Uremic Sarcopenia and Its Possible Nutritional Approach
Abstract
1. Introduction
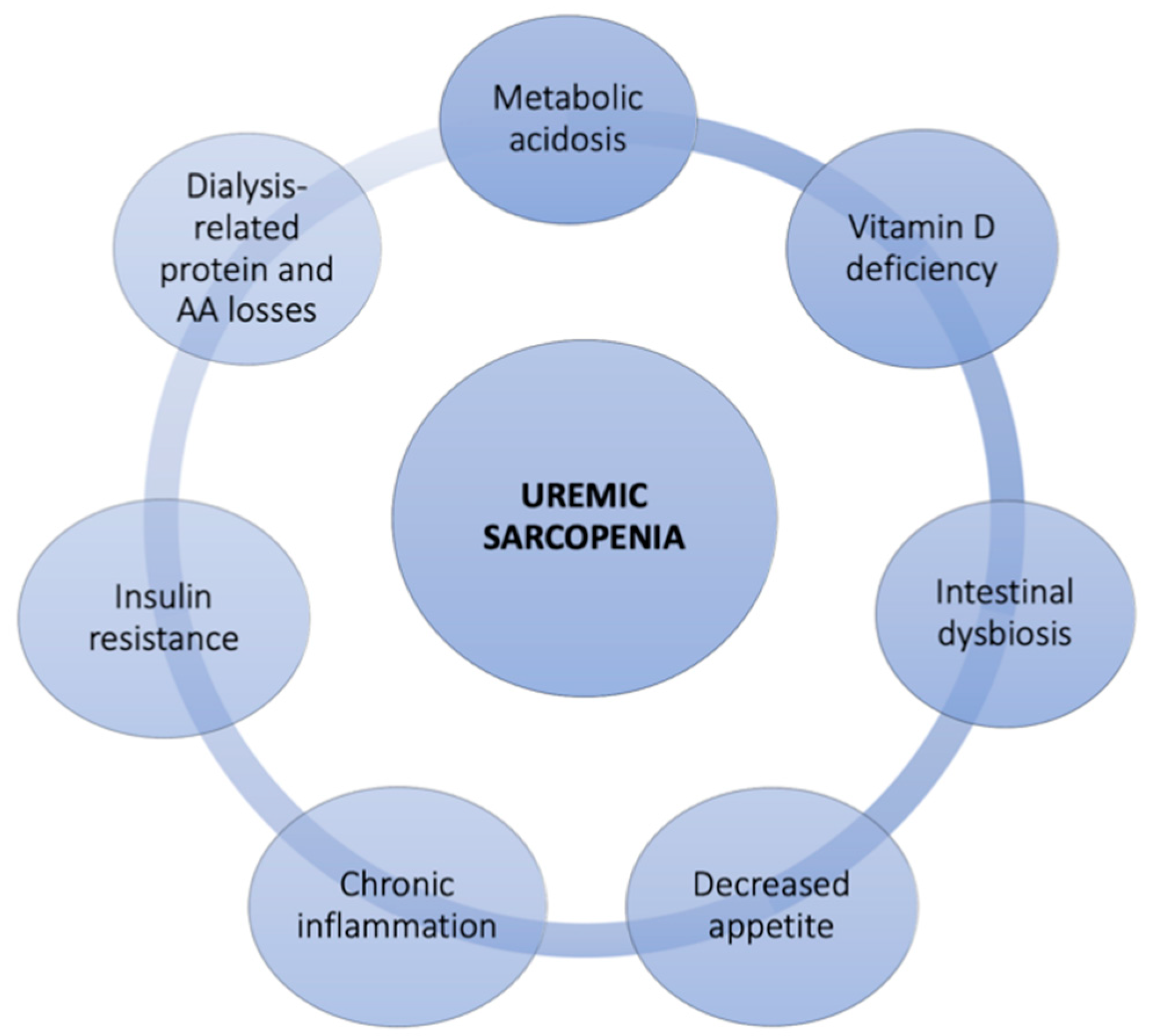
2. Sarcopenia in Chronic Kidney Disease
2.1. The Diagnosis of Sarcopenia
2.2. Possible Therapeutic Strategies for Uremic Sarcopenia
3. Nutritional Therapy
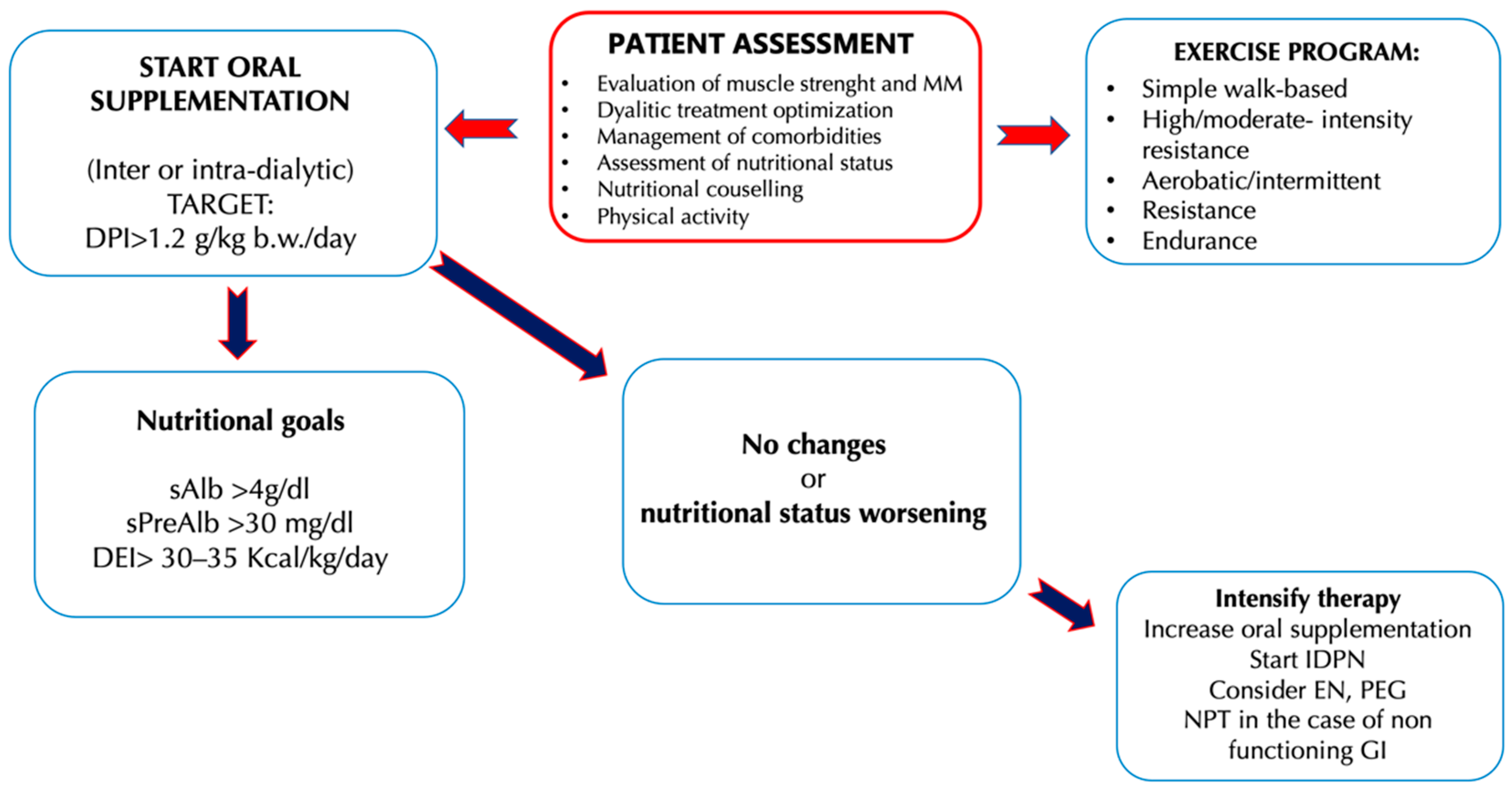
3.1. Oral Nutritional Supplements
3.2. Amino Acids Supplementation
3.3. Intra-Dialytic Parenteral Nutrition
3.4. Enteral and Total Parenteral Nutrition
4. Other Special Types of Nutritional Supplements
4.1. Ω-3 PUFAs
4.2. Fiber
5. Other Therapeutic Approaches to Uremic Sarcopenia
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BIA | Bioimpedance analysis |
| BMI | Body mass index |
| b.w. | Body weight |
| CKD | Chronic kidney disease |
| CPI | Controlled protein intake |
| CRP | C-reactive protein |
| CT | Computed tomography |
| CV | Cardiovascular |
| DHA | Docosahexaenoic acid |
| DM | Diabetes mellitus |
| DXA | Dual-energy X-ray absorptiometry |
| E3s | Ubiquitin ligases enzyme |
| EN | Enteral nutrition |
| EPA | Eicosapentaenoic acid |
| EPO | Erythropoietin |
| ESPEN | European society for clinical nutrition and metabolism |
| ESRD | End-stage renal disease |
| EWGSOP | European working group for sarcopenia for older people |
| FGF-23 | Fibroblast growth factor-23 |
| FoxO3A | Forkhead box O3A |
| GH | Growth hormone |
| GFR | Glomerular filtration rate |
| HD | Hemodialysis |
| HGS | Handgrip strength |
| HOMA | Homeostasis model of assessment |
| HPD | High-protein diet |
| IDPN | Intra-parenteral nutrition dialysis |
| IGF1 | Insulin-like growth factor-1 |
| IL | Interleukin |
| IR | Insulin resistance |
| ISRNM | International society of renal nutrition and metabolism |
| KAs | Ketoanalogues |
| LPD | Low protein diet |
| MAMC | Mid arm muscle circumference |
| MIS | Malnutrition inflammation score |
| MM | Muscle mass |
| MPS | Muscle protein synthesis |
| MRI | Magnetic resonance imaging |
| mTOR | Mammalian target of rapamycin |
| NHANES | National health and nutrition examination survey |
| ONS | Oral nutritional supplements |
| p70S6K | Ribosomal protein S6 kinase beta-1 |
| PEG | Percutaneous endoscopic gastroctomy |
| PEW | Protein-energy wasting |
| PGC-1α | Peroxisome gamma proliferator coactivator-1α |
| PI3K | Protein kinase B |
| PUFAs | Polyunsatured fatty acids |
| Qol | Quality of life |
| QUICKI | Improved quantitative insulin sensitivity check index |
| SCWD | Society on sarcopenia, cachexia and wasting disorders |
| SGA | Subject global assessment |
| SIRTs | Sirtuins |
| SOCS-3 | Suppressor of cytokine signaling-3 |
| SPPB | Short physical performance battery |
| Stat3 | Signal transducer and transcriptional activator 3 |
| TNF-α | Tumor necrosis factor-α |
| TPN | Total parenteral nutrition |
| UPS | Ubiquitin proteasome system |
| UTRs | Untranslated regions |
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Kamimura, M.A.; Lamarca, F.; Rodrigues, J.; Santin, F.; Avesani, C.M. Association of Sarcopenia with Nutritional Parameters, Quality of Life, Hospitalization, and Mortality Rates of Elderly Patients on Hemodialysis. J. Ren. Nutr. 2018, 28, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Delsante, M.; Di Motta, T.; Cantarelli, C.; Pioli, S.; Grassi, G.; Batini, V.; Gregorini, M.; Fiaccadori, E. Noninvasive evaluation of muscle mass by ultrasonography of quadriceps femoris muscle in End-Stage Renal Disease patients on hemodialysis. Clin. Nutr. 2019, 38, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Wang, C.; Ishani, A.; Collins, A.J.; Murray, A.M. Kidney function and sarcopenia in the United States general population: NHANES III. Am. J. Nephrol. 2007, 27, 279–286. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Carrero, J.J.; von Walden, F.; Ikizler, T.A.; Nader, G.A. Muscle wasting in end-stage renal disease promulgates premature death: Established, emerging and potential novel treatment strategies. Nephrol. Dial. Transplant. 2016, 31, 1070–1077. [Google Scholar] [CrossRef]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar] [CrossRef]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef]
- Von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Thome, T.; Salyers, Z.R.; Kumar, R.A.; Hahn, D.; Berru, F.N.; Ferreira, L.F.; Scali, S.T.; Ryan, T.E. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am. J. Physiol. Cell Physiol. 2019, 317, C701–C713. [Google Scholar] [CrossRef]
- Yazdi, P.G.; Moradi, H.; Yang, J.Y.; Wang, P.H.; Vaziri, N.D. Skeletal muscle mitochondrial depletion and dysfunction in chronic kidney disease. Int. J. Clin. Exp. Med. 2013, 6, 532–539. [Google Scholar]
- Afsar, B.; Afsar, R.E.; Dagel, T.; Kaya, E.; Erus, S.; Ortiz, A.; Covic, A.; Kanbay, M. Capillary rarefaction from the kidney point of view. Clin. Kidney J. 2018, 11, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.; McPhee, J.S.; Jones, D.A.; Degens, H. Five-year longitudinal changes in thigh muscle mass of septuagenarian men and women assessed with DXA and MRI. Aging Clin. Exp. Res. 2020, 32, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Rossi, A.; Di Francesco, V.; Sepe, A.; Olioso, D.; Pizzini, F.; Fantin, F.; Bosello, O.; Cominacini, L.; Harris, T.B.; et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: Relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, G.K.; Kent-Braun, J.A.; Doyle, J.W.; Shubert, T.; Gordon, P.; Johansen, K.L. Effect of diabetes mellitus on muscle size and strength in patients receiving dialysis therapy. Am. J. Kidney Dis. 2006, 47, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argiles, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyere, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertiere, M.C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, A.; Lauro, S.; Di Daniele, N.; Sorge, R.; Celi, M.; Volpe, S.L. Effect of a moderately hypoenergetic Mediterranean diet and exercise program on body cell mass and cardiovascular risk factors in obese women. Eur. J. Clin. Nutr. 2008, 62, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Chiappini, M.G.; Laviano, A.; Ammann, T.; Bollea, M.R.; Alegiani, F.; Rossi Fanelli, F.; Muscaritoli, M. Effect of intensive nutritional counseling and support on clinical outcomes of hemodialysis patients. Nutrition 2012, 28, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Fahal, I.H.; Bell, G.M.; Bone, J.M.; Edwards, R.H. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrol. Dial. Transplant. 1997, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.; Ciechanowski, K. Sarcopenia: A major challenge in elderly patients with end-stage renal disease. J. Aging Res. 2012, 2012, 754739. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Chmielewski, M.; Axelsson, J.; Snaedal, S.; Heimburger, O.; Barany, P.; Suliman, M.E.; Lindholm, B.; Stenvinkel, P.; Qureshi, A.R. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin. Nutr. 2008, 27, 557–564. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Dev, R. Measuring cachexia-diagnostic criteria. Ann. Palliat. Med. 2019, 8, 24–32. [Google Scholar] [CrossRef]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Avin, K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Sanchez-Nino, M.D. Sarcopenia in CKD: A roadmap from basic pathogenetic mechanisms to clinical trials. Clin. Kidney J. 2019, 12, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Lauro, M.; Urciuoli, S.; Pietroboni Zaitseva, A.; Wilson Jones, G.; Di Daniele, N.; Romani, A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020, 2020, 1807941. [Google Scholar] [CrossRef]
- Bocedi, A.; Noce, A.; Rovella, V.; Marrone, G.; Cattani, G.; Iappelli, M.; De Paolis, P.; Iaria, G.; Sforza, D.; Gallu, M.; et al. Erythrocyte glutathione transferase in kidney transplantation: A probe for kidney detoxification efficiency. Cell Death Dis. 2018, 9, 288. [Google Scholar] [CrossRef]
- Ochi, M.; Kohara, K.; Tabara, Y.; Kido, T.; Uetani, E.; Ochi, N.; Igase, M.; Miki, T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis 2010, 212, 327–332. [Google Scholar] [CrossRef]
- Kato, A.; Ishida, J.; Endo, Y.; Takita, T.; Furuhashi, M.; Maruyama, Y.; Odamaki, M. Association of abdominal visceral adiposity and thigh sarcopenia with changes of arteriosclerosis in haemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 1967–1976. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Rovella, V.; Cusumano, A.; Di Daniele, N.; Casasco, M. Beneficial effects of physical activity on uremic sarcopenia. Medicina Dello Sport 2018. [Google Scholar] [CrossRef]
- Noce, A.; Bocedi, A.; Campo, M.; Marrone, G.; Di Lauro, M.; Cattani, G.; Di Daniele, N.; Romani, A. A Pilot Study of a Natural Food Supplement as New Possible Therapeutic Approach in Chronic Kidney Disease Patients. Pharmaceuticals 2020, 13, 148. [Google Scholar] [CrossRef]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni-Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Qureshi, A.R.; Axelsson, J.; Heimburger, O.; Suliman, M.E.; Barany, P.; Stenvinkel, P.; Lindholm, B. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am. J. Clin. Nutr. 2007, 86, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.S.; Sun, Y.; Tzamaloukas, A.H. Hypercatabolism in dialysis patients. Curr. Opin. Nephrol. Hypertens. 2008, 17, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Workeneh, B.T.; Mitch, W.E. Review of muscle wasting associated with chronic kidney disease. Am. J. Clin. Nutr. 2010, 91, 1128S–1132S. [Google Scholar] [CrossRef]
- Testelmans, D.; Crul, T.; Maes, K.; Agten, A.; Crombach, M.; Decramer, M.; Gayan-Ramirez, G. Atrophy and hypertrophy signalling in the diaphragm of patients with COPD. Eur. Respir. J. 2010, 35, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Carrero, J.J.; Bover, J.; Chauveau, P.; Mazzaferro, S.; Torres, P.U.; European Renal Nutrition (ERN); Chronic Kidney Disease-Mineral; Bone Disorder (CKD-MBD) Working Groups of the European Renal Association-European Dialysis Transplant Association (ERA-EDTA). Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2017, 8, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.; de Boer, I.H. Impaired vitamin D metabolism in CKD. Semin. Nephrol. 2013, 33, 158–168. [Google Scholar] [CrossRef]
- Bailey, J.L.; Wang, X.; England, B.K.; Price, S.R.; Ding, X.; Mitch, W.E. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J. Clin. Investig. 1996, 97, 1447–1453. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Rondon-Berrios, H.; Zhang, L.; Hu, Z.; Ayehu, G.; Ferrando, A.; Kopple, J.D.; Wang, H.; Storer, T.; Fournier, M.; et al. Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J. Am. Soc. Nephrol. 2006, 17, 3233–3239. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Lee, I.H.; Du, J.; Mitch, W.E. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J. Clin. Investig. 2009, 119, 3059–3069. [Google Scholar] [CrossRef]
- Kerr, J.D.; Holden, R.M.; Morton, A.R.; Nolan, R.L.; Hopman, W.M.; Pruss, C.M.; Garland, J.S. Associations of epicardial fat with coronary calcification, insulin resistance, inflammation, and fibroblast growth factor-23 in stage 3-5 chronic kidney disease. BMC Nephrol. 2013, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Kanaka-Gantenbein, C.; Chrousos, G.P.; Vryonidou, A. Growth hormone axis in patients with chronic kidney disease. Hormones 2019, 18, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Frankel, J.B.; Heldt, A.M.; Grodsky, G.M. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef]
- Wolf, M.; Shah, A.; Gutierrez, O.; Ankers, E.; Monroy, M.; Tamez, H.; Steele, D.; Chang, Y.; Camargo, C.A., Jr.; Tonelli, M.; et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007, 72, 1004–1013. [Google Scholar] [CrossRef]
- Li, X.; Xiang, X.; Hu, J.; Goswami, R.; Yang, S.; Zhang, A.; Wang, Y.; Li, Q.; Bi, X. Association Between Serum Cortisol and Chronic Kidney Disease in Patients with Essential Hypertension. Kidney Blood Press Res. 2016, 41, 384–391. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P. The vulnerable man: Impact of testosterone deficiency on the uraemic phenotype. Nephrol. Dial. Transpl. 2012, 27, 4030–4041. [Google Scholar] [CrossRef]
- Raff, H.; Trivedi, H. Circadian rhythm of salivary cortisol, plasma cortisol, and plasma ACTH in end-stage renal disease. Endocr. Connect. 2013, 2, 23–31. [Google Scholar] [CrossRef]
- Sun, D.F.; Chen, Y.; Rabkin, R. Work-induced changes in skeletal muscle IGF-1 and myostatin gene expression in uremia. Kidney Int. 2006, 70, 453–459. [Google Scholar] [CrossRef]
- Deleaval, P.; Luaire, B.; Laffay, P.; Jambut-Cadon, D.; Stauss-Grabo, M.; Canaud, B.; Chazot, C. Short-Term Effects of Branched-Chain Amino Acids-Enriched Dialysis Fluid on Branched-Chain Amino Acids Plasma Level and Mass Balance: A Randomized Cross-Over Study. J. Ren. Nutr. 2020, 30, 61–68. [Google Scholar] [CrossRef]
- Mitch, W.E.; Price, S.R. Mechanisms activated by kidney disease and the loss of muscle mass. Am. J. Kidney Dis. 2001, 38, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Dias Rodrigues, J.C.; de Oliveira Santin, F.G.; Barbosa Brito Fdos, S.; Bello Moreira, A.S.; Lourenco, R.A.; Avesani, C.M. Food intake assessment of elderly patients on hemodialysis. J. Ren. Nutr. 2015, 25, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef]
- Noce, A.; Fabrini, R.; Dessi, M.; Bocedi, A.; Santini, S.; Rovella, V.; Pastore, A.; Tesauro, M.; Bernardini, S.; Di Daniele, N.; et al. Erythrocyte glutathione transferase activity: A possible early biomarker for blood toxicity in uremic diabetic patients. Acta Diabetol. 2014, 51, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J. Mechanisms of altered regulation of food intake in chronic kidney disease. J. Ren. Nutr. 2011, 21, 7–11. [Google Scholar] [CrossRef]
- Karalliedde, J.; Gnudi, L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, J.; Dong, Y.; Tweardy, D.J.; Dong, Y.; Garibotto, G.; Mitch, W.E. Stat3 activation links a C/EBPdelta to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 2013, 18, 368–379. [Google Scholar] [CrossRef]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef]
- Roshanravan, B.; Gamboa, J.; Wilund, K. Exercise and CKD: Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. Am. J. Kidney Dis. 2017, 69, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Larsson, T.E. Chronic kidney disease: A clinical model of premature aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Kooman, J.P.; Kotanko, P.; Schols, A.M.; Shiels, P.G.; Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J. Dual-Energy X-Ray Absorptiometry: Beyond Bone Mineral Density Determination. Endocrinol. Metab. (Seoul) 2016, 31, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Kehayias, J.J.; Dawson-Hughes, B.; Heymsfield, S.B. Use of dual-energy x-ray absorptiometry in body-composition studies: Not yet a “gold standard”. Am. J. Clin. Nutr. 1993, 58, 589–591. [Google Scholar] [CrossRef]
- Chertow, G.M.; Lowrie, E.G.; Wilmore, D.W.; Gonzalez, J.; Lew, N.L.; Ling, J.; Leboff, M.S.; Gottlieb, M.N.; Huang, W.; Zebrowski, B.; et al. Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J. Am. Soc. Nephrol. 1995, 6, 75–81. [Google Scholar]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. (1985) 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Zha, Y.; Qian, Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B. Editorial: Ketogenic diets: What is the benefit? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Sung, J.M.; Kao, M.D.; Wang, M.C.; Tseng, C.C.; Chen, S.T. Nonprotein calorie supplement improves adherence to low-protein diet and exerts beneficial responses on renal function in chronic kidney disease. J. Ren. Nutr. 2013, 23, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 369–384. [Google Scholar] [CrossRef]
- Cano, N.J.; Aparicio, M.; Brunori, G.; Carrero, J.J.; Cianciaruso, B.; Fiaccadori, E.; Lindholm, B.; Teplan, V.; Fouque, D.; Guarnieri, G.; et al. ESPEN Guidelines on Parenteral Nutrition: Adult renal failure. Clin. Nutr. 2009, 28, 401–414. [Google Scholar] [CrossRef]
- Cano, N.; Fiaccadori, E.; Tesinsky, P.; Toigo, G.; Druml, W.; DGEM; Kuhlmann, M.; Mann, H.; Horl, W.H.; ESPEN. ESPEN Guidelines on Enteral Nutrition: Adult renal failure. Clin. Nutr. 2006, 25, 295–310. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Sadu Singh, B.K.; Khor, B.H.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2017, 36, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Kalantar-Zadeh, K. Management of natural and added dietary phosphorus burden in kidney disease. Semin. Nephrol. 2013, 33, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Aparicio, M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Moore, L.W.; Tortorici, A.R.; Chou, J.A.; St-Jules, D.E.; Aoun, A.; Rojas-Bautista, V.; Tschida, A.K.; Rhee, C.M.; Shah, A.A.; et al. North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Peired, A.; Angelotti, M.L.; Ronconi, E.; la Marca, G.; Mazzinghi, B.; Sisti, A.; Lombardi, D.; Giocaliere, E.; Della Bona, M.; Villanelli, F.; et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J. Am. Soc. Nephrol. 2013, 24, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Bellasi, A.; Raphael, K.L.; Santoro, D.; Aucella, F.; Garofano, L.; Ceccarelli, M.; Di Lullo, L.; Capolongo, G.; Di Iorio, M.; et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: The UBI Study. J. Nephrol. 2019, 32, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef]
- Gaggl, M.; Sliber, C.; Sunder-Plassmann, G. Effect of oral alkali supplementation on progression of chronic kidney disease. Curr. Hypertens. Rev. 2014, 10, 112–120. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef]
- Scialla, J.J.; Appel, L.J.; Wolf, M.; Yang, W.; Zhang, X.; Sozio, S.M.; Miller, E.R., 3rd; Bazzano, L.A.; Cuevas, M.; Glenn, M.J.; et al. Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: The Chronic Renal Insufficiency Cohort study. J. Ren. Nutr. 2012, 22, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N. Phosphate levels and cardiovascular disease in the general population. Clin. J. Am. Soc. Nephrol. 2009, 4, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Nutrition in CKD Guideline Work Group. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.M.; Ahmadi, S.F.; Kalantar-Zadeh, K. The dual roles of obesity in chronic kidney disease: A review of the current literature. Curr. Opin. Nephrol. Hypertens. 2016, 25, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Calvani, R.; Picca, A.; Goncalves, I.O.; Landi, F.; Bernabei, R.; Cesari, M.; Uchida, M.C.; Marzetti, E. Protein-Related Dietary Parameters and Frailty Status in Older Community-Dwellers across Different Frailty Instruments. Nutrients 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Scuteri, A.; Modestino, A.; Frattari, A.; Di Daniele, N.; Tesauro, M. Occurrence of hypotension in older participants. Which 24-hour ABPM parameter better correlate with? J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 804–810. [Google Scholar] [CrossRef]
- Artaza-Artabe, I.; Saez-Lopez, P.; Sanchez-Hernandez, N.; Fernandez-Gutierrez, N.; Malafarina, V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 2016, 93, 89–99. [Google Scholar] [CrossRef]
- Caglar, K.; Fedje, L.; Dimmitt, R.; Hakim, R.M.; Shyr, Y.; Ikizler, T.A. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int. 2002, 62, 1054–1059. [Google Scholar] [CrossRef]
- Lacson, E., Jr.; Wang, W.; Zebrowski, B.; Wingard, R.; Hakim, R.M. Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: A quality improvement report. Am. J. Kidney Dis. 2012, 60, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Sezer, S.; Bal, Z.; Tutal, E.; Uyar, M.E.; Acar, N.O. Long-term oral nutrition supplementation improves outcomes in malnourished patients with chronic kidney disease on hemodialysis. JPEN J. Parenter Enteral Nutr. 2014, 38, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Benner, D.; Brunelli, S.M.; Brosch, B.; Wheeler, J.; Nissenson, A.R. Effects of Oral Nutritional Supplements on Mortality, Missed Dialysis Treatments, and Nutritional Markers in Hemodialysis Patients. J. Ren. Nutr. 2018, 28, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Leonberg-Yoo, A.K.; Wang, W.; Weiner, D.E.; Lacson, E., Jr. Oral nutritional supplements and 30-day readmission rate in hypoalbuminemic maintenance hemodialysis patients. Hemodial. Int. 2019, 23, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Marsen, T.A.; Beer, J.; Mann, H.; German IDPN-Trial Group. Intradialytic parenteral nutrition in maintenance hemodialysis patients suffering from protein-energy wasting. Results of a multicenter, open, prospective, randomized trial. Clin. Nutr. 2017, 36, 107–117. [Google Scholar] [CrossRef]
- Thabet, A.F.; Moeen, S.M.; Labiqe, M.O.; Saleh, M.A. Could intradialytic nutrition improve refractory anaemia in patients undergoing haemodialysis? J. Ren. Care 2017, 43, 183–191. [Google Scholar] [CrossRef]
- Gharekhani, A.; Khatami, M.R.; Dashti-Khavidaki, S.; Razeghi, E.; Abdollahi, A.; Hashemi-Nazari, S.S.; Mansournia, M.A. Effects of oral supplementation with omega-3 fatty acids on nutritional state and inflammatory markers in maintenance hemodialysis patients. J. Ren. Nutr. 2014, 24, 177–185. [Google Scholar] [CrossRef]
- Asemi, Z.; Soleimani, A.; Bahmani, F.; Shakeri, H.; Mazroii, N.; Abedi, F.; Fallah, M.; Mohammadi, A.A.; Esmaillzadeh, A. Effect of the omega-3 fatty acid plus vitamin E supplementation on subjective global assessment score, glucose metabolism, and lipid concentrations in chronic hemodialysis patients. Mol. Nutr. Food Res. 2016, 60, 390–398. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef]
- Donini, L.M.; Savina, C.; Cannella, C. Eating habits and appetite control in the elderly: The anorexia of aging. Int. Psychogeriatr. 2003, 15, 73–87. [Google Scholar] [CrossRef]
- Ekramzadeh, M.; Mazloom, Z.; Jafari, P.; Ayatollahi, M.; Sagheb, M.M. Major barriers responsible for malnutrition in hemodialysis patients: Challenges to optimal nutrition. Nephrourol. Mon. 2014, 6, e23158. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Peel, N.M.; Krosch, M.; Hubbard, R.E. Frailty and chronic kidney disease: A systematic review. Arch. Gerontol. Geriatr. 2017, 68, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter Enteral Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.J.; Ma, F.; Wang, Q.Y.; He, S.L. The effects of oral nutritional supplements in patients with maintenance dialysis therapy: A systematic review and meta-analysis of randomized clinical trials. PLoS ONE 2018, 13, e0203706. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Lee, H.Y.; Lin, Y.C. The Effect of Ketoanalogues on Chronic Kidney Disease Deterioration: A Meta-Analysis. Nutrients 2019, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Bolasco, P. Keto-analogues and essential aminoacids and other supplements in the conservative management of chronic kidney disease. Panminerva Med. 2017, 59, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, L.; Yang, Y.; Liu, H. Dietary supplementation with ketoacids protects against CKD-induced oxidative damage and mitochondrial dysfunction in skeletal muscle of 5/6 nephrectomised rats. Skelet. Muscle 2018, 8, 18. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Huang, J.; Yang, M.; Gu, L.J.; Ji, J.Y.; Wang, L.J.; Yuan, W.J. Effect of a low-protein diet supplemented with keto-acids on autophagy and inflammation in 5/6 nephrectomized rats. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef]
- Wang, D.T.; Lu, L.; Shi, Y.; Geng, Z.B.; Yin, Y.; Wang, M.; Wei, L.B. Supplementation of ketoacids contributes to the up-regulation of the Wnt7a/Akt/p70S6K pathway and the down-regulation of apoptotic and ubiquitin-proteasome systems in the muscle of 5/6 nephrectomised rats. Br. J. Nutr. 2014, 111, 1536–1548. [Google Scholar] [CrossRef]
- Walser, M.; Coulter, A.W.; Dighe, S.; Crantz, F.R. The effect of keto-analogues of essential amino acids in severe chronic uremia. J. Clin. Investig. 1973, 52, 678–690. [Google Scholar] [CrossRef][Green Version]
- Barsotti, G.; Guiducci, A.; Ciardella, F.; Giovannetti, S. Effects on renal function of a low-nitrogen diet supplemented with essential amino acids and ketoanalogues and of hemodialysis and free protein supply in patients with chronic renal failure. Nephron 1981, 27, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Zemchenkov, A.; Konakova, I.N. Efficacy of the Essential Amino Acids and Keto-Analogues on the CKD progression rate in real practice in Russia -city nephrology registry data for outpatient clinic. BMC Nephrol. 2016, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Antonucci, E.; Cabassi, A.; Morabito, S.; Fiaccadori, E. Intradialytic parenteral nutrition in end-stage renal disease: Practical aspects, indications and limits. J. Nephrol. 2014, 27, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Maraj, M.; Kusnierz-Cabala, B.; Dumnicka, P.; Gala-Bladzinska, A.; Gawlik, K.; Pawlica-Gosiewska, D.; Zabek-Adamska, A.; Mazur-Laskowska, M.; Ceranowicz, P.; Kuzniewski, M. Malnutrition, Inflammation, Atherosclerosis Syndrome (MIA) and Diet Recommendations among End-Stage Renal Disease Patients Treated with Maintenance Hemodialysis. Nutrients 2018, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, H.K.; Jain, D.; Chauda, R.; Bhatia, S.; Sehgal, R. Assessment of Malnutrition Inflammation Score in Different Stages of Chronic Kidney Disease. Pril (Makedon. Akad. Nauk. Umet. Odd. Med. Nauki) 2018, 39, 51–61. [Google Scholar] [CrossRef]
- Raiten, D.J.; Namaste, S.; Brabin, B.; Combs, G., Jr.; L’Abbe, M.R.; Wasantwisut, E.; Darnton-Hill, I. Executive summary--Biomarkers of Nutrition for Development: Building a Consensus. Am. J. Clin. Nutr. 2011, 94, 633S–650S. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Wilson Jones, G.; De Lorenzo, A.; Di Daniele, N. Potential Cardiovascular and Metabolic Beneficial Effects of omega-3 PUFA in Male Obesity Secondary Hypogonadism Syndrome. Nutrients 2020, 12, 2519. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Cryer, M.J.; Horani, T.; DiPette, D.J. Diabetes and Hypertension: A Comparative Review of Current Guidelines. J. Clin. Hypertens. (Greenwich) 2016, 18, 95–100. [Google Scholar] [CrossRef]
- Dessi, M.; Noce, A.; Bertucci, P.; Noce, G.; Rizza, S.; De Stefano, A.; Manca di Villahermosa, S.; Bernardini, S.; De Lorenzo, A.; Di Daniele, N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Koorts, A.M.; Viljoen, M.; Kruger, M.C. Red blood cell fatty acid profile of chronic renal failure patients receiving maintenance haemodialysis treatment. Prostaglandins Leukot. Essent. Fatty Acids 2002, 67, 13–18. [Google Scholar] [CrossRef]
- Dasgupta, A.; Kenny, M.A.; Ahmad, S. Abnormal fatty acid profile in chronic hemodialysis patients: Possible deficiency of essential fatty acids. Clin. Physiol. Biochem. 1990, 8, 238–243. [Google Scholar]
- Zambon, A.; Pirillo, A.; Zambon, S.; Norata, G.D.; Catapano, A.L. Omega n-3 Supplementation: Exploring the Cardiovascular Benefits Beyond Lipoprotein Reduction. Curr. Atheroscler. Rep. 2020, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, E.; Koukouvou, G.; Kouidi, E.; Deligiannis, A.; Tourkantonis, A. Exercise training in patients with end-stage renal disease on hemodialysis: Comparison of three rehabilitation programs. J. Rehabil. Med. 2002, 34, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Mallamaci, F.; D’Arrigo, G.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-Rey, L.; et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J. Am. Soc. Nephrol. 2017, 28, 1259–1268. [Google Scholar] [CrossRef]
- Dong, Z.J.; Zhang, H.L.; Yin, L.X. Effects of intradialytic resistance exercise on systemic inflammation in maintenance hemodialysis patients with sarcopenia: A randomized controlled trial. Int. Urol. Nephrol. 2019, 51, 1415–1424. [Google Scholar] [CrossRef]
- Lopes, L.C.C.; Mota, J.F.; Prestes, J.; Schincaglia, R.M.; Silva, D.M.; Queiroz, N.P.; Freitas, A.; Lira, F.S.; Peixoto, M. Intradialytic Resistance Training Improves Functional Capacity and Lean Mass Gain in Individuals on Hemodialysis: A Randomized Pilot Trial. Arch. Phys. Med. Rehabil. 2019, 100, 2151–2158. [Google Scholar] [CrossRef]
- Gould, D.W.; Watson, E.L.; Wilkinson, T.J.; Wormleighton, J.; Xenophontos, S.; Viana, J.L.; Smith, A.C. Ultrasound assessment of muscle mass in response to exercise training in chronic kidney disease: A comparison with MRI. J. Cachexia Sarcopenia Muscle 2019, 10, 748–755. [Google Scholar] [CrossRef]
- Nishi, H.; Takemura, K.; Higashihara, T.; Inagi, R. Uremic Sarcopenia: Clinical Evidence and Basic Experimental Approach. Nutrients 2020, 12, 1814. [Google Scholar] [CrossRef] [PubMed]
- Howden, E.J.; Leano, R.; Petchey, W.; Coombes, J.S.; Isbel, N.M.; Marwick, T.H. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1494–1501. [Google Scholar] [CrossRef]
- Van Craenenbroeck, A.H.; Van Craenenbroeck, E.M.; Van Ackeren, K.; Vrints, C.J.; Conraads, V.M.; Verpooten, G.A.; Kouidi, E.; Couttenye, M.M. Effect of Moderate Aerobic Exercise Training on Endothelial Function and Arterial Stiffness in CKD Stages 3-4: A Randomized Controlled Trial. Am. J. Kidney Dis. 2015, 66, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Fumagalli, G.; Vigo, V.; Meola, M.; Cianchi, C.; Egidi, M.F. Nutrition and physical activity in CKD patients. Kidney Blood Press Res. 2014, 39, 107–113. [Google Scholar] [CrossRef]
- Castaneda, C.; Gordon, P.L.; Uhlin, K.L.; Levey, A.S.; Kehayias, J.J.; Dwyer, J.T.; Fielding, R.A.; Roubenoff, R.; Singh, M.F. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann. Intern. Med. 2001, 135, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Pupim, L.B.; Flakoll, P.J.; Levenhagen, D.K.; Ikizler, T.A. Exercise augments the acute anabolic effects of intradialytic parenteral nutrition in chronic hemodialysis patients. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E589–E597. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Broyer, M.; Gagnadoux, M.F.; Niaudet, P.; Bresson, J.L. Alterations of protein metabolism by metabolic acidosis in children with chronic renal failure. Kidney Int. 2000, 58, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Venkatasamy, V.V.; Pericherla, S.; Manthuruthil, S.; Mishra, S.; Hanno, R. Effect of Physical activity on Insulin Resistance, Inflammation and Oxidative Stress in Diabetes Mellitus. J. Clin. Diagn. Res. 2013, 7, 1764–1766. [Google Scholar] [CrossRef]
- Mullan, R.J.; Montori, V.M.; Shah, N.D.; Christianson, T.J.; Bryant, S.C.; Guyatt, G.H.; Perestelo-Perez, L.I.; Stroebel, R.J.; Yawn, B.P.; Yapuncich, V.; et al. The diabetes mellitus medication choice decision aid: A randomized trial. Arch. Intern. Med. 2009, 169, 1560–1568. [Google Scholar] [CrossRef]
- Macdonald, J.H.; Marcora, S.M.; Jibani, M.M.; Kumwenda, M.J.; Ahmed, W.; Lemmey, A.B. Nandrolone decanoate as anabolic therapy in chronic kidney disease: A randomized phase II dose-finding study. Nephron. Clin. Pract. 2007, 106, c125–c135. [Google Scholar] [CrossRef]
- Wang, X.H.; Hu, Z.; Klein, J.D.; Zhang, L.; Fang, F.; Mitch, W.E. Decreased miR-29 suppresses myogenesis in CKD. J. Am. Soc. Nephrol. 2011, 22, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, R.; Workeneh, B.; Dong, Y.; Wang, X.; Hu, Z. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int. 2012, 82, 401–411. [Google Scholar] [CrossRef]
- Powers, S.K.; Wiggs, M.P.; Duarte, J.A.; Zergeroglu, A.M.; Demirel, H.A. Mitochondrial signaling contributes to disuse muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E31–E39. [Google Scholar] [CrossRef] [PubMed]
- Zaza, G.; Granata, S.; Masola, V.; Rugiu, C.; Fantin, F.; Gesualdo, L.; Schena, F.P.; Lupo, A. Downregulation of nuclear-encoded genes of oxidative metabolism in dialyzed chronic kidney disease patients. PLoS ONE 2013, 8, e77847. [Google Scholar] [CrossRef]
- Mercken, E.M.; Mitchell, S.J.; Martin-Montalvo, A.; Minor, R.K.; Almeida, M.; Gomes, A.P.; Scheibye-Knudsen, M.; Palacios, H.H.; Licata, J.J.; Zhang, Y.; et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 2014, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Ioannidis, J.P. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. BMJ 2013, 347, f5577. [Google Scholar] [CrossRef]
- Finch, C.F.; White, P.; Twomey, D.; Ullah, S. Implementing an exercise-training programme to prevent lower-limb injuries: Considerations for the development of a randomised controlled trial intervention delivery plan. Br. J. Sports Med. 2011, 45, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef]
| Nutritional Approaches | Author | Year | Study Population | Nutritional Treatment | Primary Outcome | Primary End-Point |
|---|---|---|---|---|---|---|
| ONS | Caglar K. et al. [110] | 2002 | 85 malnourished CHD, HD patients | ONS assumed during each HD session, containing 16.6 g of proteins, 22.7 g of lipids and 52.8 g of carbohydrates with energy content of 475 kcal. | Significant increases in serum albumin and prealbumin levels were detected. In addition, there was a 14% increase in SGA score. | ONS assumed during HD improves some nutritional biomarkers in malnourished HD patients. |
| Lacson Jr E. et al. [111] | 2012 | 5.227 HD patients with albumin level ≤3.5 g/dL vs. 5.227 patients (control group) | Four different intradialytic ONS were administered:
| A reduction in mortality was found in patients treated with ONS compared to non-treated group. | ONS treatment allows a significant increase in survival of HD patients. | |
| Wu H.L. et al. [84] | 2013 | 55 CKD patients (stage III-IV) vs. 54 patients (control group) | One daily ONS containing 0.6 g of proteins, 8.2 g of lipids, 30.9 g of carbohydrates and 1.9 g of fiber with energy content of 200 kcal. | ONS significant decreased urine protein excretion therefore, daily protein intake was lower in the ONS group. Significant decrease of creatinine and urea nitrogen levels; in addition, there was a significant increase of eGFR. | ONS has improved some blood parameters and improved the adherence to the nutritional therapy with less protein excretion. | |
| Sezer S. et al. [112] | 2014 | 32 malnourished HD patients vs. 30 patients (control group) | ONS containing 14 g of proteins, 19.2 g of lipids and 41.3 g of carbohydrates with energy content of 400 kcal. In addition, during HD sessions was served a snack containing 14 g of proteins, 10 g of lipids and 55 g of carbohydrates with energy content of 300 kcal. | Significant increases in serum albumin levels were detected. Furthermore, there was a significant increase in the dry weight of the ONS patients and a significative reduction in the dry weight of the control group. In addition, a reduction of EPO dose requirement and MIS was detected in the treated group. | ONS treatment improves serum albumin levels and allows a lower EPO dose requirement in HD patients. | |
| Benner D. et al. [113] | 2018 | 3.374 HD patients with albumin level ≤3.5 g/dL vs. 3.374 patients (control group) | Two different ONS were used:
| There was a 69% reduction in mortality and a 33% reduction in missed dialysis sessions. | ONS treatment allows a significant increase in survival in HD patients with albumin level ≤3.5 g/dL. | |
| Leonberg-Yoo A.K. et al. [114] | 2019 | 1420 HD patients vs. 4.059 patients (control group) | Six different intradialytic ONS were used:
| There was a decrease of re-hospitalization within 30 days of first discharge. | ONS treatment reduces post-discharge hospital readmission rates. | |
| IDPN | Marsen T.A. et al. [115] | 2017 | 39 HD patients with PEW vs. 44 patients (control group) | IDPN treatment three times/week containing (one dose):
| Significant increases in serum prealbumin levels were detected. | IDPN used during HD session improves prealbumin levels. |
| Thabet A.F. et al. [116] | 2017 | 20 HD patients vs. 20 patients (control group) | IDPN treatment three times/week. In addition, patients received EPO, iron dextran, folic acid and vitamin B 12. | Significant increases in hemoglobin and albumin levels were detected. In addition, there was a significant increase in BMI. Significant reduction in MIS was detected. | IDPN treatment allows an improvement of refractory anemia, as it permits an increase in hemoglobin and prealbumin levels and also an increase in body weight. It also leads to a reduction in MIS. | |
| Deleaval P. et al. [60] | 2020 | 6 HD patients | Two dialysates were used during HD treatment:
| During the HD treatment with standard dialysate a reduction in plasmatic valine was found, while with dialysate enriched in BCAA HD treatment there was an increase in plasmatic valine, isoleucine and leucine. | The use of dialysate enriched in BCAA allows the restoration of normal plasma BCAA levels. | |
| ω-3 supplementation | Gharekhani A. et al. [117] | 2014 | 27 HD patients vs. 27 patients (control group) | Six capsules per day of ω-3 supplementation (180 mg eicosapentaenoic acid and 120 mg docosahexaenoic acid in each capsule). | ω-3 supplementation is a significant independent predictor for the increase of serum prealbumin level after adjusting post-treatment nutritional markers. Significant decrease in ferritin levels and IL-10/IL-6 ratio was detected. | ω-3 supplementation in HD patients permits a slight reduction of inflammation. |
| Asemi Z. et al. [118] | 2016 | 90 HD patients vs. 30 patients (control group) | Four groups for supplementation per day:
| Significant reduction in SGA, FPG, insulin levels and HOMA-IR were detected. In addition, there was a significant enhancement in QUICKI. | ω-3 PUFA and vitamin E combined supplementation improve SGA and the metabolic profile in HD patients. | |
| Fiber | Krishnamurthy V.M.R. et al. [119] | 2012 | 1.105 CKD patients (stage IIIa-IV) vs. 13.438 subjects (control group) | Two groups were divided into two subgroups according to fiber dietary intake:
| Significant decrease in CRP was detected in CKD patients with high total fiber dietary consumption. | The high dietary fiber consumption is associated with a minor inflammation risk and mortality in CKD patients. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. https://doi.org/10.3390/nu13010147
Noce A, Marrone G, Ottaviani E, Guerriero C, Di Daniele F, Pietroboni Zaitseva A, Di Daniele N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients. 2021; 13(1):147. https://doi.org/10.3390/nu13010147
Chicago/Turabian StyleNoce, Annalisa, Giulia Marrone, Eleonora Ottaviani, Cristina Guerriero, Francesca Di Daniele, Anna Pietroboni Zaitseva, and Nicola Di Daniele. 2021. "Uremic Sarcopenia and Its Possible Nutritional Approach" Nutrients 13, no. 1: 147. https://doi.org/10.3390/nu13010147
APA StyleNoce, A., Marrone, G., Ottaviani, E., Guerriero, C., Di Daniele, F., Pietroboni Zaitseva, A., & Di Daniele, N. (2021). Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients, 13(1), 147. https://doi.org/10.3390/nu13010147





