Dietary Intake of trans Fatty Acids in the Slovenian Population
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Subjects
2.2. Food Consumption Data
2.2.1. General Questionnaire and Anthropometric Measurements
2.2.2. 24-h Dietary Recalls
2.3. Assessment of TFA Content
2.4. Final Sample for Data Analyses
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I.A.; Wanders, A.J.; Katan, M.B. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans—A quantitative review. PLoS ONE 2010, 5, e9434. [Google Scholar] [CrossRef]
- Mozaffarian, D. Trans fatty acids—Effects on systemic inflammation and endothelial function. Atheroscler. Suppl. 2006, 7, 29–32. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. European Food and Nutrition Action Plan 2015–2020; World Health Organization: Copenhagen, Denmark, 2015; Available online: https://www.euro.who.int/__data/assets/pdf_file/0008/253727/64wd14e_FoodNutAP_140426.pdf?ua=1 (accessed on 28 December 2020).
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Ghebreyesus, T.A.; Frieden, T.R. REPLACE: A roadmap to make the world trans fat free by 2023. Lancet 2018, 391, 1978–1980. [Google Scholar] [CrossRef]
- L’Abbé, M.R.; Stender, S.; Skeaff, C.M.; Ghafoorunissa, R.; Tavella, M. Approaches to removing trans fats from the food supply in industrialized and developing countries. Eur. J. Clin. Nutr. 2009, 63, S50–S67. [Google Scholar] [CrossRef]
- Restrepo, B.J.; Rieger, M. Denmark’s policy on artificial trans fat and cardiovascular disease. Am. J. Prev. Med. 2016, 50, 69–76. [Google Scholar] [CrossRef]
- World Health Organization. Eliminating Trans Fats in Europe: A Policy Brief; WHO Regional Office for Europe: Copenhagen, Denmark, 2015. [Google Scholar]
- Zuchowska-Grzywacz, M.; Kowalska, M. Trans fatty acids in food—Current legal regulations as protections for consumers and food manufacturers. Acta Aliment. 2019, 48, 105–114. [Google Scholar] [CrossRef]
- Republike Slovenije. Pravilnik o Največji Dovoljeni Vsebnosti Transmaščobnih Kislin v Živilih. Available online: https://www.uradni-list.si/glasilo-uradni-list-rs/vsebina/2018-01-0801/ (accessed on 28 December 2020).
- Zupanič, N.; Hribar, M.; Pivk Kupirovič, U.; Kušar, A.; Žmitek, K.; Pravst, I. Limiting trans fats in foods: Use of partially hydrogenated vegetable oils in prepacked foods in Slovenia. Nutrients 2018, 10, 355. [Google Scholar] [CrossRef]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Guidance on the EU Menu methodology. EFSA J. 2014, 12, 3944. [Google Scholar] [CrossRef]
- Gregorič, M.; Blaznik, U.; Delfar, N.; Zaletel, M.; Lavtar, D.; Seljak, B.K.; Golja, P.; Kotnik, K.Z.; Pravst, I.; Mis, N.F. Slovenian national food consumption survey in adolescents, adults and elderly. EFSA Supporting Publ. 2019, 16, 1729E. [Google Scholar]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Onyango, A.; Borghi, E.; Siyam, A.; Blössner, M.; Lutter, C. Worldwide implementation of the WHO child growth standards. Public Health Nutr. 2012, 15, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Korošec, M.; Golob, T.; Bertoncelj, J.; Stibilj, V.; Seljak, B.K. The Slovenian food composition database. Food Chem. 2013, 140, 495–499. [Google Scholar] [CrossRef]
- Bodner-Montville, J.; Ahuja, J.K.; Ingwersen, L.A.; Haggerty, E.S.; Enns, C.W.; Perloff, B.P. USDA food and nutrient database for dietary studies: Released on the web. J. Food Comp. Anal. 2006, 19, S100–S107. [Google Scholar] [CrossRef]
- Kušar, A.; Hribar, M.; Lavriša, Ž.; Zupanič, N.; Kupirovič, U.P.; Hristov, H.; Abramovič, H.; Vidrih, R.; Zlatić, E.; Kokalj, D. Assessment of trans-fatty acid content in a sample of foods from the Slovenian food supply using a sales-weighting approach. Public Health Nutr. 2020, 24, 12–21. [Google Scholar] [CrossRef]
- EC. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu/legal-content/EN/AUTO/?uri=CELEX:02011R1169-20180101&qid=1547797296601 (accessed on 28 December 2020).
- Dunford, E.; Neal, B.; Macgregor, G.; Czernichow, S.; Ni Mhurchu, C.; Food Monitoring Group. International collaborative project to compare and track the nutritional composition of fast foods. BMC Public Health 2012, 12, 559. [Google Scholar]
- Zupanič, N.; Hristov, H.; Gregorič, M.; Blaznik, U.; Delfar, N.; Koroušić Seljak, B.; Ding, E.L.; Fidler Mis, N.; Pravst, I. Total and Free Sugars Consumption in a Slovenian Population Representative Sample. Nutrients 2020, 12, 1729. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.; Black, A.; Jebb, S.; Cole, T.; Murgatroyd, P.; Coward, W.; Prentice, A. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar] [PubMed]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370. [Google Scholar] [CrossRef]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Harttig, U.; Haubrock, J.; Knüppel, S.; Boeing, H. The MSM program: Web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur. J. Clin. Nutr. 2011, 65, S87–S91. [Google Scholar] [CrossRef]
- Kolenikov, S. Calibrating survey data using iterative proportional fitting (raking). Stata J. 2014, 14, 22–59. [Google Scholar] [CrossRef]
- Wanders, A.J.; Zock, P.L.; Brouwer, I.A. Trans fat intake and its dietary sources in general populations worldwide: A systematic review. Nutrients 2017, 9, 840. [Google Scholar] [CrossRef]
- Temme, E.H.; Millenaar, I.L.; Van Donkersgoed, G.; Westenbrink, S. Impact of fatty acid food reformulations on intake of Dutch young adults. Acta Cardiol. 2011, 66, 721–728. [Google Scholar] [CrossRef]
- Hutchinson, J.; Rippin, H.L.; Jewell, J.; Breda, J.J.; Cade, J.E. Comparison of high and low trans-fatty acid consumers: Analyses of UK National Diet and Nutrition Surveys before and after product reformulation. Public Health Nutr. 2018, 21, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Astrup, A.; Dyerberg, J. Artificial trans fat in popular foods in 2012 and in 2014: A market basket investigation in six European countries. BMJ Open 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Destaillats, F.; Dionisi, F.; Krauss, R.M.; Baer, D.J. Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect LDL cholesterol: A double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Baehr, M.; Rohrer, C.; Jahreis, G. Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods. Eur. J. Lipid Sci. Technol. 2011, 113, 1281–1292. [Google Scholar] [CrossRef]
- Aldai Elkoro-Iribe, N.; Dugan, M.; Rolland, D.; Kramer, K. Survey of the fatty acid composition of Canadian beef: Backfat and longissimus lumborum muscle. Can. J. Anim. Sci. 2009, 89, 315–329. [Google Scholar] [CrossRef]
- Wang, X.; England, A.; Sinclair, C.; Merkosky, F.; Chan, C.B. Trans-11 vaccenic acid improves glucose homeostasis in a model of type 2 diabetes by promoting insulin secretion via GPR40. J. Funct. Foods 2019, 60, 103410. [Google Scholar] [CrossRef]
- Lim, J.-N.; Oh, J.-J.; Wang, T.; Lee, J.-S.; Kim, S.-H.; Kim, Y.-J.; Lee, H.-G. Trans-11 18: 1 vaccenic acid (TVA) has a direct anti-carcinogenic effect on MCF-7 human mammary adenocarcinoma cells. Nutrients 2014, 6, 627–636. [Google Scholar] [CrossRef]
- Bassett, C.M.; Edel, A.L.; Patenaude, A.F.; McCullough, R.S.; Blackwood, D.P.; Chouinard, P.Y.; Paquin, P.; Lamarche, B.; Pierce, G.N. Dietary vaccenic acid has antiatherogenic effects in LDLr−/− mice. J. Nutr. 2010, 140, 18–24. [Google Scholar] [CrossRef]
- Oteng, A.-B.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 2019, 11, 697–708. [Google Scholar] [CrossRef]
- Stender, S.; Astrup, A.; Dyerberg, J. What went in when trans went out? N. Engl. J. Med. 2009, 361, 314–316. [Google Scholar] [CrossRef]
- Eckel, R.H.; Borra, S.; Lichtenstein, A.H.; Yin-Piazza, S.Y. Understanding the complexity of trans fatty acid reduction in the American diet: American Heart Association Trans Fat Conference 2006: Report of the Trans Fat Conference Planning Group. Circulation 2007, 115, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Jacobson, M.F.; Greenstein, J.S. Food reformulations to reduce trans fatty acids. N. Engl. J. Med. 2010, 362, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.; L’Abbe, M.; Mozaffarian, D. Nationwide product reformulations to reduce trans fatty acids in Canada: When trans fat goes out, what goes in? Eur. J. Clin. Nutr. 2009, 63, 808–811. [Google Scholar] [CrossRef] [PubMed]
- NUTRIS. The Content of Trans Fats in Foods and Population Intakes—Public Health Implications. Available online: https://www.nutris.org/en/projects/trans-fats-in-foods (accessed on 28 December 2020).
- Poslusna, K.; Ruprich, J.; de Vries, J.H.; Jakubikova, M.; van’t Veer, P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br. J. Nutr. 2009, 101, S73–S85. [Google Scholar] [CrossRef]
- Remig, V.; Franklin, B.; Margolis, S.; Kostas, G.; Nece, T.; Street, J.C. Trans fats in America: A review of their use, consumption, health implications, and regulation. J. Am. Diet. Assoc. 2010, 110, 585–592. [Google Scholar] [CrossRef]
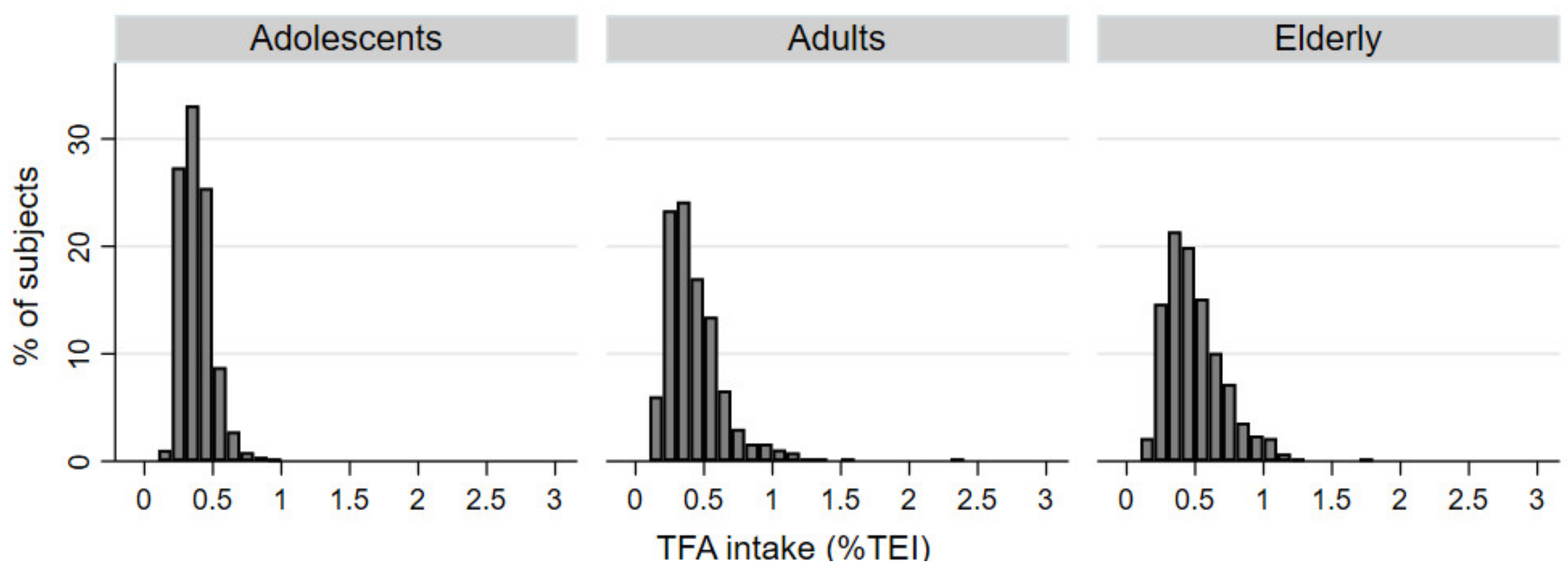
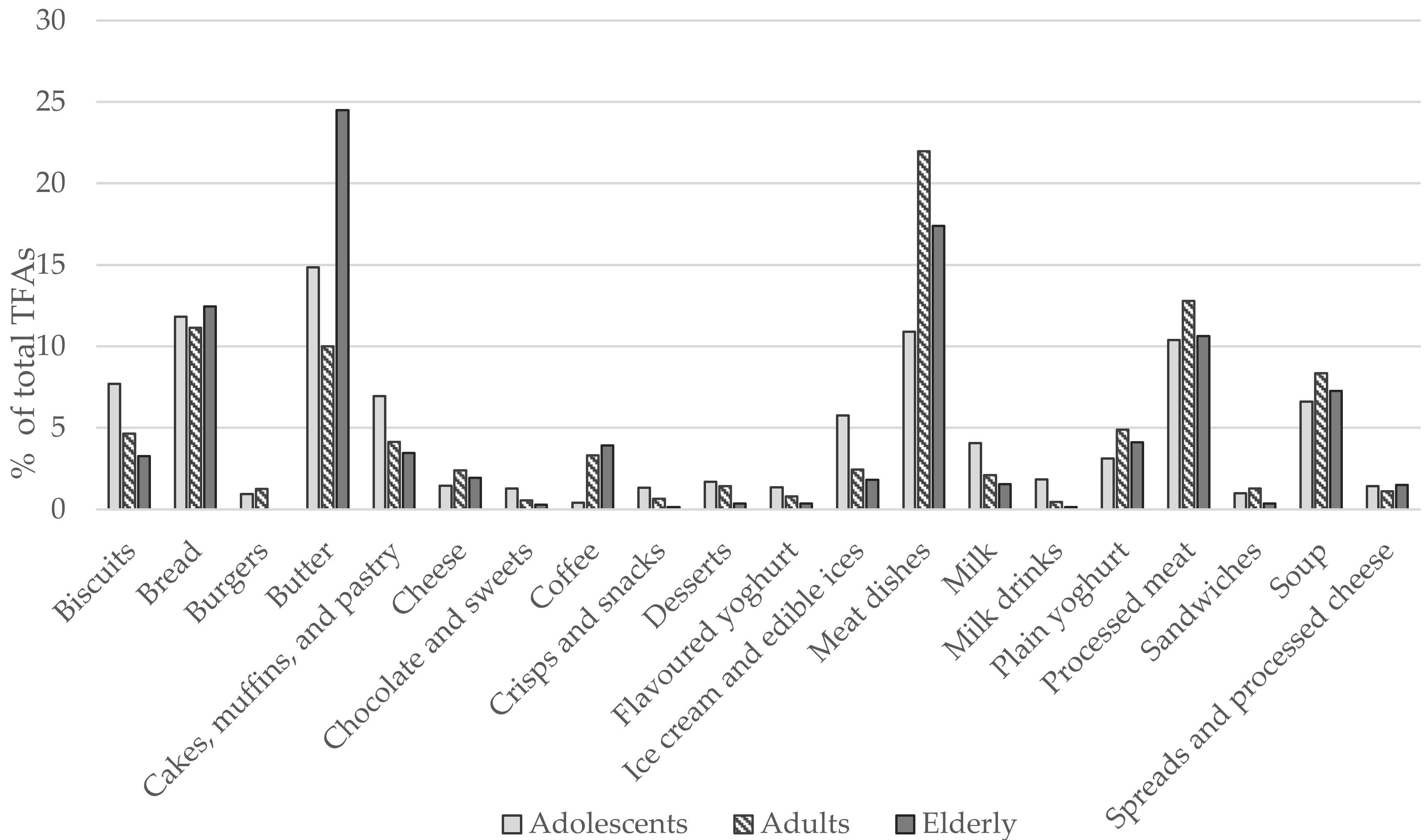
| Adolescents (10–17 years) | Adults (18–64 years) | Elderly (65–74 years) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | |
| Sample Size | |||||||||
| N (%) | 468 (100) | 238 (50.85) | 230 (49.15) | 364 (100) | 173 (47.53) | 191(52.47) | 416 (100) | 213 (51.20) | 203 (48.80) |
| Margin of error (%) | 4.53 | 6.36 | 6.47 | 5.14 | 7.45 | 7.09 | 4.81 | 6.71 | 6.88 |
| TFAs intake | |||||||||
| Mean [g/day] | 0.68 | 0.68 | 0.68 | 0.77 | 0.78 | 0.75 | 0.89 | 0.90 | 0.87 |
| Median [g/day] | 0.67 | 0.67 | 0.68 | 0.73 | 0.75 | 0.72 | 0.84 | 0.86 | 0.82 |
| Mean % TEI (95% CI) | 0.38 (0.35–0.39) | 0.33 (0.31–0.35) | 0.41 (0.38–0.45) | 0.42 (0.40–0.45) | 0.38 (0.35–0.41) | 0.46 (0.43–0.50) | 0.50 (0.47–0.53) | 0.48 (0.43–0.52) | 0.51 (0.47–0.56) |
| TEI from TFAs (%) | |||||||||
| TFA > 0.5% TEI (95% CI) | 11.50 (8.34–15.7) | 7.5 (4.6–12.2) | 15.7 (10.5–23.0) | 28.9 (23.1–33.2) | 23.0 (16.8–30.5) | 32.8 (25.9–40.6) | 43.9 (34.1–54.1) | 41.6 (24.8–60.6) | 45.9 (35.9–56.3) |
| TFA > 1.0% TEI (95% CI) | 2.51 (1.25–4.96) | 1.01 (2.47–4.06) | 4.02 (1.82–8.66) | 3.02 (1.64–5.50) | 4.18 (1.89–9.00) | 1.96 (0.74–5.14) | |||
| Variable | Adolescents (10–17 years) | Adults (18–64 years) | Elderly (65–74 years) | ||||
|---|---|---|---|---|---|---|---|
| n (%) | Adjusted | n (%) | Adjusted | n (%) | Adjusted | ||
| Overall | 468 (37.5) | 364 (29.2) | 416 (33.3) | ||||
| Sex | Male | 238 (50.9) | 0.35 (0.33–0.36) | 173 (47.5) | 0.39 (0.35–0.42) | 213 (51.2) | 0.46 (0.43–0.49) |
| Female | 230 (49.1) | 0.41 (0.40–0.43) | 191 (52.5) | 0.47 (0.44–0.51) | 203 (48.8) | 0.54 (0.50–0.57) | |
| Place of living | Rural | 270 (57.7) | 0.37 (0.37–0.39) | 202 (55.5) | 0.41 (0.38–0.45) | 229 (55.1) | 0.48 (0.45–0.51) |
| Intermediate | 76 (16.2) | 0.37 (0.34–0.39) | 56 (15.4) | 0.47 (0.40–0.53) | 71 (17.1) | 0.51 (0.46–0.56) | |
| Urban | 122 (26.1) | 0.40 (0.38–0.42) | 106 (29.1) | 0.45 (0.40–0.49) | 116 (27.9) | 0.52 (0.48–0.57) | |
| Education | No university degree | - | - | 249 (68.4) | 0.44 (0.41–0.47) | 342 (82.2) | 0.50 (0.48–0.53) |
| University degree | 115 (31.6) | 0.42 (0.38–0.47) | 74 (17.8) | 0.48 (0.43–0.54) | |||
| Family net income | Below average | - | - | 118 (38.4) | 0.44 (0.40–0.48) | 269 (71.5) | 0.51 (0.48–0.54) |
| Above average | 189 (61.6) | 0.43 (0.40–0.46) | 107 (28.5) | 0.47 (0.43–0.51) | |||
| BMI | Normal | 301 (64.3) | 0.37 (0.36–0.38) | 148 (40.7) | 0.40 (0.36–0.44) | 108 (26.0) | 0.51 (0.47–0.55) |
| Overweight/obese | 167 (35.7) | 0.39 (0.38–0.41) | 216 (59.3) | 0.46 (0.42–0.49) | 308 (74.0) | 0.50 (0.47–0.52) | |
| IPAQ | Low intensity | 108 (23.3) | 0.37 (0.35–0.39) | 127 (35.3) | 0.42 (0.38–0.46) | 137 (33.4) | 0.49 (0.46–0.53) |
| Moderate | 141 (30.5) | 0.39 (0.38–0.41) | 108 (30.0) | 0.45(0.41–0.50) | 133 (32.4) | 0.52 (0.48–0.55) | |
| High intensity | 214 (46.2) | 0.38 (0.36–0.39) | 125 (34.7) | 0.44 (0.39–0.48) | 140 (34.2) | 0.49 (0.45–0.52) | |
| Employment | Employed | - | - | 226 (62.1) | 0.42 (0.39–0.45) | - | - |
| Unemployed | 42 (11.5) | 0.40 (0.33–0.48) | |||||
| Student | 32 (8.8) | 0.43 (0.34–0.52) | |||||
| Retired | 64 (17.6) | 0.50 (0.44–0.56) | |||||
| Variable | Adolescents (10–17 Years Old) | Adults (18–64 Years Old) | Elderly (65–74 Years Old) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | >0.5% TEI n (%) | Odds Ratio * | n | >0.5% TEI n (%) | Odds Ratio * | n | >0.5% TEI n (%) | Odds Ratio * | ||
| Overall | 468 | 61 (13.03) | 364 | 107 (29.40) | 416 | 174 (41.83) | ||||
| Sex | Male | 238 | 20 (8.40) | 1 | 173 | 42 (24.28) | 1 | 213 | 64 (30.05) | 1 |
| Female | 230 | 41 (17.83) | 2.45 (1.35–4.46) | 191 | 65 (34.03) | 1.65 (0.98–2.78) | 203 | 110 (54.19) | 2.45 (1.58–3.81) | |
| Place of living | Rural | 270 | 33 (12.22) | 1 | 202 | 53 (26.24) | 1 | 229 | 84 (36.68) | 1 |
| Intermediate | 76 | 6 (7.89) | 0.65 (0.25–1.64) | 56 | 19 (33.93) | 1.48 (0.73–2.98) | 71 | 31 (43.66) | 1.06 (0.59–1.92) | |
| Urban | 122 | 22 (18.03) | 1.71 (0.92–3.15) | 106 | 35 (33.02) | 1.30 (0.74–2.30) | 116 | 59 (50.86) | 1.68 (1.01–2.80) | |
| Education | No university degree | - | - | 249 | 75 (30.12) | 1 | 342 | 146 (42.69) | 1 | |
| University degree | 115 | 32 (27.83) | 0.96 (0.53–1.75) | 74 | 28 (37.84) | 0.66 (0.36–1.24) | ||||
| Family net income | Below average (≤1300 €) | - | - | 118 | 39 (33.05) | 1 | 269 | 111 (41.26) | 1 | |
| Above average (>1300 €) | 189 | 56 (29.63) | 1.03 (0.58–1.85) | 107 | 42 (39.25) | 0.96 (0.58–1.60) | ||||
| BMI | Normal | 301 | 33(10.96) | 1 | 148 | 38 (25.68) | 1 | 108 | 55 (50.93) | 1 |
| Overweight (including obese) | 167 | 28 (16.77) | 1.64 (0.92–2.91) | 216 | 69 (31.94) | 1.66 (0.95–2.90) | 308 | 119 (38.64) | 0.75 (0.46–1.24) | |
| IPAQ | Low intensity | 108 | 13 (12.04) | 1 | 127 | 31 (24.41) | 1 | 137 | 53 (38.69) | 1 |
| Moderate | 141 | 25 (17.73) | 1.28 (0.61–2.70) | 108 | 34 (31.48) | 1.53 (0.82–2.87) | 133 | 60 (45.11) | 1.12 (0.66–1.91) | |
| High intensity | 214 | 20 (9.35) | 0.71 (0.34–1.53) | 125 | 41 (32.80) | 1.37 (0.73–2.55) | 140 | 60 (42.86) | 1.13 (0.66–1.91) | |
| Employment | Employed | - | - | 226 | 61 (26.99) | 1 | - | - | ||
| Unemployed | 42 | 13 (30.95) | 0.97 (0.40–2.33) | |||||||
| Student | 32 | 8 (25.00) | 1.51 (0.55–4.13) | |||||||
| Retired | 64 | 25 (39.06) | 1.98 (0.97–4.02) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupanič, N.; Hribar, M.; Hristov, H.; Lavriša, Ž.; Kušar, A.; Gregorič, M.; Blaznik, U.; Koroušić Seljak, B.; Golja, P.; Vidrih, R.; et al. Dietary Intake of trans Fatty Acids in the Slovenian Population. Nutrients 2021, 13, 207. https://doi.org/10.3390/nu13010207
Zupanič N, Hribar M, Hristov H, Lavriša Ž, Kušar A, Gregorič M, Blaznik U, Koroušić Seljak B, Golja P, Vidrih R, et al. Dietary Intake of trans Fatty Acids in the Slovenian Population. Nutrients. 2021; 13(1):207. https://doi.org/10.3390/nu13010207
Chicago/Turabian StyleZupanič, Nina, Maša Hribar, Hristo Hristov, Živa Lavriša, Anita Kušar, Matej Gregorič, Urška Blaznik, Barbara Koroušić Seljak, Petra Golja, Rajko Vidrih, and et al. 2021. "Dietary Intake of trans Fatty Acids in the Slovenian Population" Nutrients 13, no. 1: 207. https://doi.org/10.3390/nu13010207
APA StyleZupanič, N., Hribar, M., Hristov, H., Lavriša, Ž., Kušar, A., Gregorič, M., Blaznik, U., Koroušić Seljak, B., Golja, P., Vidrih, R., Žmitek, K., & Pravst, I. (2021). Dietary Intake of trans Fatty Acids in the Slovenian Population. Nutrients, 13(1), 207. https://doi.org/10.3390/nu13010207











