The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Procedure
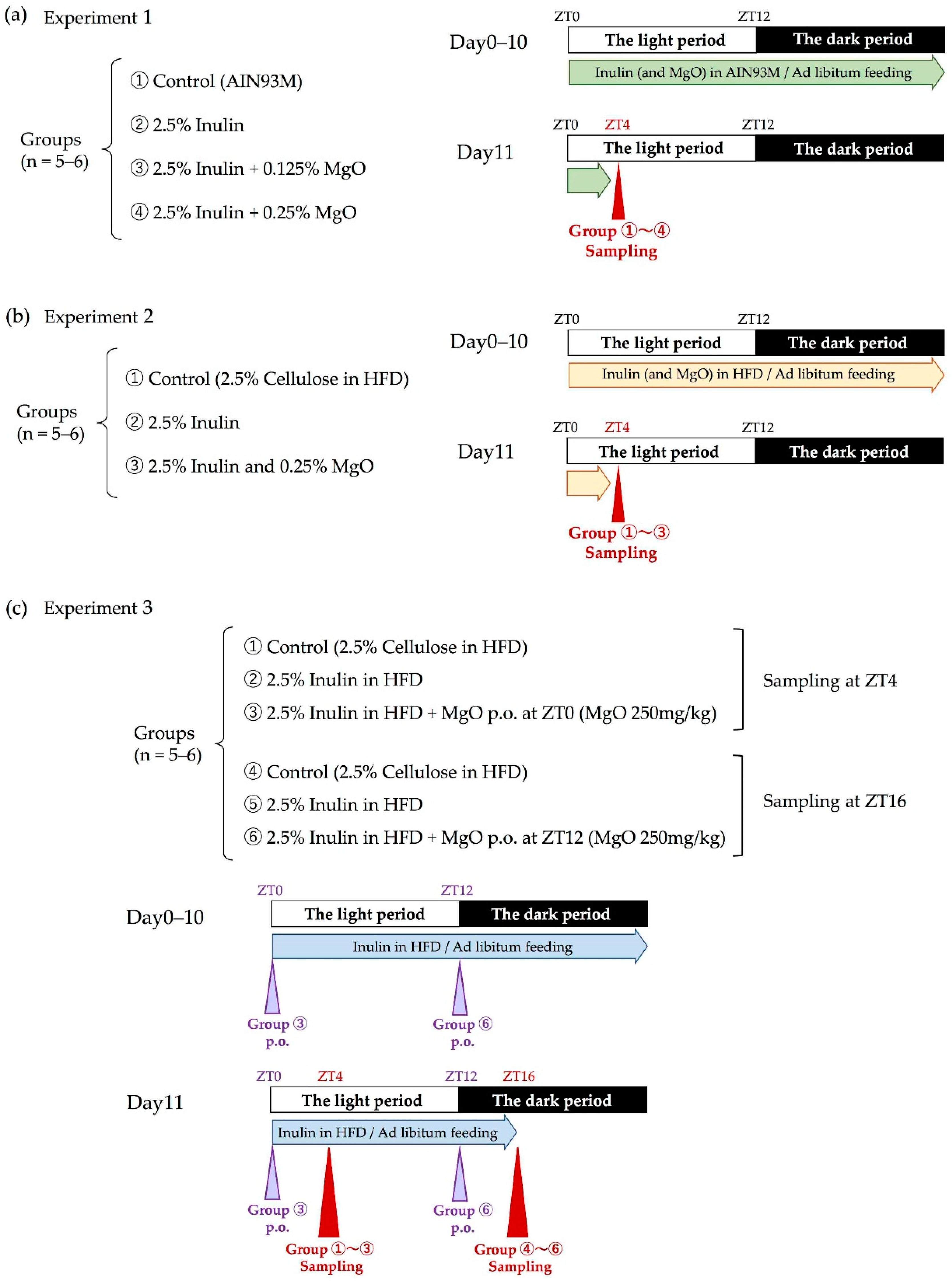
2.2.1. Experimental Design
2.2.2. Measuring the Cecal pH
2.2.3. Measuring the SCFA and Lactic Acid Levels in the Cecum
2.2.4. Fecal DNA Extraction
2.2.5. 16S rDNA Sequencing
2.2.6. Analysis of 16S rDNA Gene Sequence
2.2.7. Statistical Analysis
3. Results
3.1. Effects of Inulin and MgO Combination on Normal Intestinal Environments
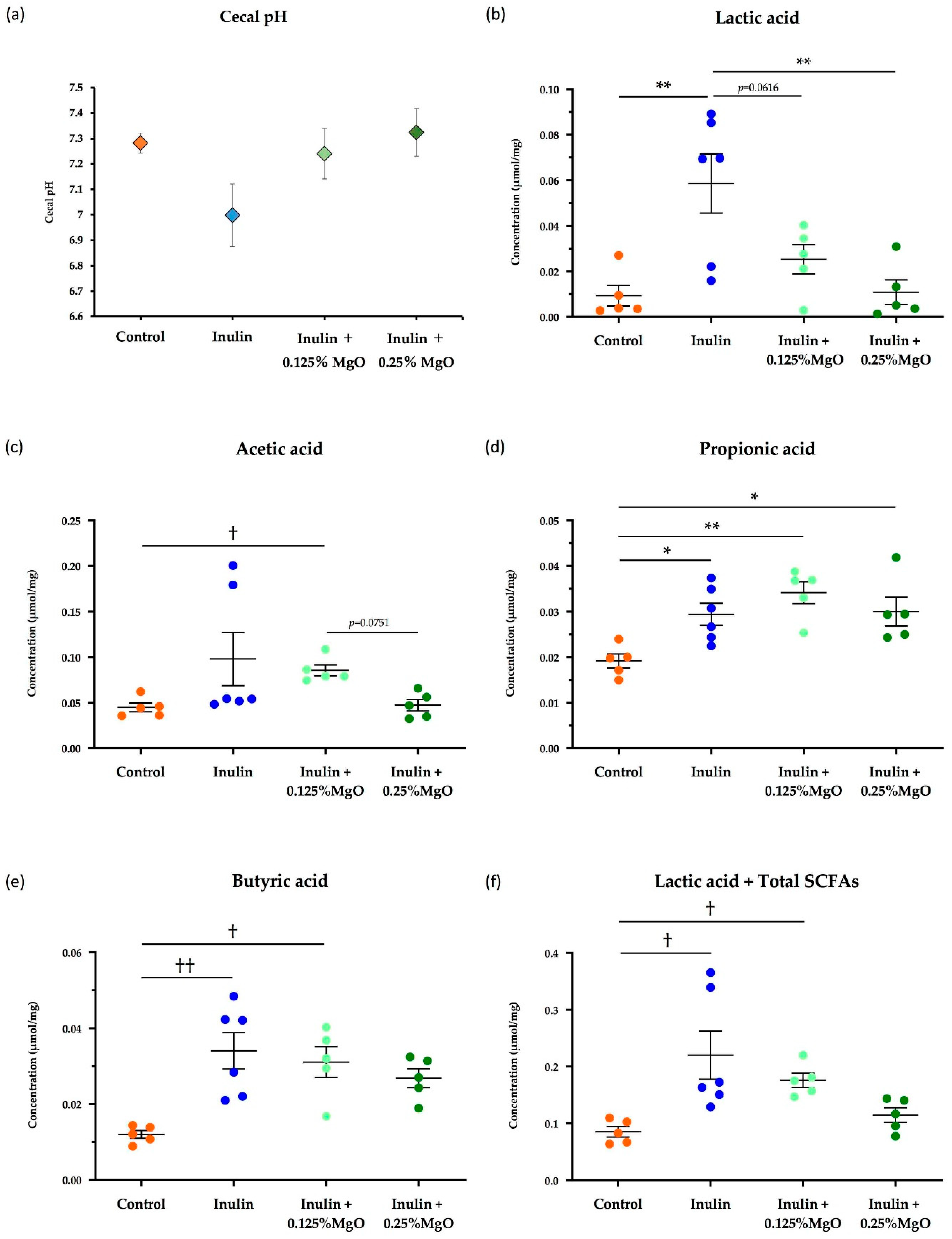
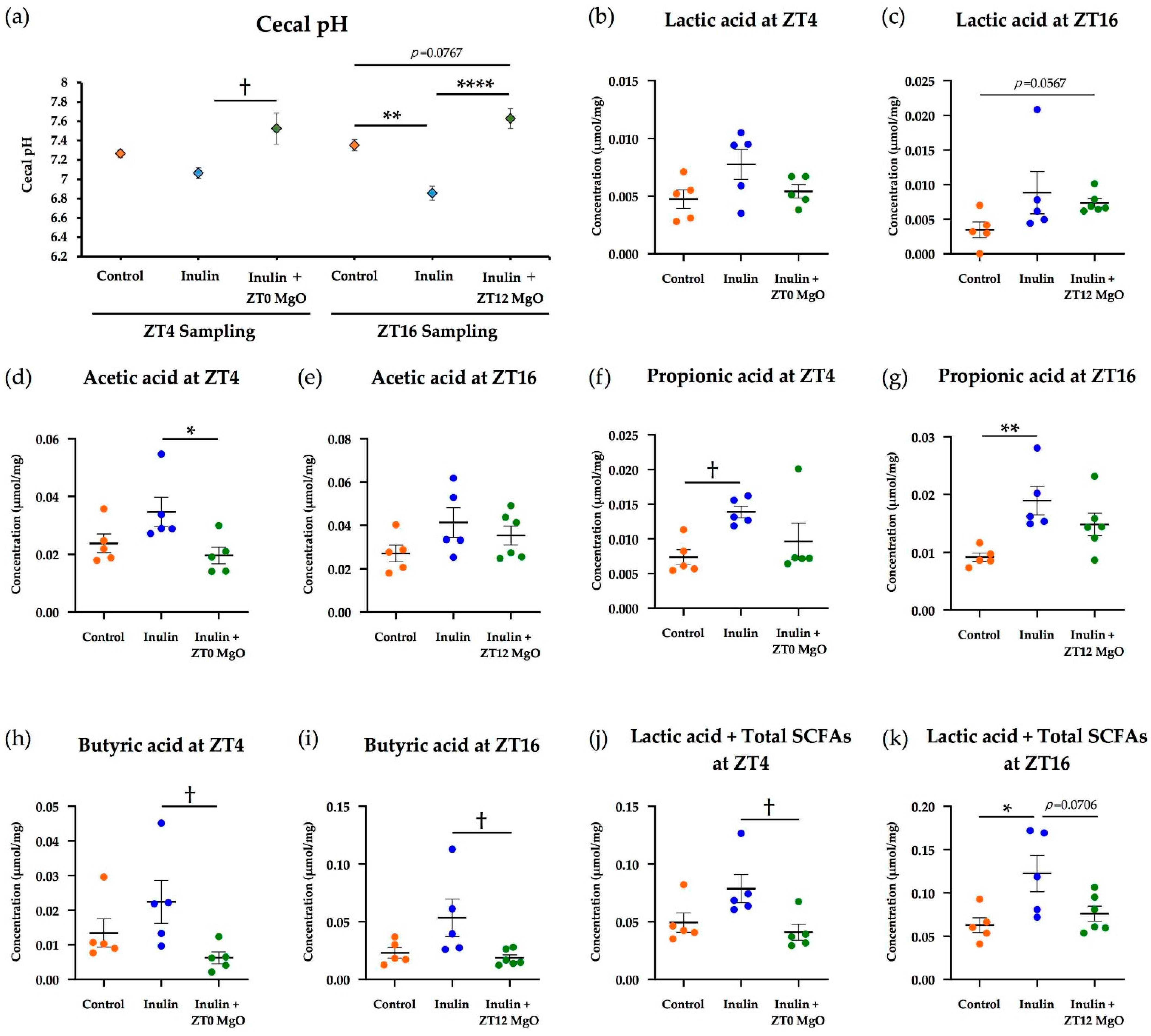
3.1.1. The Combined Inulin and MgO Effects on Cecal pH
3.1.2. The Combined Inulin and MgO Effects on Cecal SCFAs
3.2. The Combined Inulin and MgO Effects on the Intestinal Environment and Microbiota during HFD Feeding
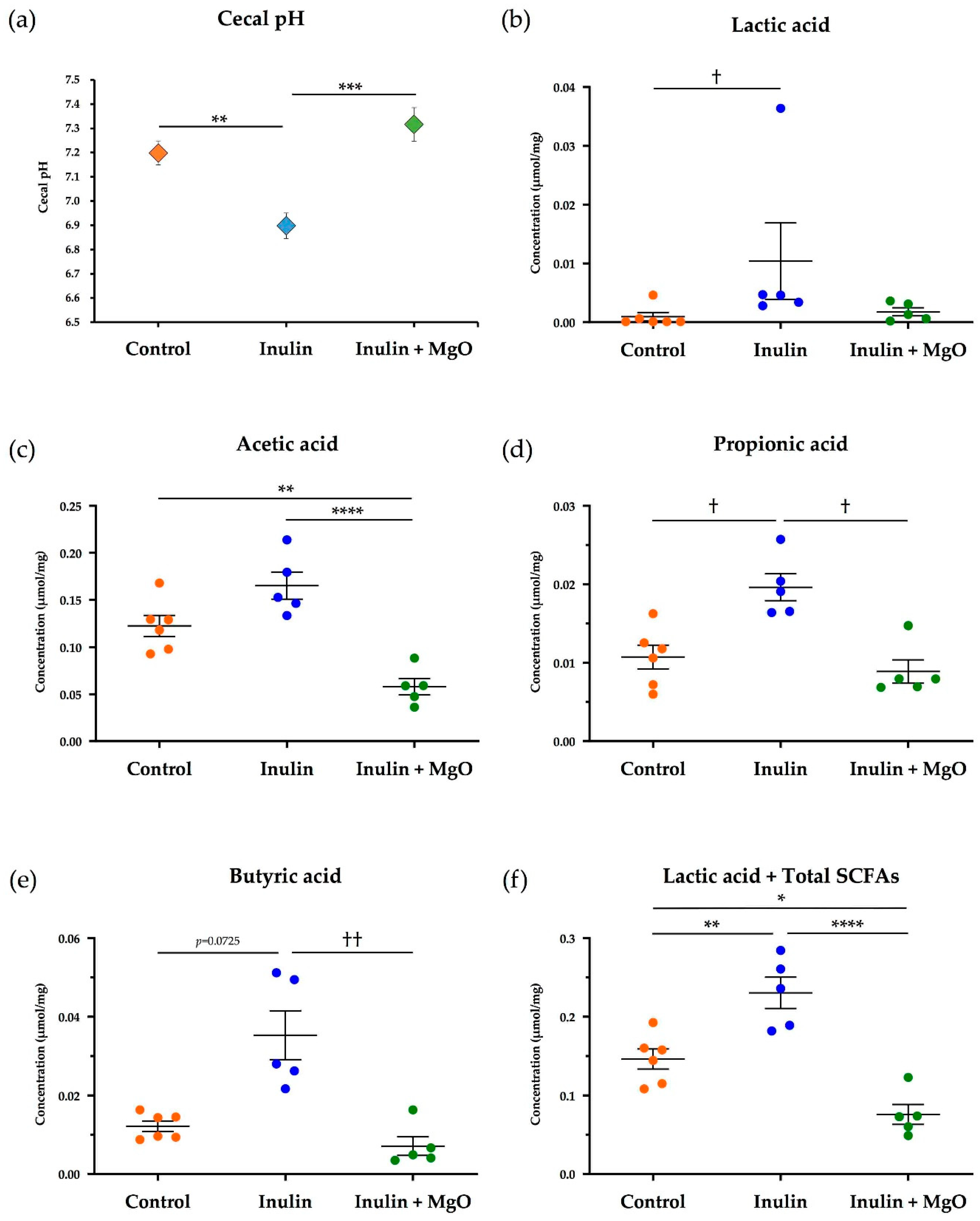
3.2.1. The Combined Inulin and MgO Effects on Cecal pH during HFD Feeding
3.2.2. The Combined Inulin and MgO Effects on Cecum SCFAs during HFD Feeding
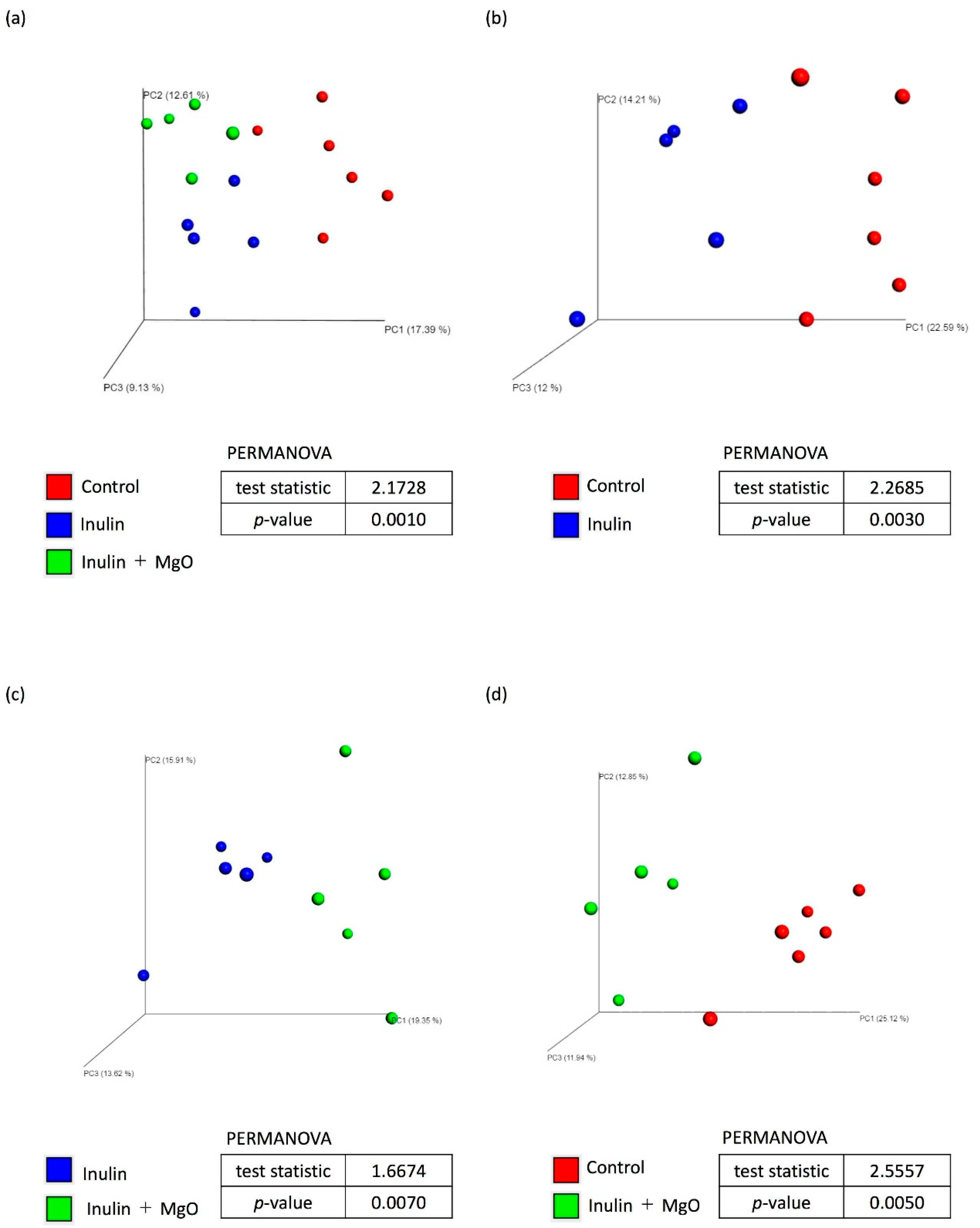
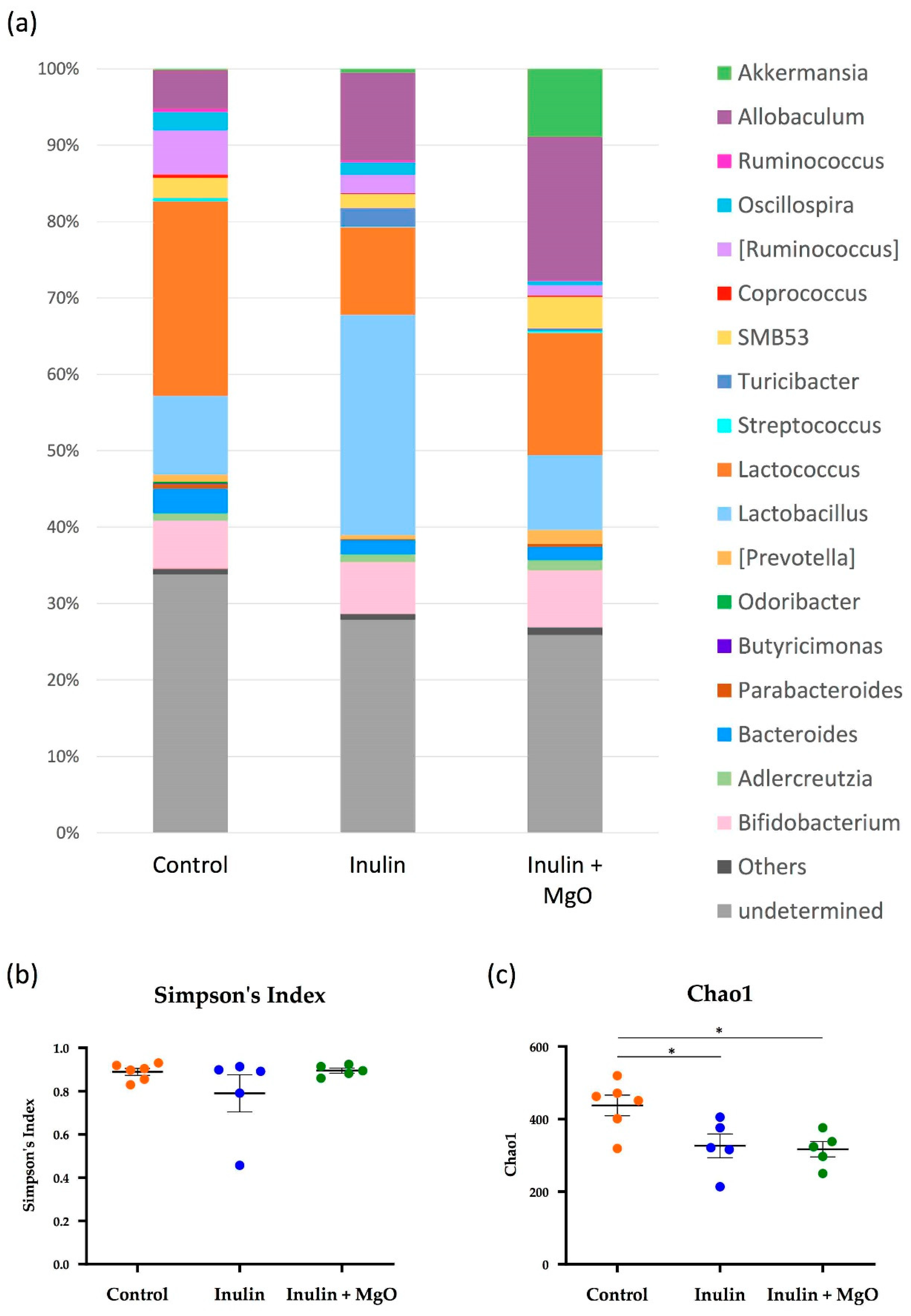
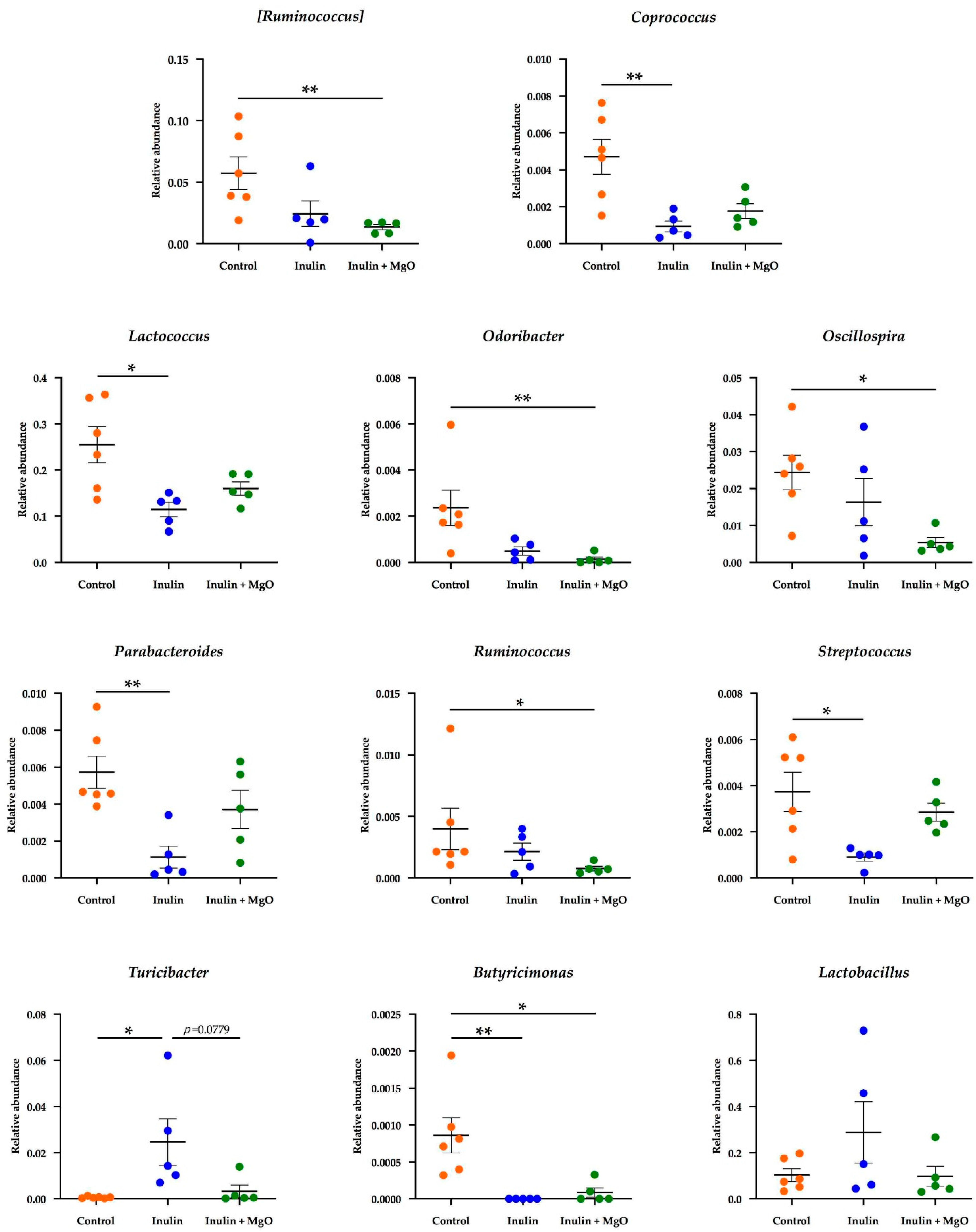
3.2.3. The Combined Inulin and MgO Effects on the Microbiota during HFD Feeding
3.3. The Relationship between the MgO Effect on the Intestinal Environment and Administration Timing
4. Discussion
4.1. Combined Inulin and MgO Effect on the SCFA Concentrations in the Cecum
4.2. Changes in Cecal pH and Intestinal Flora Composition
4.3. Microbiota α-Diversity
4.4. Combined Inulin and MgO Effect on Intestinal Bacteria at the Genus Level
4.5. MgO Administration Timing Effects on Cecal pH and Cecal SCFAs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tamura, A.; Tomita, T.; Oshima, T.; Toyoshima, F.; Yamasaki, T.; Okugawa, T.; Kondo, T.; Kono, T.; Tozawa, K.; Ikehara, H.; et al. Prevalence and self-recognition of chronic constipation: Results of an internet survey. J. Neurogastroenterol. Motil. 2016, 22, 677–685. [Google Scholar] [CrossRef]
- Higgins, P.; Johanson, J. Epidemiology of constipation in North America: A systematic review. Am. J. Gastroenterol. 2004, 99, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.X.; DiBonaventura, M.; Purayidathil, F.W.; Wagner, J.-S.; Dabbous, O.; Mody, R. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: An analysis of the national health and wellness survey. Dig. Dis. Sci. 2011, 56, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Research Society for the Diagnosis and Treatment of Chronic Constipation. Evidence-Based Clinical Practice Guidelines for Chronic Constipation; Nankodo: Tokyo, Japan, 2017. [Google Scholar]
- Wakai, E.; Ikemura, K.; Sugimoto, H.; Iwamoto, T.; Okuda, M. Risk factors for the development of hypermagnesemia in patients prescribed magnesium oxide: A retrospective cohort study. J. Pharm. Health Care Sci. 2019, 5, 4. [Google Scholar] [CrossRef]
- Mizukami, K. “Manseibenpisho no kizonchiryo to sono mondaiten” [Existing treatments for chronic constipation and their problems.]. In Gastroenterology: “Manseibenpisho Shinryo Guideline Zidai no Benpi Shinryo” [Constipation Treatment in the Guideline Era for Chronic Constipation Treatment.]; Nakajima, A., Ed.; Igaku Shuppan: Tokyo, Japan, 2020; Volume 2, pp. 38–44. (In Japanese) [Google Scholar]
- Saito, N. Influence of impaired renal function and magnesium oxide administration on serum magnesium levels in elderly inpatients. Nihon Ronen Igakkai Zasshi (Jpn. J. Geriatr.) 2011, 48, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Fujisaki, K.; Yamamoto, T.; Shinohata, Y. Pharmacokinetic studies of orally administered magnesium oxide in rats. Yakugaku Zasshi 2017, 137, 581–587. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare (Japan). Dietary Reference Intakes for Japanese 2020. Available online: https://www.mhlw.go.jp/stf/newpage_08517.html (accessed on 28 December 2020).
- Lindberg, G.; Hamid, S.; Malfertheiner, P.; Thomsen, O.; Fernandez, L.B.; Garisch, J.; Thomson, A.; Goh, K.-L.; Tandon, R.; Fedail, S.; et al. World gastroenterology organisation global guideline: Constipation-a global perspective. J. Clin. Gastroenterol. 2011, 45, 483–487. [Google Scholar]
- Rolhion, N.; Chassaing, B. When pathogenic bacteria meet the intestinal microbiota. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150504. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–67. [Google Scholar] [CrossRef]
- Koh, A.; Vadder, F.D.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Watabe, J. Carbohydrate fermentation in the colon. J. Investig. Microbiol. 2005, 19, 169–177. [Google Scholar]
- Cruz-Ortiz, R.; Gutiérrez-Dorado, R.; Osorio-Díaz, P.; Alvarado-Jasso, G.M.; Tornero-Martínez, A.; Guzmán-Ortiz, F.A.; Mora-Escobedo, R. Optimized germinated soybean/cornstarch extrudate and its in vitro fermentation with human inoculum. J. Food Sci. Technol. 2020, 57, 848–857. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Fructo-oligosaccharide malabsorption: Benefit for gastrointestinal functions. Curr. Opin. Gastroenterol. 2000, 16, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.H.; Gu, F.; Schols, H.A.; Verkade, H.J.; Tietge, U.J.F. Effect of the prebiotic fiber inulin on cholesterol metabolism in wildtype mice. Sci. Rep. 2018, 8, 13238. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Annunziata, G.; Laudisio, D.; Pugliese, G.; Salzano, C.; Colao, A.; Savastano, S. From gut microbiota dysfunction to obesity: Could short-chain fatty acids stop this dangerous course? Hormones 2019, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Gumz, M.L. Advances in understanding the peripheral circadian clocks. FASEB J. 2012, 26, 3602–3613. [Google Scholar] [CrossRef]
- Tahara, Y.; Aoyama, S.; Shibata, S. The mammalian circadian clock and its entrainment by stress and exercise. J. Physiol. Sci. 2017, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shibata, S. Chrono-bioligy, chrono-pharmacology, and chrono-nutrition. J. Pharmacol. Sci. 2014, 124, 320–335. [Google Scholar] [CrossRef]
- Narishige, S.; Kuwahara, M.; Shinozaki, A.; Okada, S.; Ikeda, Y.; Kamagata, M.; Tahara, Y.; Shibata, S. Effects of caffeine on circadian phase, amplitude and period evaluated in cells in vitro and peripheral organs in vivo in PER2::LUCIFERASE mice. Br. J. Pharmacol. 2014, 171, 5858–5869. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shiraishi, T.; Kikuchi, Y.; Haraguchi, A.; Kuriki, D.; Sasaki, H.; Motohashi, H.; Sakai, T.; Shibata, S. Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Sci. Rep. 2015, 5, 11417. [Google Scholar] [CrossRef] [PubMed]
- Furutani, A.; Ikeda, Y.; Itokawa, M.; Nagahama, H.; Ohtsu, T.; Furutani, N.; Kamagata, M.; Yang, Z.-H.; Hirasawa, A.; Tahara, Y.; et al. Fish oil accelerates diet-induced entrainment of the mouse peripheral clock via GPR120. PLoS ONE 2015, 10, e0132472. [Google Scholar] [CrossRef]
- Tahara, Y.; Shibata, S. Circadian rhythms of liver physiology and disease: Experimental and clinical evidence. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 217–226. [Google Scholar] [CrossRef]
- Albrecht, U. Molecular mechanisms in mood regulation involving the circadian clock. Front. Neurol. 2017, 8, 30. [Google Scholar] [CrossRef]
- McGinnis, G.R.; Young, M.E. Circadian regulation of metabolic homeostasis: Causes and consequences. Nat. Sci. Sleep 2016, 8, 163–180. [Google Scholar]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Ohishi, R.; Hirose, Y.; Torii-Goto, A.; Park, S.J.; Miura, N.; Yoshikawa, M. Chronopharmacology of dapagliflozin-induced antihyperglycemic effects in C57BL/6J mice. Obes. Res. Clin. Pract. 2019, 13, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Sasaki, H.; Miyakawa, H.; Nakayama, Y.; Lyu, Y.; Shibata, S. Effect of dose and timing of burdock (Arctium lappa) root intake on intestinal microbiota of mice. Microorganisms 2020, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Sasaki, H.; Shiga, K.; Miyakawa, H.; Shibata, S. The timing effects of soy protein intake on mice gut microbiota. Nutrients 2020, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffman, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.-Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.e1-2. [Google Scholar] [CrossRef]
- Sasaki, H.; Lyu, Y.; Nakayama, Y.; Nakamura, F.; Watanabe, A.; Miyakawa, H.; Nakao, Y.; Shibata, S. Combinatorial Effects of Soluble, Insoluble, and Organic Extracts from Jerusalem Artichokes on Gut Microbiota in Mice. Microorganisms 2020, 8, 954. [Google Scholar] [CrossRef]
- Huazano-García, A.; López, M.G. Metabolism of short chain fatty acids in the colon and faeces of mice after a supplementation of diets with agave fructans. In Lipid Metabolism; Baez, R.V., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.-W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA. Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods. 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than blast. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhu, L.; Yang, X.; He, F.; Wang, T.; Bao, T.; Lu, H.; Wang, H.; Yang, S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharmacol. 2020, 78, 106062. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G506–G513. [Google Scholar] [CrossRef] [PubMed]
- Nadatani, Y.; Watanabe, T.; Suda, W.; Nakata, A.; Matsumoto, Y.; Kosaka, S.; Higashimori, A.; Otani, K.; Hosomi, S.; Tanaka, F.; et al. Gastric acid inhibitor aggravates indomethacin-induced small intestinal injury via reducing Lactobacillus johnsonii. Sci. Rep. 2019, 9, 17490. [Google Scholar] [CrossRef] [PubMed]
- Tropini, C.; Moss, E.L.; Merrill, B.D.; Ng, K.M.; Higginbottom, S.K.; Casavant, E.P.; Gonzalez, C.G.; Fremin, B.; Bouley, D.M.; Elias, J.E.; et al. Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell 2018, 173, 1742–1754. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, N.; Nam, R.H.; Park, J.H.; Choi, S.I.; Park, Y.-T.; Kim, Y.-R.; Seok, Y.-J.; Shin, C.M.; Lee, D.H. Gut microbiota and butyrate level changes associated with the long-term administration of proton pump inhibitors to old rats. Sci. Rep. 2019, 9, 6626. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Cai, S.-T.; Tian, Y.-P.; Zhao, H.-J.; Zhang, Y.-B.; Chen, J.; Ren, R.-R.; Luo, X.; Peng, L.-H.; Sun, G.; et al. Effects of proton pump inhibitors on the gastrointestinal microbiota in gastroesophageal reflux disease. Genom. Proteom. Bioinform. 2019, 17, 52–63. [Google Scholar] [CrossRef]
- Hojo, M.; Asahara, T.; Nagahara, A.; Takeda, T.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Asaoka, D.; Takahashi, T.; Nomoto, K.; et al. Gut microbiota composition before and after use of proton pump inhibitors. Dig. Dis. Sci. 2018, 63, 2940–2949. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omori, K.; Miyakawa, H.; Watanabe, A.; Nakayama, Y.; Lyu, Y.; Ichikawa, N.; Sasaki, H.; Shibata, S. The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids. Nutrients 2021, 13, 152. https://doi.org/10.3390/nu13010152
Omori K, Miyakawa H, Watanabe A, Nakayama Y, Lyu Y, Ichikawa N, Sasaki H, Shibata S. The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids. Nutrients. 2021; 13(1):152. https://doi.org/10.3390/nu13010152
Chicago/Turabian StyleOmori, Kanako, Hiroki Miyakawa, Aya Watanabe, Yuki Nakayama, Yijin Lyu, Natsumi Ichikawa, Hiroyuki Sasaki, and Shigenobu Shibata. 2021. "The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids" Nutrients 13, no. 1: 152. https://doi.org/10.3390/nu13010152
APA StyleOmori, K., Miyakawa, H., Watanabe, A., Nakayama, Y., Lyu, Y., Ichikawa, N., Sasaki, H., & Shibata, S. (2021). The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids. Nutrients, 13(1), 152. https://doi.org/10.3390/nu13010152




