | Antioxidant, Anti-inflammatory, neuroprotection | no benefits reported | [89,90] | |
| Vitamin D3 | NA | NA | Antioxidant, neuroprotection | Lowered serum levels of Vita D3 in PD patients. High Vit D levels are protective against PD | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunaratne, T.B.; Okereke, C.; Seamon, M.; Purohit, S.; Wakade, C.; Sharma, A. Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease. Nutrients 2021, 13, 28. https://doi.org/10.3390/nu13010028
Karunaratne TB, Okereke C, Seamon M, Purohit S, Wakade C, Sharma A. Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease. Nutrients. 2021; 13(1):28. https://doi.org/10.3390/nu13010028
Chicago/Turabian StyleKarunaratne, Tennekoon B., Chijioke Okereke, Marissa Seamon, Sharad Purohit, Chandramohan Wakade, and Amol Sharma. 2021. "Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease" Nutrients 13, no. 1: 28. https://doi.org/10.3390/nu13010028
APA StyleKarunaratne, T. B., Okereke, C., Seamon, M., Purohit, S., Wakade, C., & Sharma, A. (2021). Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease. Nutrients, 13(1), 28. https://doi.org/10.3390/nu13010028





