Abstract
Polyphenols are a group of phytochemicals with potential health-promoting effects. They are classified as flavonoid (flavonols, flavanols, flavones, flavanones, isoflavones, and anthocyanins) and non-flavonoid molecules (phenolic acids, hydroxycinnamic acids, lignans, stilbenes, and tannins). Although an increasing number of trials have shown a correlation among polyphenol consumption and a reduction in risk factors for chronic diseases, discrepancies in explaining their positive effects have been found in terms of the bioavailability. In fact, polyphenols show a low bioavailability due to several factors: interaction with the food matrix, the metabolic processes mediated by the liver (phase I and II metabolism), intestine and microbiota. On the other hand, the biological activities of phenol compounds may be mediated by their metabolites, which are produced in vivo, and recent studies have confirmed that these molecules may have antioxidant and anti-phlogistic properties. This review discusses the studies performed in vivo, which consider the polyphenol bioavailability and their different food sources. Factors influencing the biological effects of the main classes of polyphenols are also considered.
1. Introduction
It is known that an appropriate diet and lifestyle are essential for preserving well-being and preventing illnesses. Due to their abundance in foods derived from plants (e.g., vegetables, fruits, and beverages) and their potential antioxidant activity, polyphenols have been considerably studied in the past years as adjuvants in attenuating the risk factors for disabling diseases (mainly cardiovascular disease (CVD), diabetes, cancer, and cognitive disorders) [1]. Polyphenols are phenylpropanoids synthetized by plants as secondary metabolites, in adverse situations, such as in the presence of pathogens or under adverse climatic conditions. More than 8000 phenolic molecules have been identified, which must contain at least one aromatic nucleus and one or more -OH groups [2]. Polyphenols are commonly categorized as flavonoids, characterized by a C6-C3-C6 structure, and non-flavonoids. Flavonoids generally present in foods are anthocyanins, flavonols, flavan-3-ols, flavones, isoflavones, flavanones, and stilbenes (Figure 1).
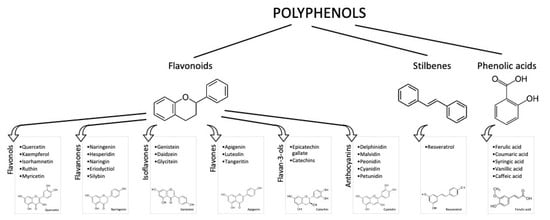
Figure 1.
Polyphenol classes and chemical structures of some of their main compounds.
Most flavonoids found in foods are conjugated with sugars, acids, or alcohols. Non-flavonoids include phenolic acids, in particular, hydroxybenzoic acids (i.e., vanillic and gallic acids), and cinnamic acids (i.e., ferulic and caffeic acids). All of these molecules have proven to have biological activities [3,4]; however, most of them have been shown using in vitro models and pure compounds, where the metabolism and matrix effect were not taken into account. This is due to the fact that in vitro approaches enable the specific mechanisms of action of each molecule/group of molecules to be identified, with relatively low costs. Unfortunately, in vitro studies do not take into account the metabolic transformations and physiological concentrations [5].
As regards in vivo studies, although several epidemiological and clinical studies have evaluated the polyphenol intake, several of them present several limitations, such as a low number of participants, no controls, the use of different methodologies to assess dietary habits, heterogeneous types of database to determine the consumption, etc. Despite the fact that other large and well-designed studies (for example, the PREDIMED study) have shown that the Mediterranean diet, characterized by foods rich in polyphenols, is associated with a reduction in cardiovascular risk and a better cognitive function in the elderly, strong evidence supporting its effects on human health is still not clear [6]. This is also due to the scarcity of knowledge regarding polyphenol bioavailability, together with the difficulty in determining which specific molecule is involved in the biological effect when different phenol compounds are present concomitantly. To date, only cocoa and extra virgin olive oil have thus received the approval of a health claim related to the content in phenol compounds.
Lastly, besides the polyphenol content in foods, in order to establish a correlation between the bioavailability and health effects, their mean intake in humans has to be considered. In a recent systematic literature revision, including more than 90 human studies, the polyphenol intake was estimated according to different dietary habits. Total polyphenol intake for the general population (inclusive of young people, adults, and the elderly) was estimated to be 0.9 g per day, where the main dietary founts were coffee, tea, wine (especially red wine), fruits, and vegetables. Total flavonoids and specific subclasses (flavonols, anthocyanidins, proanthocyanidins, flavan-3-ols, flavones, and flavanones) were associated with reduced CVD, diabetes mellitus (T2D), and mortality for all causes [7]. However, a correlation with the bioavailability of these compounds was not evaluated.
The goal of the present review was to assess the human bioavailability of the main classes of polyphenols, taking into account their food sources and the main factors affecting their in vivo accessibility.
2. Materials and Methods
The most important life-science databases of references and abstracts (PubMed, MEDLINE, Embase, and CAB-Abstract) were methodically analyzed (from database beginning to October 2020), using the following terms: “polyphenols”, “anthocyanins”, “flavanols”, “flavonols”, “flavans”, “stilbenes”, “flavanones” in combination with “bioavailability”, “disease”, “health” and refining the results for “human studies” AND “controlled trials”.
A search by title and abstract led to the collection of 98 relevant publications on the association between polyphenols and bioavailability. By removing duplicates and non-relevant papers (papers investigating the effects of polyphenols without considering bioavailability data, studies performed on animals, or using in vitro models), in total, 37 publications were included in this review.
3. Results and Discussion
3.1. Anthocyanins
Anthocyanins are pigments soluble in water contributing to the red, violet, and blue colors in flowers and fruits. At low pH, the anthocyanin chemical structure presents a positive charge at the oxygen atom of the C-ring, called the flavylium ion, and appears as red pigments. Anthocyanins are classified on the basis of the number and position of -OH groups on the flavonoid molecule. To date, more than 600 anthocyanin compounds have been identified [2]. Of these, the glycosylated form of cyanidin, delphinidin, malvidin, peonidin, petunidin, and pelargonidin are the most abundant. The most common sugar in anthocyanidin glycosides is glucose; however, rhamnose, galactose, and rutinose may also be present. The sugar group can be acylated, generally at C3 position, by aromatic acids such as hydroxycinnamic acids (ferulic, caffeic, and coumaric).
Anthocyanins are among the polyphenol compounds with the highest concentration in foods, with an average concentration of 115 ± 259 mg 100 g−1 [8]. The richest sources of anthocyanins (as glycosides) are black elderberries (1316 mg 100 g−1), black chokeberry (878 mg 100 g−1), and black currant (595 mg 100 g−1). Apart from red fruits, important sources of anthocyanins are represented by red wine, colored beans, and vegetables such as red oranges, red lettuce, or red onions. Like other flavonoids, the mean daily intake of anthocyanins can vary among countries, depending on the nutritional habits and cultural differences. It has been calculated that the daily intake of anthocyanins is between 6.8 mg per day in Brazil (where the most important dietary sources are citrus and tropical fruits) and Australia, and 133 mg per day in Italy (the main dietary sources are seasonal fruits, citrus fruits, leafy vegetables, and wine) [9,10,11].
Anthocyanin bioavailability is extremely low: only about 1–2% maintain their original structure after ingestion [12]. Anthocyanins exist in different chemical structures depending on the pH. In the stomach, at pH 1.5–3, the main chemical forms are flavylium cations, while in the intestinal environment, the carbinol forms predominate, with lower absorption. In addition, other biotransformation steps occur during gastrointestinal digestion, such as phase II metabolism processes (glucuronidation, sulphation, and methylation), enzymatic and microbiota catabolism [12]. These lead to several chemical compounds, namely anthocyanin glucuronides, phenolic acids (ferulic acid, caffeic acid, vanillic acid, gallic acid, protocatechuic acid, syringic acid, and 4-hydroxybenzoic acid), and aldehydes (phloroglucinaldehyde and phloroglucinaldehyde) [12]. Nevertheless, there is a remarkable inter- and intra-variability in the bioavailability of anthocyanins, due to several factors, such as the food matrix or technological/processing conditions, enzymatic patterns, and microbiota composition. Only a few studies have evaluated a correlation among the results obtained and the bioavailability data. Table 1 summarizes the human trials where the health properties of anthocyanins are correlated with bioavailability studies. The literature data show that the anthocyanin daily doses used in the clinical trials ranged between 2.1 and 94.47 mg. These amounts were generally provided by food (blackcurrant and orange juice); and only the study by Xie et al. (2017) used a food supplement [13]. When anthocyanins were consumed in food, they did not affect the biomarkers investigated, mainly associated with the cardiovascular function, such as the oxidative status, inflammation and vascular reactivity. Only in the study by Xie et al., (2017) 500 mg/day of Aronia extract (Aronia melanocarpa, also known as black chokeberry), containing 45.1 mg anthocyanins, 35.7 mg hydroxycinnamic acids, and 41.9 mg proanthocyanidins, improved the total plasma cholesterol and LDL (Low-Density Lipoproteins) receptor in peripheral blood mononuclear cells [13]. This positive effect was due to the duration of the study (12 weeks vs acute consumption or a maximum of four weeks in the other studies) and by the synergistic effect of other phenol compounds present in the extract (hydrocynnamic acids and proanthocyanidins). These results are also supported by the literature data, where the chronic consumption, from six weeks to two months, of Aronia berry extracts (255–300 mg/day) decreased several biomarkers of cardiometabolic diseases, due to the high Aronia polyphenol content [14,15]. In addition, the studies were generally performed on healthy subjects, thus making it more difficult to measure evident effects on the health status.

Table 1.
Anthocyanin’s beneficial effects and bioavailability in human subjects.
Regarding the bioavailability data, the amounts of anthocyanins found in plasma or urine were generally low, which was correlated with the dose taken and the kind of food provided in the studies. Jin et al. (2011) hypothesized that blackcurrant juice did not ameliorate the vascular reactivity in healthy subjects, due to the low levels of anthocyanins in the juice (20%) [16]. This observation has been supported by other authors, who reported that a consistent elevation of plasma anthocyanins and, as a consequence, significative health effects, can be observed only when anthocyanins are consumed at pharmacological levels (500–1500 mg/day) [17,18]. It is also noticeable that anthocyanin compounds are affected by high instability and susceptibility to degradation, particularly at the gastrointestinal level [19]. In addition, the short periods of supplementation or juice ingestion (maximum 12 weeks) were also found to influence the lack of a significant reduction in oxidative status and CVD biomarkers. In urine, the percentage of anthocyanins varied between 0.009 ± 0.002 and 0.79 ± 0.90% of the dose taken, in line with the literature data [20,21]. Despite the great variability in anthocyanin food content, cyanidin and peonidin glucosides were generally considered the most available, since their metabolites were the only ones measurable in plasma or urine. In their clinical trial, Xie et al. (2017) speculated that the 8% reduction in fasting plasma total cholesterol after 12 weeks of supplementation with Aronia extract was also due to cyanidin methylated metabolite and 3-(4-hydroxyphenyl)propionic acid derived from microbiota fermentation [13]. However, the mechanism by which these metabolites affect lipid metabolism needs to be further explored. Despite the general lack of positive effects on CVD biomarkers, several trials have described positive effects of anthocyanins when included in the daily diet. For example, Wedick et al. (2012) reported that the anthocyanin intake of about 22.3 mg per day and an anthocyanin-rich fruit consumption (≥5 times per week) was correlated with a minor risk of developing type-2 diabetes [22]. Cassidy et al. (2016) reported a decreased risk of myocardial infarction in normotensive patients > 65 years, when high levels of anthocyanins were consumed (>35 mg/day) [23]. Other studies reported positive effects of food supplements containing anthocyanins on ocular function, showing encouraging results in relation to glaucoma and in the reduction of retinal oxidative stress due to aging. However, in these studies, anthocyanin bioavailability was not considered. Manach et al. (2005) partially explained the discrepancies in the positive effects of anthocyanin despite their low anthocyanin bioavailability, by considering the following: (1) the possible presence of anthocyanin metabolites not measured in biological samples, such as microbiota metabolites; (2) the instability of anthocyanin metabolites (glucuronides and sulfates), which can extensively degrade in frozen urine, during storage [20].
3.2. Flavanols
Flavanol compounds are included in a wide of range of foods. The main sources of flavanols are cocoa (3411 mg 100 g−1) and dark chocolate (1590 mg 100 g−1). Berries are also an important source of flavanols, containing 659, 330, and 139 mg 100 g−1 for black chokeberry, blueberry, and blackcurrant, respectively. Strawberry (148 mg 100 g−1) and apple (111 mg 100 g−1), as well as hazelnut, pecan nut, pistachio, and almonds (181–496 mg 100 g−1), are other important sources. Black tea, green tea, and red wine contain high levels of flavanols, in particular, catechins and proanthocyanidin dimers, with estimated mean amounts of 18–50 mg in the daily diet [20].
However, the content of some flavanols is often underestimated, since generally the methods used (e.g., HPLC) only evaluate monomers and proanthocyanidin dimers and trimers. The intake of flavanols in the European Union, according to the EFSA (European Food Safety Authority) Comprehensive European Food Consumption Database, and the average intake of flavan-3-ol monomers, theaflavins, and proanthocyanidins ranges from 181 mg per day (Czech Republic) to 793 mg per day (Ireland) [28].
The highest intakes of flavan-3-ols (monomers) and theaflavins were detected in Ireland (191 and 505 mg per day, respectively) and the lowest in Spain (24 and 9 mg per day, respectively). On the other hand, the highest daily intake of proanthocyanidins (PA) was found in Spain (175 mg per day) and lowest in The Netherlands (96 mg per day). The most important sources were tea (62%), pome fruits (such as apples and pears) (11%), berries (3%), and cocoa derivatives (3%). Tea was the principal contributor to monomer intake (75%), followed by pome fruits (6%). In addition, pome fruits were the most important source of proanthocyanidins (28%). Table 2 reports the clinical trials where the flavanol intakes and their bioavailability were correlated with beneficial effects. Flavanol intake ranged between 28.3 to 907.5 mg/day. These amounts were chosen on the basis of the mean dietary intake of flavonosl among the population included in the studies, and thei consumption with food (enriched chocolate or coffee). Participants in the studies were generally healthy or had stage 1 hypertension without concomitant risk factors.

Table 2.
Flavanols’ beneficial effects and bioavailability in human subjects.
Dower et al. (2016) compared the chocolate consumption (containing 150 mg epicatechin, EC) with pure epicatechin supplementation (100 mg) [29]. The length of treatment varied between acute consumption to 18 weeks. The principal outcomes investigated were associated with cardiovascular function (e.g., blood pressure, flow-mediated dilation, and platelet function) and antioxidant activity (e.g., LDL oxidation or plasma 8-isoprostane). A significant improvement in cardiovascular biomarkers was also generally observed for the consumption of low levels of flavanols (28.3 mg/day). A significant improvement in cardiovascular biomarkers was also generally observed for the consumption of low levels of flavanols (28.3 mg/day), despite the EFSA highlighting of a cause-effect correlation between cocoa consumption and endothelium vasodilation for a daily intake of 200 mg [30]. In contrast, only a few studies have measured notable effects on oxidative stress, suggesting that other mechanisms can be involved in the improvement of cardiovascular function [31,32].
According to Taubert et al. (2007), the positive results were generally associated with the monomers epicatechin/catechin and the dimers procyanidin B2/procyanidin B2 gallate, which were the only monomers dosed in plasma, at a concentration ranging between 0.14 (±0.06) ng/mL of procyanidin B2 gallate and 3.63 (±1.02) ng/mL (from 1 to 6 h after consumption) [32]. These amounts reduced blood pressure by 1.9 (±1) mmHg in patients with stage 1 hypertension probably due to the “chronic” increase in endothelium NO production. Similar effects were observed in other studies where higher levels of flavan-3-ols were used, due to the shorter period of intake. Interestingly, in the clinical trial by Dower et al. (2016), the positive effect of epicatechin (EC) on flow-mediated dilatation (FMD) from dark chocolate was not observed for pure epicatechin administered in association with white chocolate, suggesting a significant impact of the food matrix and sugar content on epicatechin bioavailability [29].
Urinary flavan-3-ols were measured only by Ostertag et al. (2013), who described a maximum catechin excretion of 13.4 mmol/mol creatinine 2–6 h after dark chocolate intake (containing 907.5 mg total catechins) [33]. As regards platelet function, flavan-3-ols affected platelet aggregation 120 min after intake, when a peak plasma concentration was obtained. However, bleeding time was affected only after 360 min, when the colonic metabolites kicked in Reference [33]. Interestingly, a gender-specific modulation of platelet aggregation reduction was also observed, probably due the formation of larger platelet aggregates after adenosine diphosphate (ADP) stimulation in women. It should also be noted that flavan-3-ol bioavailability varies markedly among the different subclasses. Manach et al. (2005) reported that galloylated catechins are poorly absorbed, explaining the higher bioavailability and the activity of epicatechin described in the clinical trials [20]. In addition, epicatechin glucuronide and sulfate metabolites, together with valerolactone microbial derivatives—not measured in the clinical trials included in this review—account for 6–39% of the ingested epicatechin, thus prolonging its biological effects [34].
As regards procyanidins, these compounds have a reduced bioavailability, which is about 100-fold lower than monomers. The biological effects are generally due to the monomers formed after gastric degradation, which can be rapidly absorbed in the gut. In addition, the gut microbiota is responsible for the metabolite formation (m-hydroxyphenylpropionic acid, m-hydroxyphenylacetic acid, phenylpropionic acid, phenylacetic acid, hydroxyphenylvaleric acid, and benzoic acid among others), which could also be responsible for various biological effects [20].
3.3. Flavonols
Fruits, vegetables, and some beverages, like tea and red wine, are the main source of flavonols, for which the intake is estimated between 18 (USA) and 58 mg (Japan) per day [28]. However, these intake levels generally refer only to the three main flavonols, namely quercetin, myricetin, and kaempferol. In fruits and vegetables, the highest quercetin content is found in cranberry (149 mg/100 g) and onions (65 mg 100 g−1), while in green tea and red wine, the mean contents are 2.5 and 1.6 mg/100 mL, respectively [36]. Eleven studies have explored the different health effects of flavonol intake, mainly on the reduction in CVD risk factors (homocysteine and LDL-oxidation levels, homocysteine, plasmatic High Density Lipoproteins (HDL) and LDL cholesterol, blood pressure, NO production, and platelet aggregation), inflammatory biomarkers (C-reactive protein and endothelin-1 expression), and antioxidant activity (e.g., excretion of urinary F2-isoprostanes and plasma malondialdehyde (MDA)) (Table 3). One study also investigated the effect of an enzymatically modified quercetin on cognitive function [37]. These effects have generally been measured in healthy subjects, apart from three studies involving subjects at cardiovascular risk [37,38,39].

Table 3.
Flavonols’ beneficial effects and bioavailability in human subjects.
In most studies, flavonols were administered by extracts, given alone or mixed with food; only in three studies they were provided using food preparations (onion soup or cake) [40,41,42]. Flavonol intake ranged between 16.7 to 400 mg/day and included mainly quercetin and its derivatives, isorhamnetin and kaempferol. The data in Table 3 show that cardiovascular parameters, as well as oxidative stress biomarkers, were generally not affected by flavonols. Suomela et al. (2006) and Larmo et al. (2009) used a sea buckthorn extract (Hippophae rhamnoides L.) as the source of flavonols (78 and 16.7 mg per day, respectively), administered with meals [38,43]. Both flavonol intakes failed to reduce oxidative stress, total and LDL cholesterol, or C reactive protein (CRP) concentration. Sea buckthorn was administered because of its traditional use in Eastern countries and the encouraging results from clinical and epidemiological studies reporting a reduction in cardiovascular risk factors [44]. The authors explained the different outcomes of their studies, using a moderate berry dose, similar to the average consumption in the daily diet and to the dosage (3–9 g) prescribed by the Chinese Pharmacopoeia for ameliorating hematic circulation [45]. These amounts were lower than those used in other trials (600 mg sea buckthorn flavonols), where positive results were reported [46].
Despite the negative results, plasma flavonols (quercetin, kaempferol and isorhamnetin glucuronides, and sulfates) significantly increased after the treatment, with respect to placebo (p < 0.005). This means that the initial dose taken correlates closely with the biological effects but is not always explained by the bioavailability of flavonols and the duration of intake. In fact, although the flavonol plasma levels were always measurable, they were not sufficient to affect specific biomarkers. Quercetin, especially in its glucosidic form, is generally efficiently absorbed and bioavailable. In addition, quercetin metabolite elimination is quite low (t1/2 11–28 h), leading to their accumulation in plasma with repeated intakes [20]. Positive results were obtained on FMD and platelet aggregation.
Bondonno et al. (2016) observed no improvement in blood pressure or FMD in healthy subjects, when increasing amounts of quercetin-3-O-glucoside (50–400 mg/day) were provided [47], but only when quercetin was enzymatically modified to obtain a more bioavailable α-oligoglucosylated quercetin derivative, isoquercitrin. Supplementation with 4.89 mg of this compound significantly increased FMD response by 1.8% compared with the placebo (p = 0.025), but with different mechanisms not involving NO production increase. In contrast, cognitive function was not affected. The different form of quercetin used makes it difficult to compare it with other studies; however, the maximum plasma concentration reached 144 ± 12.3 nM, which is higher than the values measured in other studies where quercetin was administered [48,49].
Perez et al. (2014) postulated that the vasodilator effects can be mediated by quercetin-3-O-glucuronide (Q3GA) deconjugation mediated by plasmatic glucuronidase [50]. This is because a plasmatic dose-dependent increase in this metabolite was detected after 200 and 400 mg supplementation with quercetin, which was not detected with other metabolites (quercetin aglycone, isorhamnetin aglycone, and their glucuronide forms). The enzyme glucuronidase is present in lysosomes involved in the glycosaminoglycans cleavage. An extensive inter-individual variability in the activity of glucuronidase has also been described, which may be attributed by variations in its gene sequence or expression. This enzyme hydrolyzes glucuronidated metabolites at the vascular level, producing the parent aglycone, which, due to its elevated liposolubility, accumulates in tissues and performing its biological activity. The effect on arterial diameter was not associated with either the early changes flavonoid plasmatic levels or the glucuronidase activity. Nevertheless, these effects were associated with a combination of both factors. Quercetin provided by food (high-quercetin onion soup) or supplements (providing 138 and 150 mg quercetin, respectively) was shown to be effective in inhibiting platelet aggregation mediated by collagen. The inhibition correlated highly with the AUC of the quercetin metabolites, isorhamnetin and tamarixetin (4-O-methyl-quercetin) [40,48].
3.4. Phenolic Acids
Phenolic acids are a class of secondary metabolites, highly distributed among plants. According to their chemical structure, phenolic acids can be divided in benzoic and cinnamic acids. The main benzoic groups are gallic, protocatechuic, and p-hydroxybenzoic acids, mainly as conjugates. The highest concentration (fresh weight) of benzoic acids has been calculated in Apiaceae species (spices and herbs): anise 730–1080 mg kg−1, cumin up to 42 mg kg−1, fennel up to 106 mg kg−1, and parsley up to 30 mg kg−1 [51,52]. Cinnamic acids are widely distributed in plants, as esters or amides. The most representative are caffeic, chlorogenic, and ferulic acids. High concentrations of cinnamic acids are coffee, tea, wine, cocoa, fruits, vegetables, and cereals. Some of the most important sources of caffeic acid are wild blueberry (1470 mg kg−1), coffee (870 mg kg−1), carrots (260 mg kg−1), plum (234 mg kg−1), and eggplant (210 mg kg−1).
One of the most important derivatives of caffeic acid is caftaric acid, a representative polyphenol in wine (6–73 mg L−1 in white wine, 46–141 mg L−1 in red wine), while chlorogenic acid is present in considerable levels in coffee (depending on the climatic and processing conditions, and procedures for coffee preparation) [53,54]. The chlorogenic acid content in roasted coffee beans varies depending on the roasting extent, in the range of 2.3–80 g/kg (dried weight) and 890–8130 mg/L in espresso coffee [53]. The intake of chlorogenic acid can be very high; it has been estimated to be up to 0.8 g per day among coffee drinkers [20].
Cereals are the most important source of ferulic acid, derivative of cinnamic acid derivative (for which the intake ranges from about 0.092 to 0.32 g) [49]. Table 4 shows the studies correlating phenolic acid intake, their bioavailability, and different health effects, mainly focused on blood pressure, vasodilation, antioxidant activity, and inflammation. Six of ten studies included in this review investigated the effects of chlorogenic acid and its metabolites in coffee or in beverages prepared in order to mimic coffee intake; one study used purified caffeoylquinic acid (5-CQA); one study included whole grain; and two studies included a blueberry drink. Phenolic acids were administered to healthy subjects in the range of 138.7 and 900 mg/day and were tested both in acute and chronic consumption (maximum eight weeks). Generally speaking, vascular function was positively affected by chlorogenic acid (CGA) provided by decaffeinated coffee intake (50 mL) or purified caffeoylquinic acid (5-CQA), confirming previous studies showing that phenolic compounds, other than caffeine, can contribute to vasoactive efficacy [55,56]. Observational studies indicate that moderate coffee intake (4 cups), containing from 105 to 500 mg of CGA, is associated with a lower CVD risk [57].

Table 4.
Phenolic acids’ beneficial effects and bioavailability in human subjects.
Potential mechanisms by which CGA and its main plasma metabolites (5-cholorgenic acid, ferulic-4′ -O-sulfate, and isoferulic-3′-O-glucuronide) mediate the vascular effects include the inhibition of NAPDH oxidase, leading to a reduction in superoxide production and, as a consequence, to an increase in endothelium NO bioavailability [58]. Conflicting positions have been taken regarding the possible agonistic or antagonist effects of caffeine on vascular function. Agudelo-Ochoa et al. (2016), postulated that caffeine could be responsible for the negative effects observed after 400 mL coffee intake (caffeine content < 300 mg), since it can interfere with the mechanism of action of chlorogenic acid, thus decreasing NO production [59]. In contrast, Boon et al. (2017) noticed a vasodilator effect only in subjects consuming caffeinated coffee (270 mg caffeine) but not decaffeinated coffee, although the CGA levels were comparable (300 and 287 mg CGA, respectively) [60]. In plasma, an increase in CGA metabolites (5-CGA) was always measured, even when no significant effects on vascular function were observed, suggesting that synergistic effects on different polyphenol compounds can occur. Rodriguez-Mateos et al. (2013) also observed an amelioration of endothelial function after the acute intake of blueberry drinks containing different levels of polyphenols, from 766 to 1791 mg [61]. Phenolic acid metabolites (caffeic acid, ferulic acid, iso-ferulic acid, vanillic acid, benzoic acid, and 2-hydroxybenzoic acid) were the only ones measured in plasma, while no flavonols or anthocyanins were detected, thus suggesting that these compounds were not responsible for the positive effects noticed. The intake of phenolic acids with whole grains (138 mg/70 g) for eight weeks was associated with a reduced inflammatory status in overweight subjects by Vitaglione et al. (2015), compared with equal amounts of refined wheat (2.6 mg ferulic acid) [62].
Considering the bioavailability data, this effect was mainly associated with ferulic acid, whose concentrations were two-fold higher in the feces of subjects consuming whole grains. Interestingly, this result was explained by the fact that the release of FA in the colon could be due to wheat bran polysaccharide fermentation and mediated by bacterial enzymes xylanase and ferulic acid esterase. These enzymes are mainly synthetized by Lactobacilli (Firmicutes), Bifidobacteria (Actinobacteria), Bacteroides, and Prevotella (Bacteroidetes) when arabinoxylans with esterified ferulic acids are introduced [66,67]. Since overweight or obese individuals show reduced amounts of Bacteroidetes and Bifidobacteriales, Firmicutes are considered the main responsible for the fermentation of whole-grain polysaccharides and ferulic acid liberation [62,66].
3.5. Stilbenes, Isoflavones, and Flavanones
Only a few studies were found correlating the effects of stilbenes (resveratrol), isoflavones, and flavanones with their bioavailability (Table 5). Resveratrol is a phytoalexin that is widely distributed in the plant kingdom. It is found in more than 70 species, but grapes and wine are the most important sources. The mean levels of total resveratrol in red wine is 7 mg L−1; rose wine has a total of 2 mg L−1, and white wine has and 0.5 mg L−1 [60]. Resveratrol supplementation (250–500 mg/day for 7 and 28 days, respectively) was investigated in healthy subjects as regards cognitive function and cerebral ematic circulation. Despite total resveratrol metabolites (resveratrol 4′glucuronide, 3′ glucuronide, and sulfate) being ten-fold higher in the treatment group, supplementation failed to improve cognitive function but increased cerebral flow and reduced fatigue levels [68,69]. These effects could also be mediated by unmetabolized resveratrol, since the literature data indicate that it can be found in plasma bound to albumin or LDL, and that it elicits its biological function after interaction with cells that have receptors for albumin and LDL [70]. In brain, resveratrol contributed to vasorelaxation, oxygenation, and sirtuin (SIRT)-mediated increases in mitochondrial gene expression in brain [68].

Table 5.
Stilbenes’, isoflavones’, and flavanones’ beneficial effects and bioavailability in human subjects.
The only source of isoflavones are products derived from soybeans. Depending on the kind of soy preparation, isoflavones can be present as aglycones or glycosides. One study evaluated the impact of isoflavones on triglycerides and oxidative biomarkers [71]. Only two studies investigated the effects of isoflavones (supplemented for 12 months at doses of 60 and 96 mg isoflavone aglycones/day, respectively) on bone density in post-menopausal women with contradictory results. In the study by Lambert et al. (2017), the positive effects were explained by the use of lactic acid probiotic bacteria in the treatment group in association with isoflavones, which mediated equol production [72]. Equol is a derivative of daidzein produced by anaerobic bacteria with great estrogenic potential. Since none of the subjects was able to produce equol at the beginning of the study and, after six months of treatment, 55% of individuals in the Red Clover Extract (RCE) group produced equol, it is plausible that that the probiotics positively affected the participant intestinal bacterial pattern, promoting more positive conditions for equol production. This hypothesis seems to be confirmed by the fact that high plasma levels were found in both studies; however, in the study by Brink et al. (2008), bone density was not affected by isoflavones [73]. However, a higher number of studies is necessary to confirm these results.
Only one study investigated the effect of flavanones, in particular hesperidin on vascular function, providing 320 mg with 767 mL orange juice/day as acute consumption. Despite the detection of several hesperidin metabolites in plasma (hesperidin-glucuronide, naringenin-7-O-glucuronide, dihydroferulic acid, dihydroferulic acid–glucuronide, hippuric acid, and vanillic acid 4-hydroxyphenylacetic acid) after 5 h, no significant effects were measured. Despite previous studies showing that plasma hesperidin metabolites were correlated with health effects on the endothelium after flavanone acute consumption [75,76], further investigations are necessary to evaluate and support the biological effects of these polyphenols.
4. Conclusions
The data reported in this review highlight that, despite the very large number of studies investigating the health effects of polyphenols in humans, only a few have considered their bioavailability in order to partially support the associated bio-efficacy. The bioavailability of polyphenols varies among the different classes and ranks as follows: phenolic acids > isoflavones > flavonols > catechins > flavanones, proanthocyanidins > anthocyanins, confirming data from previous pharmacokinetic studies [20]. Apart from favanols and flavonols, the amounts of polyphenols used in studies were considered to be too low to reach significant plasma concentrations that would provide beneficial effects. However, the amounts were chosen on the basis of the mean consumption in the daily diet of the populations included in the studies. Another point to be considered is that healthy subjects were mainly included in clinical trials. On the one hand, it makes it more difficult to measure significant changes in biomarkers generally associated with pathological conditions; on the other hand, it suggests a potential role of polyphenols provided with the daily diet or supplementation in maintaining the health status. In fact, positive variations of physiological parameters generated by polyphenol intake could help in improving or modulating specific functions and reducing some risk factors for chronic diseases. Cardiovascular function was the main health area investigated. Vasodilator effects were found for phenolic acids (mainly chlorogenic acid and ferulic acid) and flavanols (in particular catechins and proanthocyanidins), which were partially explained by their bioavailability.
As regards anthocyanins, their plasmatic levels were too low to affect the biomarkers considered; however, cyanidin and peonidin were the most available. Future research should focus on confirming and integrating the data discussed in this review, particularly for stilbenes, isoflavones, and flavanones, since few studies have associated their bioavailability with health effects. In addition, the biological effects of phenol metabolites derived from microbiota fermentation should be more extensively studied, since several data suggest their role in mediating the benefits of polyphenols.
Finally, since polyphenol bioavailability can be affected by food matrix components, specific strategies could be considered in order to increase their in vivo delivery (e.g., fermentation or exploiting the association among foods), or to protect them from degradation (e.g., microencapsulation).
Author Contributions
Writing—original draft preparation, C.D.L.; methodology, F.C.; data curation, C.S. and S.B.; supervision, P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tufarelli, V.; Casalino, E.; D’Alessandro, A.G.; Laudadio, V. Dietary Phenolic Compounds: Biochemistry, Metabolism and Significance in Animal and Human Health. Curr. Drug Metab. 2017, 18, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Stockley, C.; Teissedre, P.L.; Boban, M.; Di Lorenzo, C.; Restani, P. Bioavailability of wine-derived phenolic compounds in humans: A review. Food Funct. 2012, 3, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 Polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef]
- Pounis, G.; Tabolacci, C.; Costanzo, S.; Cordella, M.; Bonaccio, M.; Rago, L.; D’Arcangelo, D.; Filippo Di Castelnuovo, A.; de Gaetano, G.; Donati, M.B.; et al. Reduction by coffee consumption of prostate cancer risk: Evidence from the Moli-sani cohort and cellular models. Int. J. Cancer 2017, 141, 72–82. [Google Scholar] [CrossRef]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Dietary intake and food contributors of polyphenols in adults and elderly adults of Sao Paulo: A population-based study. Br. J. Nutr. 2016, 115, 1061–1070. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.E.; Lee, S.; Mond, J.; Russell, J.; Mitchell, P.; Flood, V.M. Dietary flavonoid intake in older adults: How many days of dietary assessment are required and what is the impact of seasonality? Nutr. J. 2018, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Broncel, M.; Koziróg, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, CR28–CR34. [Google Scholar] [PubMed]
- Skoczyńska, A.; Jedrychowska, I.; Poreba, R.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Andrzejak, R. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol. Rep. 2007, 59, 177–182. [Google Scholar]
- Jin, Y.; Alimbetov, D.; George, T.; Gordon, M.H.; Lovegrove, J.A. A randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur. J. Clin. Nutr. 2011, 65, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Anthocyanins are detected in human plasma after oral administration of an elderberry extract. Clin. Chem. 1999, 45, 574–576. [Google Scholar] [CrossRef]
- Murkovic, M.; Toplak, H.; Adam, U.; Pfannhauser, W. Analysis of anthocyanins in plasma for determination of their bioavailability. J. Food Compos. Anal. 2000, 13, 291–296. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef]
- Hollands, W.; Brett, G.M.; Radreau, P.; Saha, S.; Teucher, B.; Bennett, R.N.; Kroon, P.A. Processing blackcurrants dramatically reduces the content and does not enhance the urinary yield of anthocyanins in human subjects. Food Chem. 2008, 108, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; Van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Jenkinson, A.M.E.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Coletta, W.; Tamburrelli, C.; D’Imperio, M.; Crescente, M.; Silvestri, C.; Rapisarda, P.; Reforgiato Recupero, G.; De Curtis, A.; Iacoviello, L.; et al. Four-week ingestion of blood orange juice results in measurable anthocyanin urinary levels but does not affect cellular markers related to cardiovascular risk: A randomized cross-over study in healthy volunteers. Eur. J. Nutr. 2012, 51, 541–548. [Google Scholar] [CrossRef]
- Riso, P.; Visioli, F.; Gardana, C.; Grande, S.; Brusamolino, A.; Galvano, F.; Galvano, G.; Porrini, M. Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress. J. Agric. Food Chem. 2005, 53, 941–947. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Luben, R.N.; Lentjes, M.A.H.; Heiss, C.; Kelm, M.; Merx, M.W.; Spencer, J.P.E.; Schroeter, H.; Kuhnle, G.G.C. Assessment of the dietary intake of total flavan-3-ols, monomeric flavan-3-ols, proanthocyanidins and theaflavins in the European Union. Br. J. Nutr. 2014, 111, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Kroon, P.A.; Philo, M.; Mensink, M.; Kromhout, D.; Hollman, P.C.H. Does epicatechin contribute to the acute vascular function effects of dark chocolate? A randomized, crossover study. Mol. Nutr. Food Res. 2016, 60, 2379–2386. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006 following a request in accordan. EFSA J. 2014, 12, 3654. [Google Scholar] [CrossRef]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.-K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, L.M.; Kroon, P.A.; Wood, S.; Horgan, G.W.; Cienfuegos-Jovellanos, E.; Saha, S.; Duthie, G.G.; De Roos, B. Flavan-3-ol-enriched dark chocolate and white chocolate improve acute measures of platelet function in a gender-specific way-a randomized-controlled human intervention trial. Mol. Nutr. Food Res. 2013, 57, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Danese, E.; Angelino, D.; Mena, P.; Rosi, A.; Benati, M.; Gelati, M.; Salvagno, G.L.; Favaloro, E.J.; Del Rio, D.; et al. Dark chocolate modulates platelet function with a mechanism mediated by flavan-3-ol metabolites. Medicine 2018, 97, e13432. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism chemistry and structure of the flavonoids. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically modified isoquercitrin improves endothelial function in volunteers at risk of cardiovascular disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Suomela, J.P.; Ahotupa, M.; Yang, B.; Vasankari, T.; Kallio, H. Absorption of flavonols derived from sea buckthorn (Hippophaë rhamnoides L.) and their effect on emerging risk factors for cardiovascular disease in humans. J. Agric. Food Chem. 2006, 54, 7364–7369. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Wolffram, S.; de Vos, R.; Bovy, A.; Gibbins, J.M.; Lovegrove, J.A. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: A pilot study. Br. J. Nutr. 2006, 96, 482–488. [Google Scholar] [CrossRef]
- Beatty, E.R.; O’Reilly, J.D.; England, T.G.; McAnlis, G.T.; Young, I.S.; Halliwell, B.; Geissler, C.A.; Sanders, T.A.B.; Wiseman, H. Effect of dietary quercetin on oxidative DNA damage in healthy human subjects. Br. J. Nutr. 2000, 84, 919–925. [Google Scholar] [CrossRef]
- O’Reilly, J.D.; Mallet, A.I.; McAnlis, G.T.; Young, I.S.; Halliwell, B.; Sanders, T.A.B.; Wiseman, H. Consumption of flavonoids in onions and black tea: Lack of effect on F2-isoprostanes and autoantibodies to oxidized LDL in healthy humans. Am. J. Clin. Nutr. 2001, 73, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Larmo, P.S.; Yang, B.; Hurme, S.A.M.; Alin, J.A.; Kallio, H.P.; Salminen, E.K.; Tahvonen, R.L. Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonols in healthy adults. Eur. J. Nutr. 2009, 48, 277–282. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission of the PRC. Pharmacopoeia of the People’s Republic of China (The First Part); People’s Medical Publishing House: Beijing, China, 2000; Volume I, ISBN 9787117069823. (In English)
- Wang, J.; Zhang, M.; Xu, Z.; Zhang, T.; Cheng, Y. Clinical observation on effects of sea buckthorn total flavones on ischemic heart diseases. Shanxi Med. Res. 1985, 2, 60–67. [Google Scholar]
- Bondonno, N.P.; Bondonno, C.P.; Rich, L.; Mas, E.; Shinde, S.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Acute effects of quercetin-3-O-glucoside on endothelial function and blood pressure: A randomized dose-response study. Am. J. Clin. Nutr. 2016, 104, 97–103. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Wolffram, S.; Lovegrove, J.A.; Gibbins, J.M. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J. Thromb. Haemost. 2004, 2, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef]
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J.; Perez-Vizcaino, F. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharmacol. Res. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Nagel, C.W. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.J.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Flury, A.; Marmet, C.; Poquet, L.; Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Brenner, R.; Allemann, Y.; Zimmermann, D.; et al. Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials. Clin. Nutr. 2017, 36, 1520–1529. [Google Scholar] [CrossRef]
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Nam, Y.; Kim, J.; Choi, H.; Won, C. Coffee consumption and stroke risk: A meta-analysis of epidemiologic studies. Korean J. Fam. Med. 2012, 33, 356–365. [Google Scholar] [CrossRef]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzmán, O.J.; Muñoz-Durango, K. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef]
- Boon, E.A.J.; Croft, K.D.; Shinde, S.; Hodgson, J.M.; Ward, N.C. The acute effect of coffee on endothelial function and glucose metabolism following a glucose load in healthy human volunteers. Food Funct. 2017, 8, 3366–3373. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P.E. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Hodgson, J.M.; Woodman, R.J.; Zimmermann, D.; Poquet, L.; Leveques, A.; Actis-Goretta, L.; Puddey, I.B.; Croft, K.D. Acute effects of chlorogenic acids on endothelial function and blood pressure in healthy men and women. Food Funct. 2016, 7, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Álvarez-Quintero, R.; Osorio, E.; Naranjo-Cano, M.; Muñoz-Durango, K. GC/MS method to quantify bioavailable phenolic compounds and antioxidant capacity determination of plasma after acute coffee consumption in human volunteers. Food Res. Int. 2016, 89, 219–226. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Guy, P.A.; Marmet, C.; Fraering, A.L.; Longet, K.; Moulin, J.; Enslen, M.; Barron, D.; Dionisi, F.; Cavin, C.; et al. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: Small intestine and colon are key sites for coffee metabolism. Mol. Nutr. Food Res. 2010, 54, 760–766. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.F.; Restani, P.; Di Lorenzo, C.; Orgiu, F.; Teissedre, P.L.; Stockley, C.; Ruf, J.C.; Quini, C.I.; Garcìa Tejedor, N.; Gargantini, R.; et al. Resveratrol, human health and winemaking perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 1237–1255. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, H.E.C.; Kay, C.D.; Lampe, J.W.; Holub, B.J.; Duncan, A.M. Acute fish oil and soy isoflavone supplementation increase postprandial serum (n-3) polyunsaturated fatty acids and isoflavones but do not affect triacylglycerols or biomarkers of oxidative stress in overweight and obese hypertriglyceridemic men. J. Nutr. 2009, 139, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Brink, E.; Coxam, V.; Robins, S.; Wahala, K.; Cassidy, A.; Branca, F. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: A randomized, double-blind, placebo controlled study. Am. J. Clin. Nutr. 2008, 87, 761–770. [Google Scholar] [CrossRef]
- Schar, M.Y.; Curtis, P.J.; Hazim, S.; Ostertag, L.M.; Kay, C.D.; Potter, J.F.; Cassidy, A. Orange juice-derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: A randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease1-5. Am. J. Clin. Nutr. 2015, 101, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, C.; Dong, H.; Saunders, C.; Harkness, L.; Blaze, M.; Hou, Y.; Belanger, R.L.; Corona, G.; Lovegrove, J.A.; Spencer, J.P.E. Flavanone-rich citrus beverages counteract the transient decline in postprandial endothelial function in humans: A randomised, controlled, double-masked, cross-over intervention study. Br. J. Nutr. 2016, 116, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).