Effect of the Natural Sweetener Xylitol on Gut Hormone Secretion and Gastric Emptying in Humans: A Pilot Dose-Ranging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Subjects
2.3. Study Design and Experimental Procedure
- 7 g xylitol dissolved in 300 mL tap water
- 17 g xylitol dissolved in 300 mL tap water
- 35 g xylitol dissolved in 300 mL tap water
- 300 mL tap water (placebo)
2.4. Materials
2.5. Blood Sample Collection and Processing
2.6. Assessment of Gastric Emptying Rates
2.7. Laboratory Analysis
2.8. Statistics
3. Results
3.1. Fasting Values
3.2. Gut Hormone Secretion
3.3. Minimum Detectable Difference
3.4. Dose–Response Evaluation
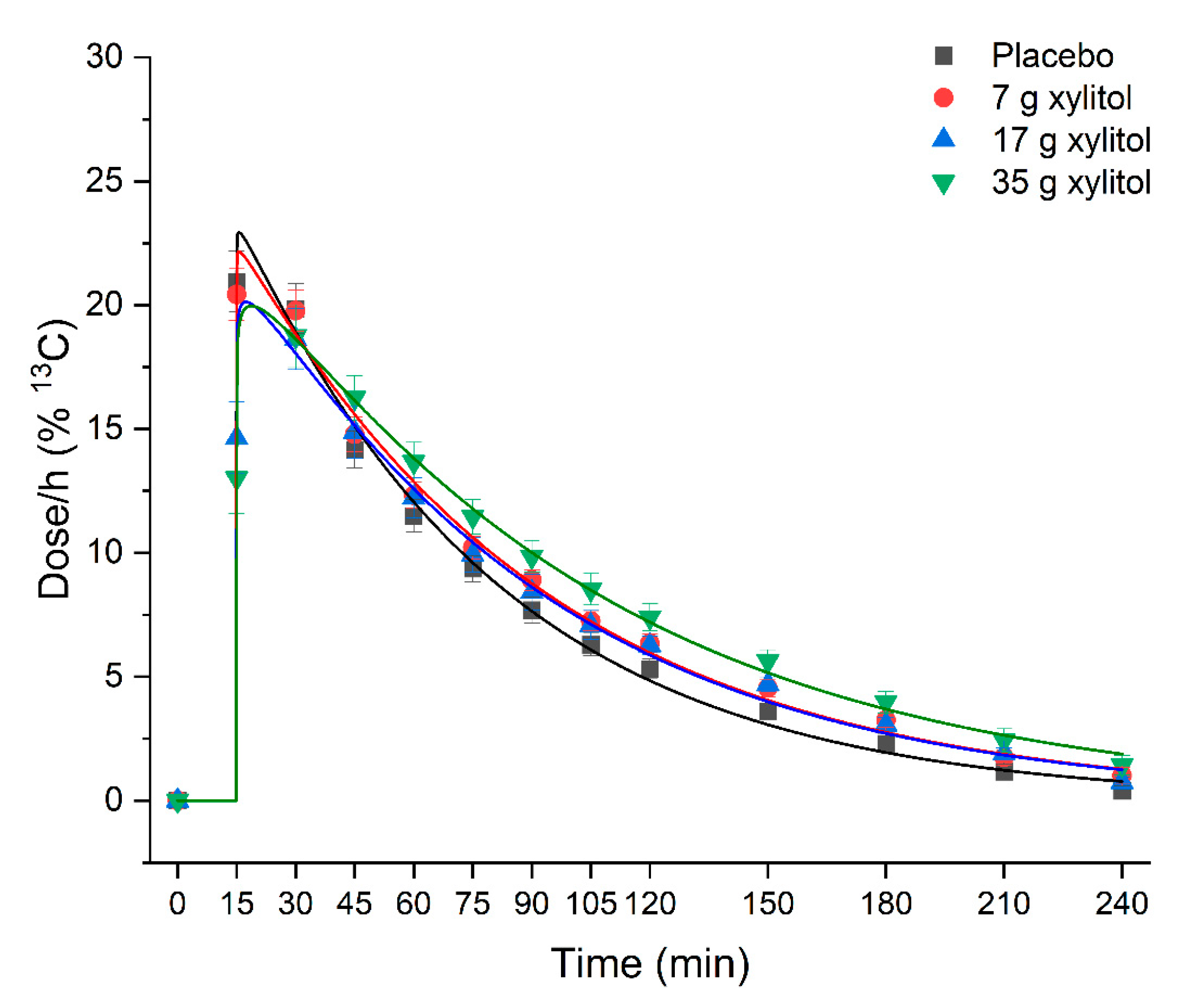
3.5. Gastric Emptying
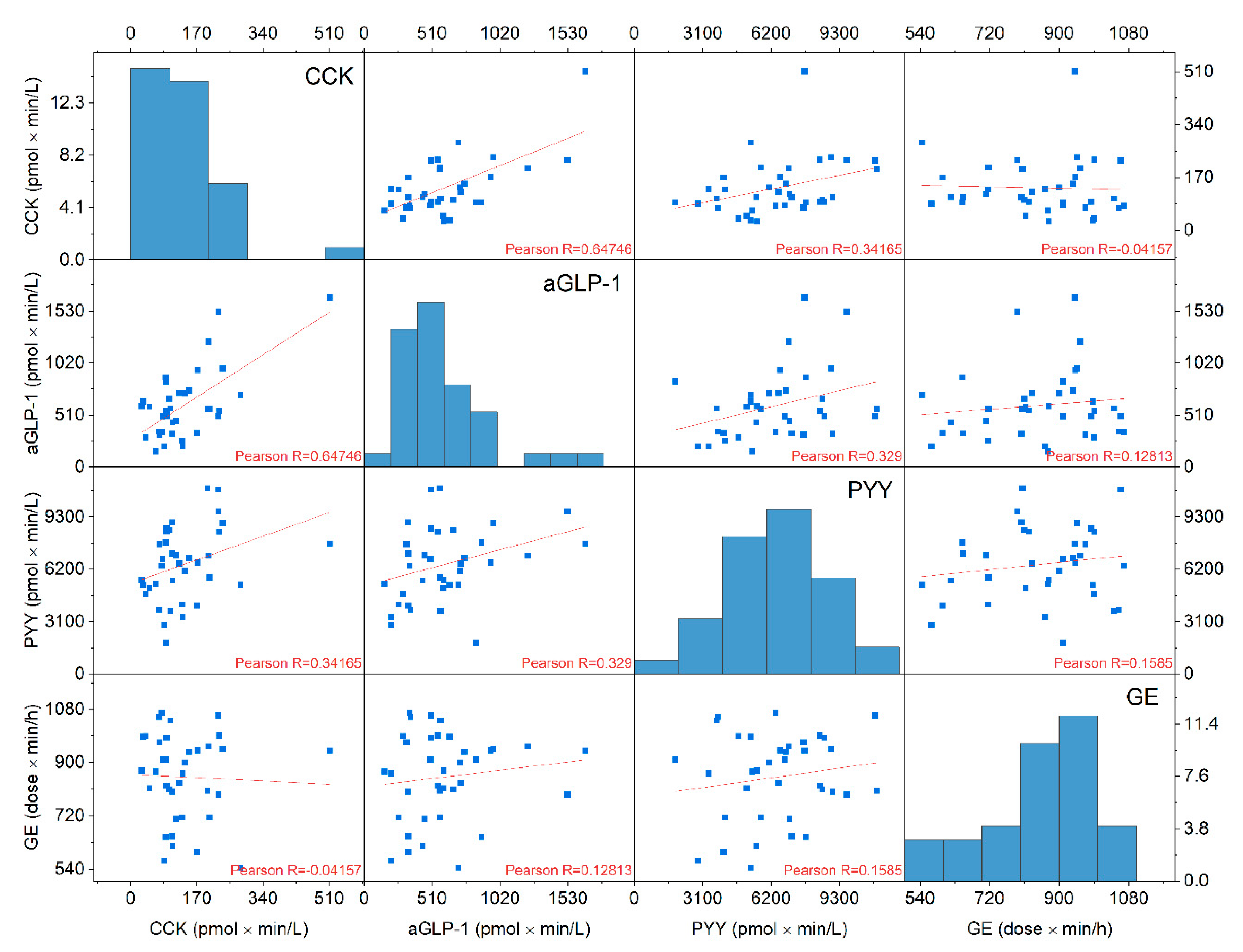
3.6. Correlations Gastric Emptyimg Rates and Gut Hormone Concentrations
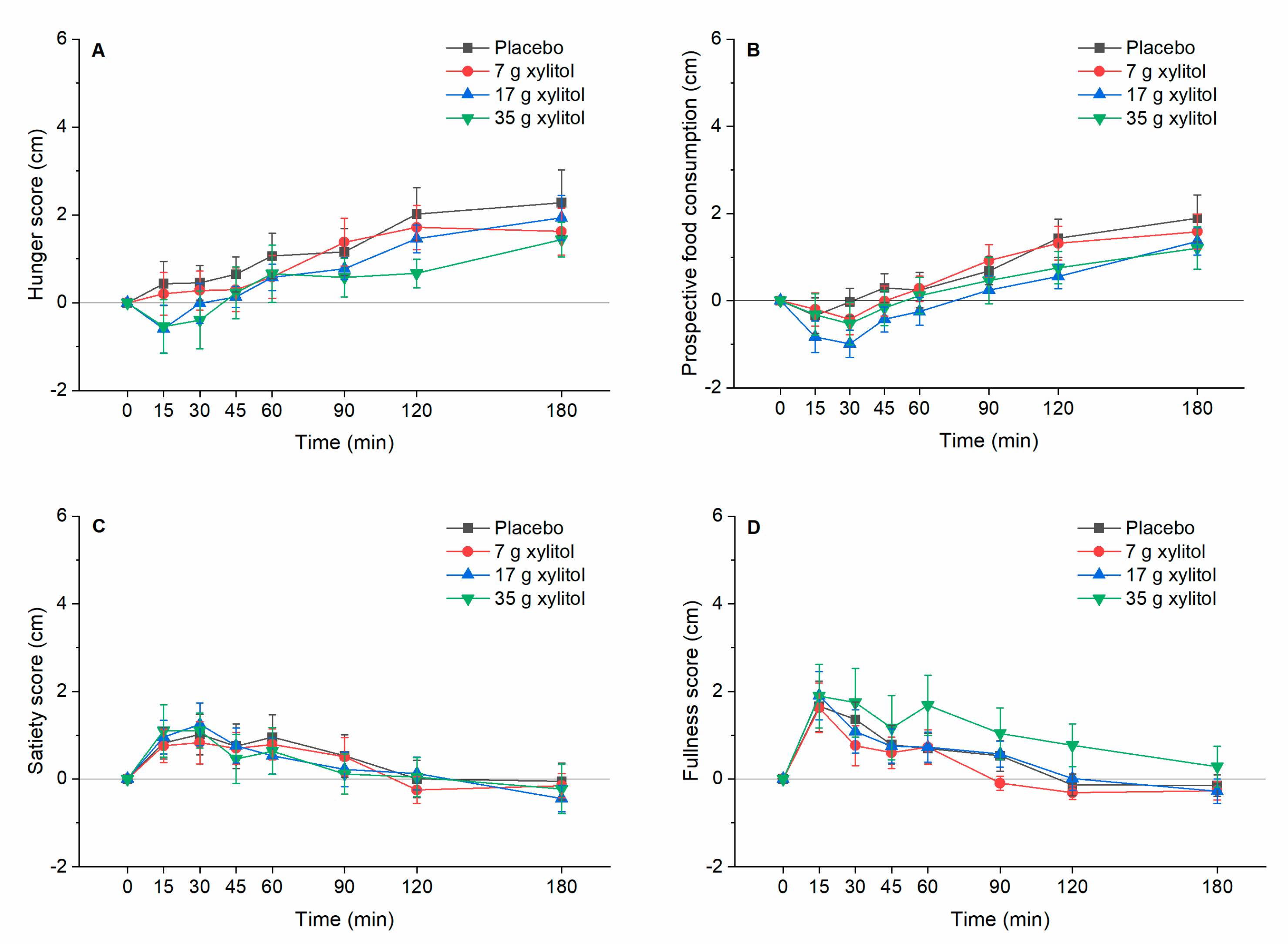
3.7. Appetite-Realted Sensations
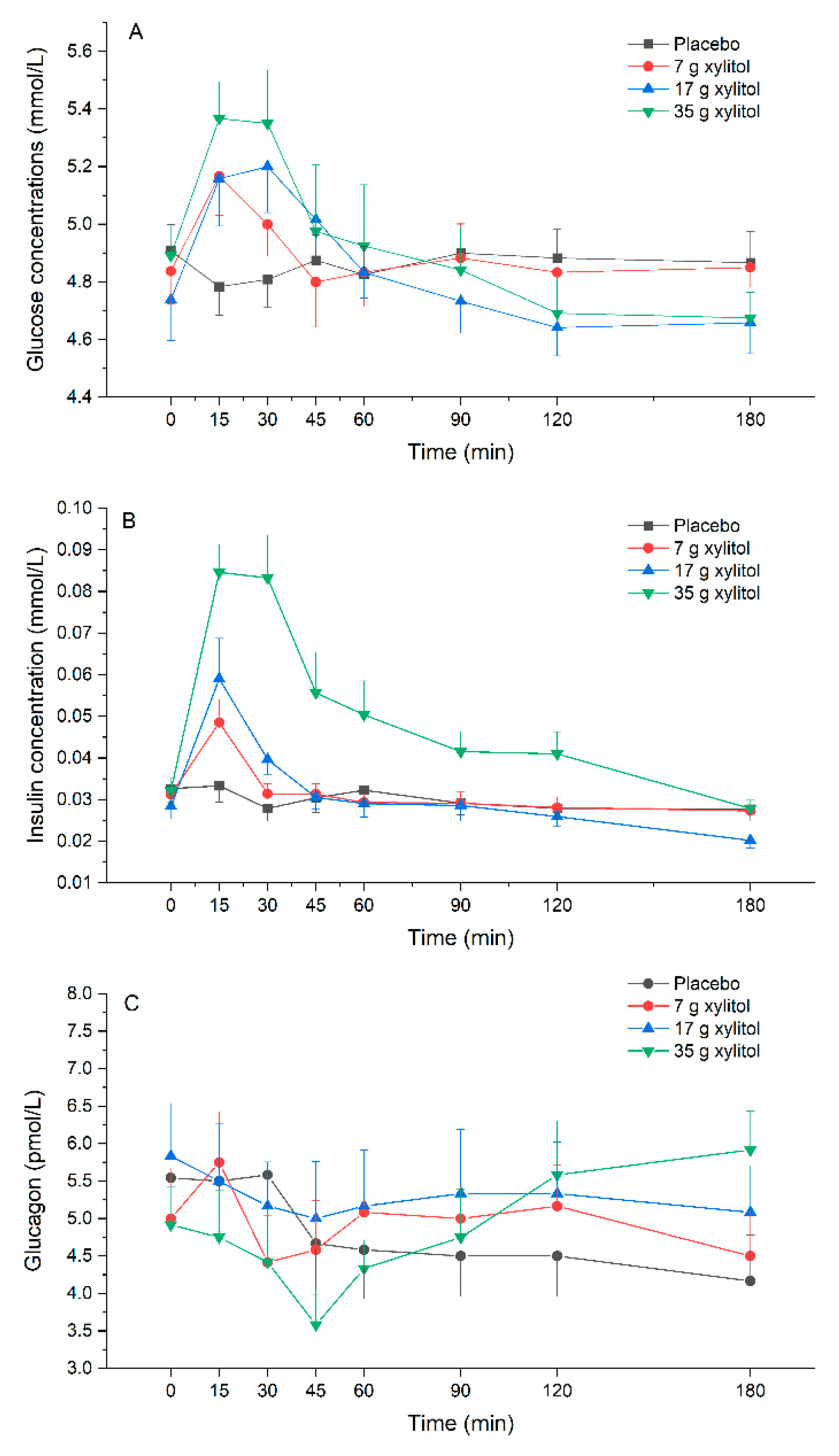
3.8. Plasma Glucose, Insulin and Glucagon Release
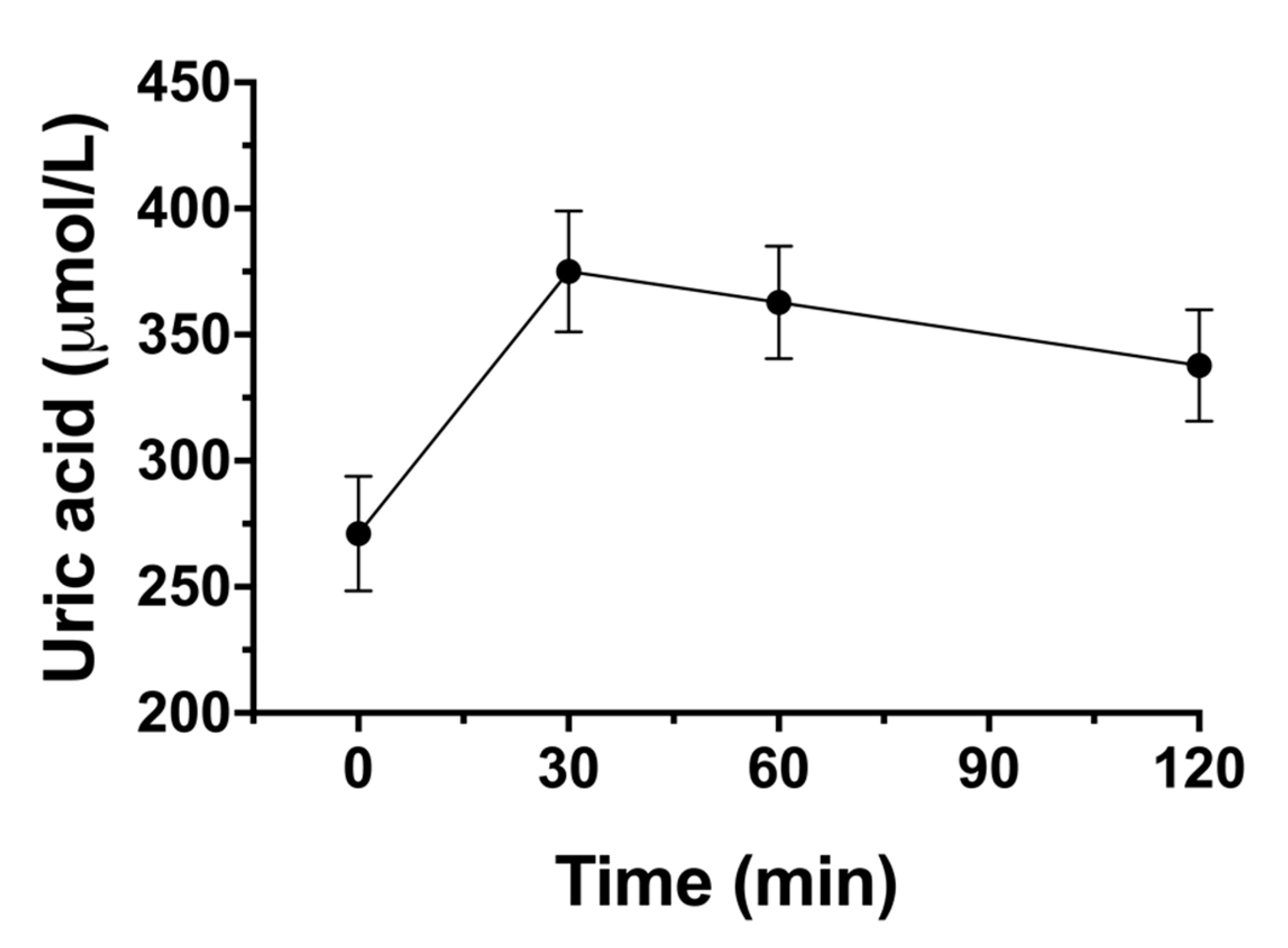
3.9. Impact on Blood Lipid Profil, Uric Acid and Gastrointestinal Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
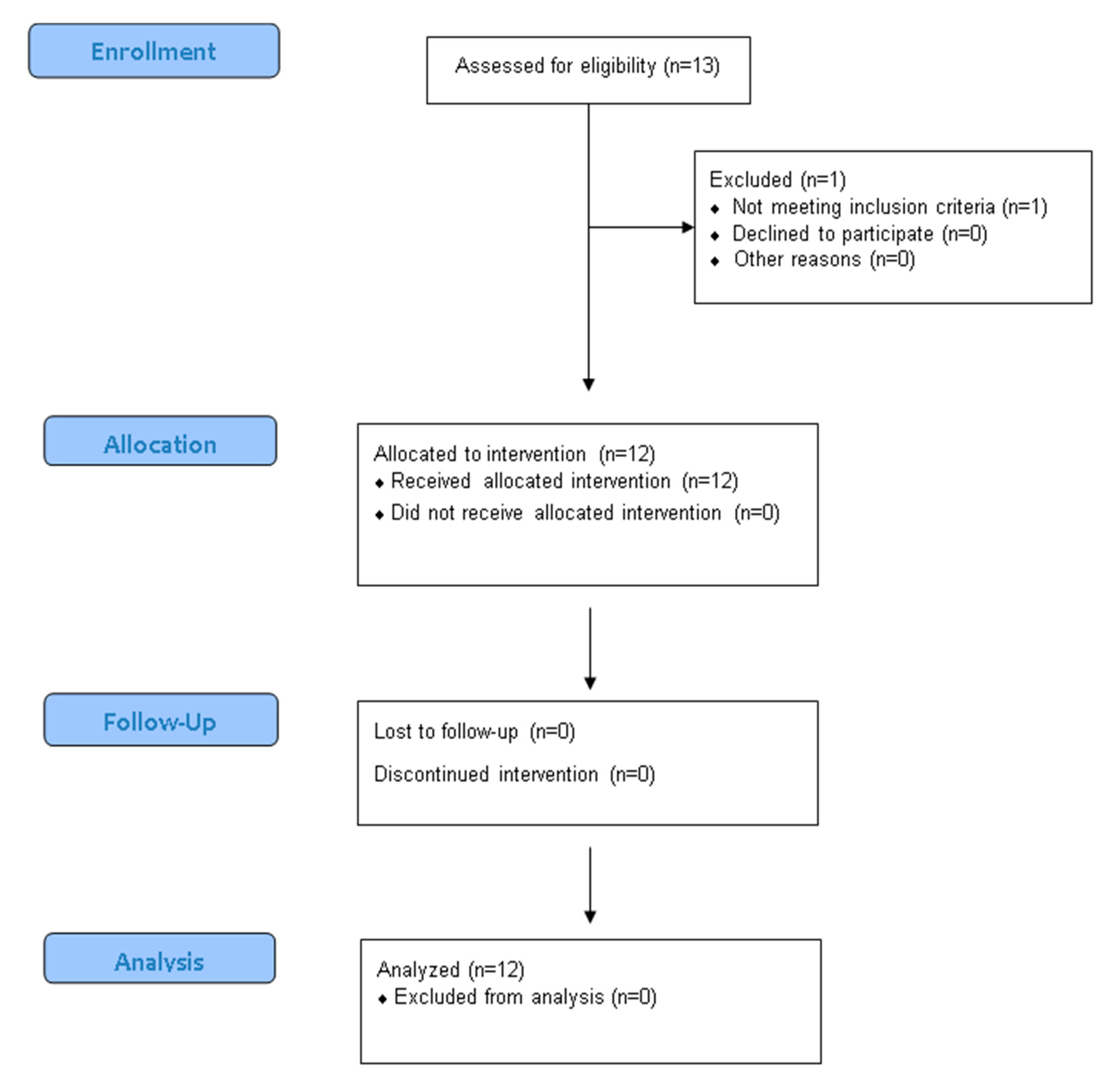
References
- WHO. Sugars Intake for Adults and Children: Guideline; World Health Organization: Geneva, Switzerland, 2015; ISBN 9789241549028. [Google Scholar]
- Hunter, S.R.; Reister, E.J.; Cheon, E.; Mattes, R.D. Low Calorie Sweeteners Differ in Their Physiological Effects in Humans. Nutrients 2019, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Salminen, E.K.; Salminen, S.J.; Porkka, L.; Kwasowski, P.; Marks, V.; Koivistoinen, P.E. Xylitol vs glucose: Effect on the rate of gastric emptying and motilin, insulin, and gastric inhibitory polypeptide release. Am. J. Clin. Nutr. 1989, 49, 1228–1232. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1053–E1061. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef]
- Jameel, F.; Phang, M.; Wood, L.G.; Garg, M.L. Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis. 2014, 13, 195. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sanchez-Lozada, L.G.; Kang, D.H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef]
- Forster, H.; Ziege, M. Increase of plasma uric acid concentration after oral administration of fructose, sorbit and xylit. Z. fur Ernahr. 1971, 10, 394–396. [Google Scholar]
- Yamamoto, T.; Moriwaki, Y.; Takahashi, S.; Suda, M.; Higashino, K. Xylitol-induced increase in the concentration of oxypurines and its mechanism. Int. J. Clin. Pharmacol. Ther. 1995, 33, 360–365. [Google Scholar]
- Forster, H.; Boecker, S.; Walther, A. Use of xylitol as sugar substitute in diabetic children. Fortschr. Med. 1977, 95, 99–102. [Google Scholar]
- Huttunen, J.K.; Makinen, K.K.; Scheinin, A. Turku sugar studies XI. Effects of sucrose, fructose and xylitol diets on glucose, lipid and urate metabolism. Acta Odontol. Scand. 1976, 34, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Sonnichsen, A.C.; Ritter, M.M.; Richter, W.O.; Schwandt, P. Influence of fiber, xylitol and fructose in enteral formulas on glucose and lipid metabolism in normal subjects. Clin. Investig. 1993, 71, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Forster, H.; Quadbeck, R.; Gottstein, U. Metabolic tolerance to high doses of oral xylitol in human volunteers not previously adapted to xylitol. Int. J. Vitam. Nutr. Res. Suppl. 1982, 22, 67–88. [Google Scholar] [PubMed]
- Crum, A.J.; Corbin, W.R.; Brownell, K.D.; Salovey, P. Mind over milkshakes: Mindsets, not just nutrients, determine ghrelin response. Health Psychol. 2011, 30, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Ghoos, Y.F.; Maes, B.D.; Geypens, B.J.; Mys, G.; Hiele, M.I.; Rutgeerts, P.J.; Vantrappen, G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993, 104, 1640–1647. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Accurate measurement of cholecystokinin in plasma. Clin. Chem. 1998, 44, 991–1001. [Google Scholar] [CrossRef]
- Bormans, V.; Peeters, T.L.; Janssens, J.; Pearce, D.; Vandeweerd, M.; Vantrappen, G. In man, only activity fronts that originate in the stomach correlate with motilin peaks. Scand. J. Gastroenterol. 1987, 22, 781–784. [Google Scholar] [CrossRef]
- Holst, J.J. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33–69) of glicentin. Biochem. J. 1982, 207, 381–388. [Google Scholar] [CrossRef]
- Wewer Albrechtsen, N.J.; Veedfald, S.; Plamboeck, A.; Deacon, C.F.; Hartmann, B.; Knop, F.K.; Vilsboll, T.; Holst, J.J. Inability of Some Commercial Assays to Measure Suppression of Glucagon Secretion. J. Diabetes Res. 2016, 2016, 8352957. [Google Scholar] [CrossRef] [PubMed]
- Raybould, H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010, 153, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F. Incretin physiology beyond glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: Cholecystokinin and gastrin peptides. Acta Physiol. 2011, 201, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Frey, F.; Topfer, A.; Drewe, J.; Beglinger, C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br. J. Nutr. 2011, 105, 1320–1328. [Google Scholar] [CrossRef]
- Ma, J.; Bellon, M.; Wishart, J.M.; Young, R.; Blackshaw, L.A.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G735–G739. [Google Scholar] [CrossRef]
- Meyer-Gerspach, A.C.; Biesiekierski, J.R.; Deloose, E.; Clevers, E.; Rotondo, A.; Rehfeld, J.F.; Depoortere, I.; Van Oudenhove, L.; Tack, J. Effects of caloric and noncaloric sweeteners on antroduodenal motility, gastrointestinal hormone secretion and appetite-related sensations in healthy subjects. Am. J. Clin. Nutr. 2018, 107, 707–716. [Google Scholar] [CrossRef]
- Veedfald, S.; Wu, T.; Bound, M.; Grivell, J.; Hartmann, B.; Rehfeld, J.F.; Deacon, C.F.; Horowitz, M.; Holst, J.J.; Rayner, C.K. Hyperosmolar Duodenal Saline Infusion Lowers Circulating Ghrelin and Stimulates Intestinal Hormone Release in Young Men. J. Clin. Endocrinol. Metab. 2018, 103, 4409–4418. [Google Scholar] [CrossRef]
- Peeters, T.L.; Vantrappen, G.; Janssens, J. Fasting plasma motilin levels are related to the interdigestive motility complex. Gastroenterology 1980, 79, 716–719. [Google Scholar] [CrossRef]
- Vantrappen, G.; Janssens, J.; Peeters, T.L.; Bloom, S.R.; Christofides, N.D.; Hellemans, J. Motilin and the interdigestive migrating motor complex in man. Dig. Dis. Sci. 1979, 24, 497–500. [Google Scholar] [CrossRef]
- Deloose, E.; Vos, R.; Corsetti, M.; Depoortere, I.; Tack, J. Endogenous motilin, but not ghrelin plasma levels fluctuate in accordance with gastric phase III activity of the migrating motor complex in man. Neurogastroenterol. Motil. 2015, 27, 63–71. [Google Scholar] [CrossRef]
- Deloose, E.; Vos, R.; Janssen, P.; Van den Bergh, O.; Van Oudenhove, L.; Depoortere, I.; Tack, J. The motilin receptor agonist erythromycin stimulates hunger and food intake through a cholinergic pathway. Am. J. Clin. Nutr. 2016, 103, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I.; Islam, M.S. Effects of xylitol on carbohydrate digesting enzymes activity, intestinal glucose absorption and muscle glucose uptake: A multi-mode study. Food Funct. 2015, 6, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Islam, M.S. Xylitol improves pancreatic islets morphology to ameliorate type 2 diabetes in rats: A dose response study. J. Food Sci. 2014, 79, H1436–H1442. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I.; Islam, S. Xylitol Improves Anti-Oxidative Defense System in Serum, Liver, Heart, Kidney and Pancreas of Normal and Type 2 Diabetes Model of Rats. Acta Pol. Pharm. 2017, 74, 817–826. [Google Scholar] [PubMed]
- Mäkinen, K.K.; Scheinin, A. Turku sugar studies VI. The administration of the trial and the control of the dietary regimen. Acta Odontol. Scand. 1975, 33, 105–127. [Google Scholar] [CrossRef] [PubMed]
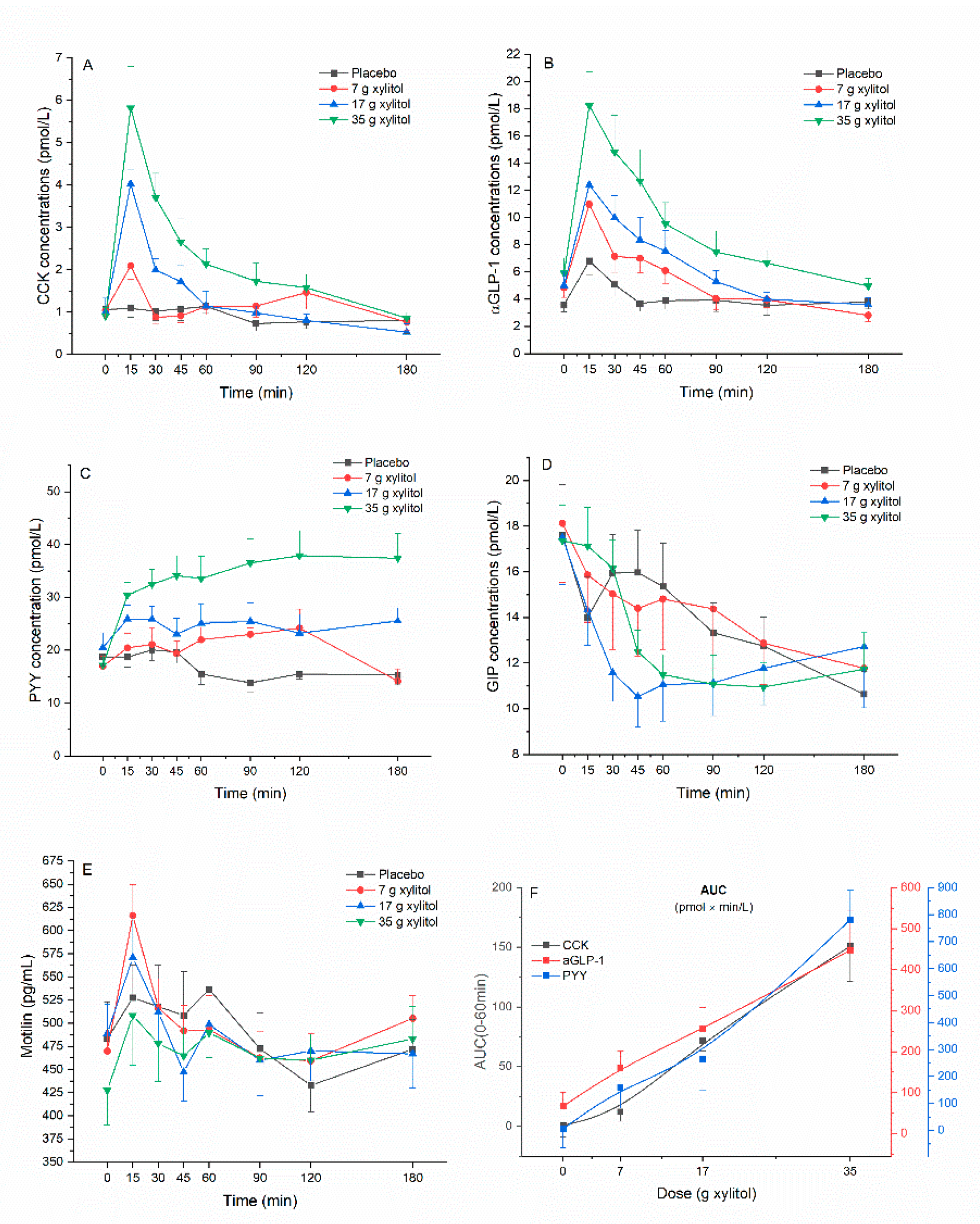
| Parameters | A: Placebo (n = 12) | B: Xylitol 7 g (n = 12) | C: Xylitol 17 g (n = 12) | D: Xylitol 35 g (n = 12) | p-Values (Overall) | p-Values (post-hoc) | |
|---|---|---|---|---|---|---|---|
| CCK | Fasting values (pmol/L) | 1.1 ± 0.8 | 1.0 ± 0.6 | 1.0 ± 1.1 | 0.9 ± 0.7 | NS | |
| iAUC (0–60 min) (pmol × min/L) | 0.6 ± 32.6 | 12.1 ± 27.6 | 71.8 ± 30.2 | 150.8 ± 102.8 | p < 0.001 | A vs. C: p < 0.001 A vs. D: p = 0.002 B vs. C: p = 0.009 B vs. D: p = 0.010 | |
| iAUC (0–180 min) (pmol × min/L) | −29.2 ± 108.9 | 27.1 ± 112.4 | 49.7 ± 89.4 | 222.2 ± 187.6 | p = 0.026 | A vs. D: p = 0.023 | |
| iCmax (pmol/L) | 0.6 ± 0.6 | 1.4 ± 1.1 | 3.0 ± 1.2 | 5.1 ± 3.1 | p < 0.001 | A vs. C: p < 0.001 A vs. D: p = 0.002 B vs. C: p = 0.009 B vs. D: p = 0.010 | |
| Tmax (min) | 40.0 ± 52.1 | 32.5 ± 41.4 | 15.0 ± 0.0 | 25.0 ± 30.2 | NS | ||
| iCmin (pmol/L) | −0.7 ± 0.6 | −0.5 ± 0.4 | −0.6 ± 0.7 | −0.2 ± 0.4 | NS | ||
| Tmin (min) | 90.0 ± 56.1 | 113.8 ± 74.4 | 127.5± 62.8 | 80.0 ± 86.1 | NS | ||
| aGLP | Fasting values (pmol/L) | 3.6 ± 1.9 | 4.9 ± 2.5 | 5.0 ± 3.4 | 5.9 ± 3.8 | NS | |
| iAUC (0–60 min) (pmol × min/L) | 66.5 ± 119.1 | 160.6 ± 170.2 | 254.7 ± 182.2 | 447.0 ± 332.1 | p = 0.002 | A vs. C: p = 0.008 A vs. D: p = 0.006 | |
| iAUC (0–180 min) (pmol × min/L) | 71.8 ± 269.4 | 53.6 ± 392.4 | 215.1 ± 330.4 | 553.5 ± 612.8 | NS | ||
| iCmax (pmol/L) | 3.5 ± 2.8 | 6.9 ± 5.4 | 8.2 ± 4.2 | 13.0 ± 7.3 | p = 0.002 | A vs. C: p = 0.013 A vs. D: p = 0.003 | |
| Tmax (min) | 31.3 ± 49.3 | 28.8 ± 30.3 | 25.0 ± 14.8 | 28.8 ± 30.3 | NS | ||
| iCmin (pmol/L) | −1.4 ± 1.0 | −2,5 ± 2.5 | −2.1 ± 2.5 | −1.9 ± 2.6 | NS | ||
| Tmin (min) | 98.8 ± 60.2 | 112.5 ± 69.0 | 91.3 ± 79.0 | 105.0 ± 84.4 | NS | ||
| PYY | Fasting values (pmol/mL) | 18.7 ± 6.3 | 17.0 ± 6.8 | 20.5 ± 9.2 | 17.2 ± 6.2 | NS | |
| iAUC (0–60 min) (pmol × min/L) | 5.6 ± 240.0 | 159.3 ± 241.4 | 263.1 ± 380.6 | 780.8 ± 378.6 | p < 0.001 | A vs. D: p = 0.002 B vs. D: p < 0.001 C vs. D: p = 0.005 | |
| iAUC (0–180 min) (pmol × min/L) | −256.5 ± 775.1 | 537.1 ± 935.6 | 835.9 ± 1420.4 | 3148.2 ± 2037.9 | p = 0.004 | A vs. D: p = 0.003 B vs. D: p < 0.015 C vs. D: p = 0.007 | |
| iCmax (pmol/L) | 5.1 ± 3.6 | 13.3 ± 7.4 | 12.5 ± 8.9 | 25.7 ± 12.4 | p = 0.001 | A vs. B: p = 0.030 A vs. D: p = 0.002 C vs. D: p = 0.024 | |
| Tmax (min) | 30.0 ± 15.7 | 91.4 ± 47.6 | 72.3 ± 54.4 | 88.8 ± 72.8 | p < 0.001 | A vs. B: p = 0.009 | |
| iCmin (pmol/L) | −8.4 ± 6.6 | −6.9 ± 6.7 | −4.4 ± 5.9 | −0.3 ± 0.8 | p = 0.007 | A vs. D: p = 0.012 | |
| Tmin (min) | 115.0 ± 52.5 | 111.8 ± 82.0 | 47.7 ± 63.9 | 30.0 ± 70.1 | p = 0.030 | A vs. D: p = 0.047 | |
| GIP | Fasting values (pmol/L) | 17.6 ± 7.67 | 18.12 ± 8.93 | 17.51 ± 7.16 | 17.36 ± 5.44 | NS | |
| iAUC (0–60 min) (pmol × min/L) | −120.8 ± 295.6 | −160.92 ± 234.1 | −307.6 ± 194.2 | −138.2 ± 191.8 | NS | ||
| iAUC (0–180 min) (pmol × min/L) | −710.9 ± 1186.3 | −749.4 ± 925.1 | −990.7 ± 815.0 | −871.5 ± 727.1 | NS | ||
| iCmax (pmol/L) | 3.1 ± 4.6 | 3.0 ± 3.1 | 3.2 ± 4.3 | 3.1 ± 3.5 | NS | ||
| Tmax (min) | 37.5 ± 43.6 | 56.3 ± 58.7 | 65.0 ± 70.7 | 30.0 ± 53.5 | NS | ||
| iCmin (pmol/L) | −8.9 ± 6.3 | −9.2 ± 6.3 | −10.8 ± 5.3 | −8.7 ± 4.3 | NS | ||
| Tmin (min) | 122.5 ± 62.9 | 117.5 ± 67.3 | 102.5 ± 54.6 | 123.8 ± 46.2 | NS | ||
| Motilin | Fasting values (pg/mL) | 483 ± 136 | 470 ± 79 | 489 ± 108 | 428 ± 136 | NS | |
| iAUC (0–60 min) (pg × min/mL) | 1957 ± 6146 | 3392 ± 3231 | 896 ± 4037 | 2973 ± 4027 | NS | ||
| iAUC (0−180 min) (pg × min/mL) | −182 ± 10963 | 4091 ± 6991 | −1493 ± 11287 | 8074 ± 11825 | NS | ||
| iCmax (pg/mL) | 117.3 ± 116.6 | 165.6 ± 92.0 | 119.0 ± 79.2 | 131.4 ± 75.6 | NS | ||
| Tmax (min) | 40.0 ± 23.4 | 43.8 ± 54.4 | 36.3 ± 37.5 | 52.5 ± 53.3 | NS | ||
| iCmin (pg/mL) | −81.9 ± 51.8 | −67.8 ± 33.9 | −102.3 ± 78.5 | −45.0 ± 45.0 | NS | ||
| Tmin (min) | 2.3 ± 56.3 | 21.8 ± 76.9 | 30.5 ± 91.7 | 45.4 ± 76.1 | NS | ||
| A: Placebo (n = 12) | B: Xylitol 7 g (n = 12) | C: Xylitol 17 g (n = 12) | D: Xylitol 35 g (n = 12) | p-Values (Overall) | p-Values (post-hoc) | |
|---|---|---|---|---|---|---|
| iAUC (0–60 min) (dose × min/h) | 911 ± 129 | 917 ± 101 | 814 ± 164 | 823 ± 154 | NS | |
| iAUC (0–180 min) (dose × min/h) | 1609 ± 270 | 1733 ± 221 | 1614 ± 319 | 1767 ± 302 | NS | |
| Tmax (min) | 21.25 ± 7.7 | 21.25 ± 7.7 | 27.50 ± 5.8 | 30.00 ± 11.1 | p = 0.039 | B vs. C: p = 0.099 |
| T50% (time 50% emptied) (min) | 56.6 ± 13.0 | 65.3 ± 11.1 | 66.9 ± 13.3 | 74.0 ± 18.1 | p = 0.026 | A vs. D: p = 0.029 |
| Parameters | A: Placebo (n = 12) | B: Xylitol 7 g (n = 12) | C: Xylitol 17 g (n = 12) | D: Xylitol 35 g (n = 12) | p-Values (Overall) | p-Values (post-hoc) | |
|---|---|---|---|---|---|---|---|
| Glucose | Fasting values (mmol/L) | 4.9 ± 0.3 | 4.8 ± 0.4 | 4.7 ± 0.5 | 4.9 ± 0.4 | NS | |
| iAUC (0–60 min) (mmol × min/L) | −4.5 ± 4.0 | 6.8 ± 18.6 | 18.2 ± 0.3 | 15.5 ± 22.3 | p < 0.001 | A vs. C: p < 0.001 | |
| iAUC (0–180 min) (mmol × min/L) | −8.4 ± 14.1 | −8.3 ± 56.4 | 12.8 ± 35.4 | −1.0 ± 49.4 | NS | ||
| iCmax (mmol/L) | 0.07 ± 0.1 | 0.37 ± 0.33 | 0.55 ± 0.21 | 0.67 ± 0.39 | p < 0.001 | A vs. C: p < 0.001 A vs. D: p = 0.001 | |
| Tmax (min) | 43.8 ± 48.9 | 31.3 ± 47.6 | 23.8 ± 10.0 | 22.5 ± 15.0 | NS | ||
| iCmin (mmol/L) | −0.19 ± 0.07 | −0.23 ± 0.25 | −0.21 ± 0.16 | −0.44 ± 0.31 | NS | ||
| Tmin (min) | 55.0 ± 55.9 | 50.0 ± 54.0 | 102.5 ± 62.0 | 97.5 ± 59.8 | NS | ||
| Insulin | Fasting values (mmol/L) | 0.033± 0.012 | 0.031 ± 0.009 | 0.028 ± 0.010 | 0.032 ± 0.011 | NS | |
| iAUC(0–60 min) (mmol × min/L) | −0.1 ± 0.4 | 0.3 ± 0.4 | 0.7 ± 0.4 | 2.0 ± 1.1 | p < 0.001 | A vs. C: p < 0.001 A vs. D: p < 0.001 B vs. D: p = 0.001 C vs. D: p = 0.004 | |
| iAUC (0–180 min) (mmol × min/L) | −0.6 ± 1.2 | −0.18 ± 0.3 | 0.3 ± 0.95 | 2.9 ± 2.5 | p = 0.008 | A vs. D: p = 0.010 B vs. D: p = 0.006 C vs. D: p = 0.008 | |
| iCmax (mmol/L) | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.03 | 0.07 ± 0.02 | p < 0.001 | A vs. C: p = 0.030 A vs. D: p < 0.001 B vs. D: p = 0.001 C vs. D: p = 0.036 | |
| Tmax (min) | 41.3 ± 66.8 | 20.0 ± 22.5 | 15.0 ± 0.0 | 25.0 ± 14.8 | NS | ||
| iCmin (mmol/L) | −0.01 ± 0.01 | −0.01 ± 0.01 | −0-01 ± 0-011 | −0.01 ± 0.01 | NS | ||
| Tmin (min) | 96.3 ± 72.8 | 88.8 ± 67.2 | 118.8 ± 77.9 | 126.3 ± 73.9 | NS | ||
| Glucagon | Fasting values (pmol/L) | 5.54 ± 1.50 | 5.00 ± 2.35 | 5.83 ± 2.42 | 4.92 ± 1.74 | NS | |
| iAUC (0–60 min) (pmol × min/L) | −20.3 ± 78.9 | −3.1 ± 64.7 | −32.5 ± 36.8 | 34.4 ± 62.2 | NS | ||
| iAUC (0–180 min) (pmol × min/L) | −154.1 ± 268.1 | −9.4 ± 233.6 | −102.5 ± 147.7 | 11.88 ± 170.4 | NS | ||
| iCmax (pmol/L) | 1.04 ± 1.14 | 1.25 ± 0.78 | 0.75 ± 0.45 | 1.92 ± 0.97 | p = 0.011 | C vs D: p = 0.012 | |
| Tmax (min) | 22.5 ± 18.6 | 31.3 ± 38.1 | 50.0 ± 39.1 | 75.0 ± 56.1 | p = 0.018 | A vs D: p = 0.082 | |
| iCmin (pmol/L) | −2.00 ± 1.28 | −1.54 ± 1.53 | −1.75 ± 1.06 | 1.83 ± 0.86 | NS | ||
| Tmin (min) | 52.8 ± 34.1 | 56.38 ± 52.1 | 537 ± 52.1 | 46.3 ± 47.6 | NS | ||
| Placebo (n = 12) | Xylitol 7 g (n = 12) | Xylitol 17 g (n = 12) | Xylitol 35 g (n = 12) | ||
|---|---|---|---|---|---|
| Abdominal pain | No of subjects with symptom | 0 | 0 | 0 | 0 |
| max severity * | - | - | - | - | |
| Nausea | No of subjects with symptom | 0 | 0 | 0 | 0 |
| max severity | - | - | - | - | |
| Vomiting | No of subjects with symptom | 0 | 0 | 0 | 0 |
| max severity | - | - | - | - | |
| Diarrhea | No of subjects with symptom | 0 | 0 | 0 | 0 |
| max severity | - | - | - | - | |
| Bowel sounds | No of subjects with symptom | 5 | 9 | 8 | 9 |
| max severity | 1.4 | 1.0 | 1.25 | 1.0 | |
| Bloating | No of subjects with symptom | 0 | 0 | 1 | 1 |
| max severity | - | - | 1.0 | 1.0 | |
| Eructation | No of subjects with symptom | 0 | 1 | 0 | 0 |
| max severity | - | 1.0 | - | - | |
| Flatulence | No of subjects with symptom | 0 | 0 | 0 | 0 |
| max severity | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer-Gerspach, A.C.; Drewe, J.; Verbeure, W.; Roux, C.W.l.; Dellatorre-Teixeira, L.; Rehfeld, J.F.; Holst, J.J.; Hartmann, B.; Tack, J.; Peterli, R.; et al. Effect of the Natural Sweetener Xylitol on Gut Hormone Secretion and Gastric Emptying in Humans: A Pilot Dose-Ranging Study. Nutrients 2021, 13, 174. https://doi.org/10.3390/nu13010174
Meyer-Gerspach AC, Drewe J, Verbeure W, Roux CWl, Dellatorre-Teixeira L, Rehfeld JF, Holst JJ, Hartmann B, Tack J, Peterli R, et al. Effect of the Natural Sweetener Xylitol on Gut Hormone Secretion and Gastric Emptying in Humans: A Pilot Dose-Ranging Study. Nutrients. 2021; 13(1):174. https://doi.org/10.3390/nu13010174
Chicago/Turabian StyleMeyer-Gerspach, Anne Christin, Jürgen Drewe, Wout Verbeure, Carel W. le Roux, Ludmilla Dellatorre-Teixeira, Jens F. Rehfeld, Jens J. Holst, Bolette Hartmann, Jan Tack, Ralph Peterli, and et al. 2021. "Effect of the Natural Sweetener Xylitol on Gut Hormone Secretion and Gastric Emptying in Humans: A Pilot Dose-Ranging Study" Nutrients 13, no. 1: 174. https://doi.org/10.3390/nu13010174
APA StyleMeyer-Gerspach, A. C., Drewe, J., Verbeure, W., Roux, C. W. l., Dellatorre-Teixeira, L., Rehfeld, J. F., Holst, J. J., Hartmann, B., Tack, J., Peterli, R., Beglinger, C., & Wölnerhanssen, B. K. (2021). Effect of the Natural Sweetener Xylitol on Gut Hormone Secretion and Gastric Emptying in Humans: A Pilot Dose-Ranging Study. Nutrients, 13(1), 174. https://doi.org/10.3390/nu13010174







