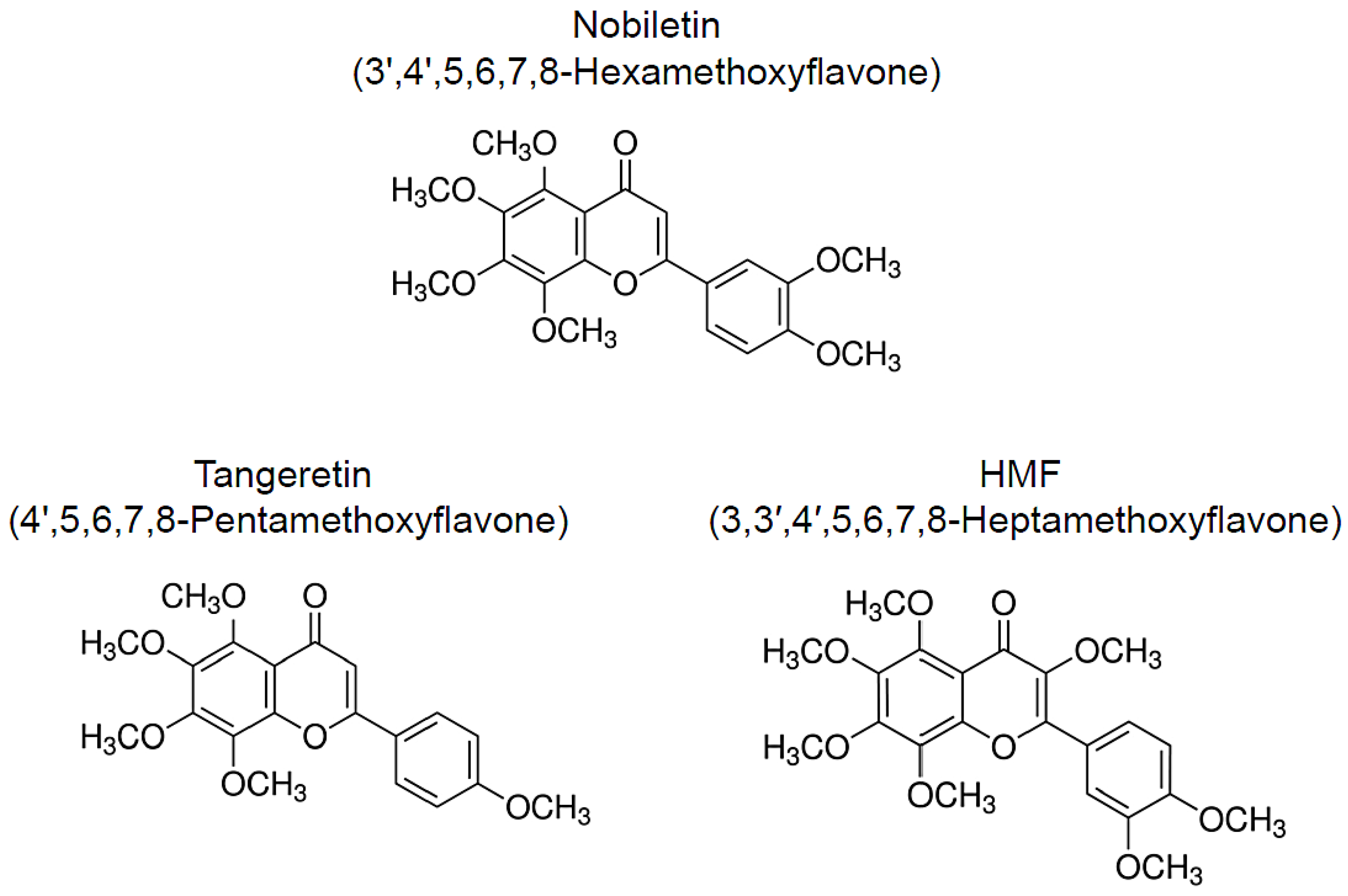
Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders
Abstract
1. Introduction
2. Nobiletin
2.1. AD Model Animals
2.2. PD Model Animals
2.3. Ischemic Injury Models
2.4. Lipopolysaccharide (LPS)-Induced Inflammation
2.5. Animal Model for Multiple Sclerosis
2.6. Chronic Unpredictable Mild Stress (CUMS)-Induced Synaptic Dysfunction and Depression-Like Behavior
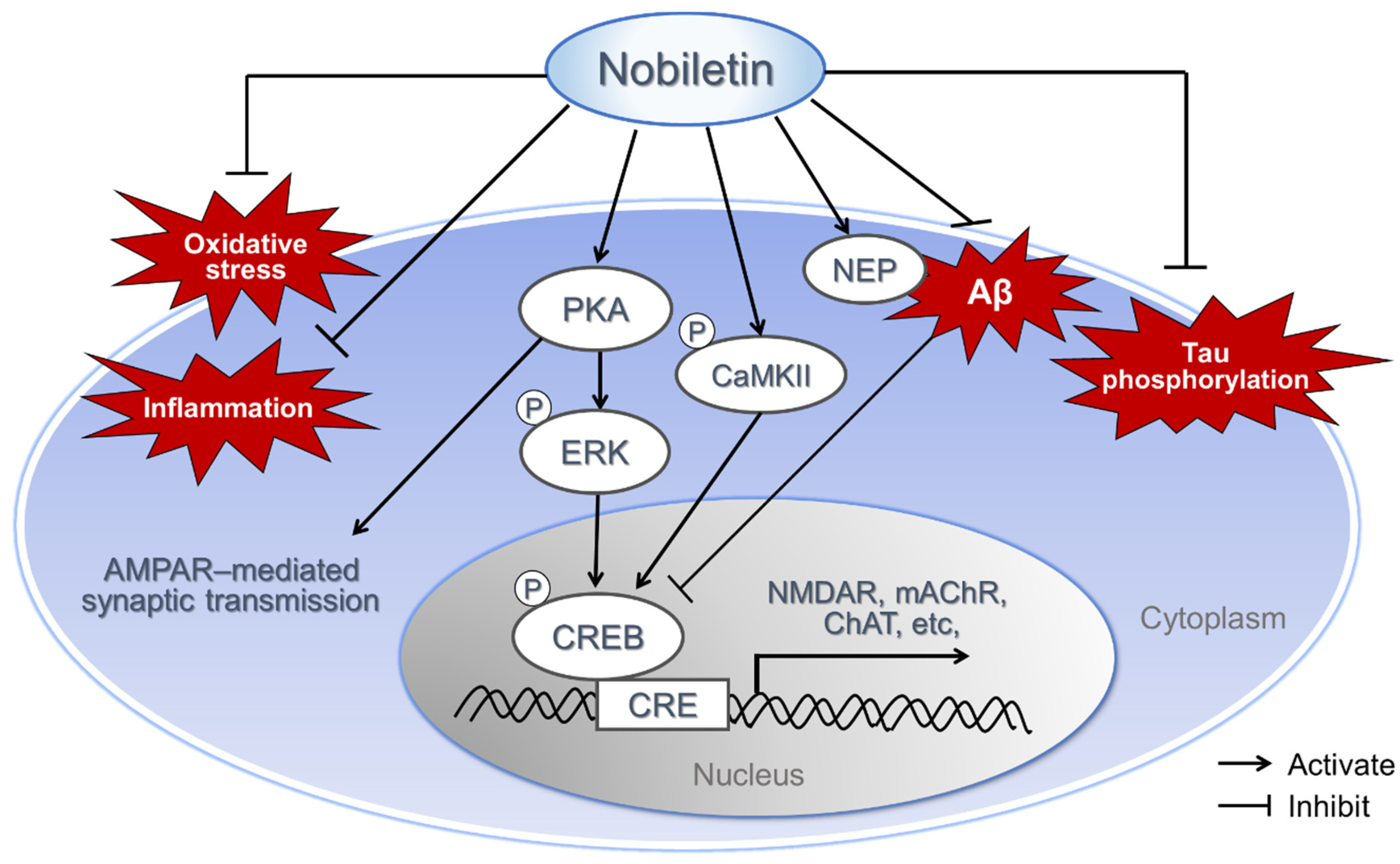
2.7. Various Mechanism of Neuroprotective Effect of Nobiletin
3. Tangeretin
3.1. PD Models
3.2. Cerebral Ischemia–Reperfusion Injury Model
3.3. Epilepsy Model Rats
3.4. Nephrectomized Rats
3.5. Posttraumatic Stress Disorder (PTSD) Model Rats
3.6. Antioxidative and Antineuroinflammatory Effect of Tangeretin
4. HMF
4.1. Cerebral Ischemia Mouse
4.2. MK-801–Induced Memory Deficits and Locomotive Hyperactivity
4.3. Antineuroinflammatory Effect
4.4. Stress-Induced Depression Models
4.5. Neurotrophic Effect of HMF
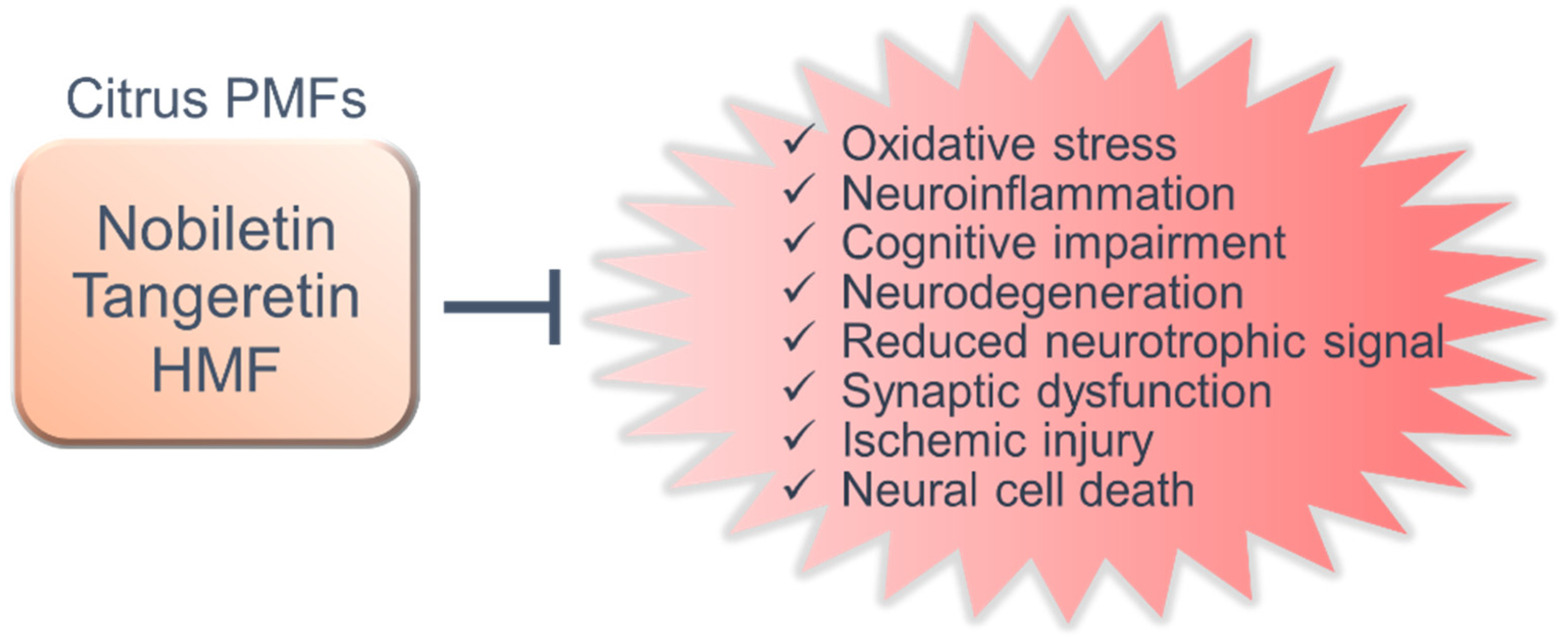
5. Shared Functions and New Research Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Australia, D.; Baker, S.; Banerjee, S. Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to dementia. In Alzheimer’s Disease International; Alzheimer’s Disease International: London, UK, 2019. [Google Scholar]
- Robinson, L.; Tang, E.; Taylor, J.-P. Dementia: Timely diagnosis and early intervention. BMJ 2015, 350, h3029. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Switzerland, Geneva, 2019. [Google Scholar]
- Selkoe, D.J.; Lansbury, P.J. Alzheimer’s disease is the most common neurodegenerative disorder. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, J.G., Agranoff, B.W., Albers., R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Rabbito, A.; Dulewicz, M.; Kulczyńska-Przybik, A.; Mroczko, B. Biochemical Markers in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1989. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [PubMed]
- Mhatre, M.; Floyd, R.A.; Hensley, K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: Common links and potential therapeutic targets. J. Alzheimers Dis. 2004, 6, 147–157. [Google Scholar] [CrossRef]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar]
- Ohyagi, Y.; Asahara, H.; Chui, D.H.; Tsuruta, Y.; Sakae, N.; Miyoshi, K.; Yamada, T.; Kikuchi, H.; Taniwaki, T.; Murai, H.; et al. Intracellular Abeta42 activates p53 promoter: A pathway to neurodegeneration in Alzheimer’s disease. FASEB J. 2005, 19, 255–257. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Picone, P.; Di Carlo, M.; Nuzzo, D. Obesity and Alzheimer’s disease: Molecular bases. Eur. J. Neurosci. 2020, 52, 3944–3950. [Google Scholar] [CrossRef]
- Vinciguerra, F.; Graziano, M.; Hagnäs, M.; Frittita, L.; Tumminia, A. Influence of the Mediterranean and Ketogenic Diets on Cognitive Status and Decline: A Narrative Review. Nutrients 2020, 12, 1019. [Google Scholar] [CrossRef]
- Ohizumi, Y. A New Strategy for Preventive and Functional Therapeutic Methods for Dementia—Approach Using Natural Products. Yakugaku Zasshi 2015, 135, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Yano, S.; Sumiyoshi, E.; Shido, O.; Katsube, T.; Tabata, M.; Okuda, M.; Sugimoto, H.; Yoshino, K.; Hashimoto, M. Long-Term Ultra-High Hydrostatic Pressurized Brown Rice Intake Prevents Bone Mineral Density Decline in Elderly Japanese Individuals. J. Nutr. Sci. Vitaminol. 2019, 65, S88–S92. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Matsuzaki, K.; Sumiyoshi, E.; Hossain, M.E.; Hashimoto, M.; Katakura, M.; Sugimoto, N.; Shido, O. Theobromine Improves Working Memory by Activating the CaMKII/CREB/BDNF Pathway in Rats. Nutrients 2019, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, E.; Matsuzaki, K.; Sugimoto, N.; Tanabe, Y.; Hara, T.; Katakura, M.; Miyamoto, M.; Mishima, S.; Shido, O. Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial. Nutrients 2019, 11, 2800. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Matsuzaki, K.; Shido, O.; Yoshino, K. The journey from white rice to ultra-high hydrostatic pressurized brown rice: An excellent endeavor for ideal nutrition from staple food. Crit. Rev. Food Sci. Nutr. 2020, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tanabe, Y.; Hossain, S.; Matsuzaki, K.; Ohno, M.; Kato, S.; Katakura, M.; Shido, O. Intake of Alpha-Linolenic Acid-Rich Perilla frutescens Leaf Powder Decreases Home Blood Pressure and Serum Oxidized Low-Density Lipoprotein in Japanese Adults. Molecules 2020, 25, 2099. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Choi, S.Y.; Hwang, J.H.; Ko, H.C.; Park, J.G.; Kim, S.J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-kappaB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef]
- Hirata, Y.; Masuda, Y.; Kakutani, H.; Higuchi, T.; Takada, K.; Ito, A.; Nakagawa, Y.; Ishii, H. Sp1 is an essential transcription factor for LPS-induced tissue factor expression in THP-1 monocytic cells, and nobiletin represses the expression through inhibition of NF-κB, AP-1, and Sp1 activation. Biochem. Pharmacol. 2008, 75, 1504–1514. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.-T.; Li, H.-B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.-Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M.; et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar] [PubMed]
- Wang, Z.; Yang, B.; Chen, X.; Zhou, Q.; Li, H.; Chen, S.; Yin, D.; He, H.; He, M. Nobiletin Regulates ROS/ADMA/DDAHII/eNOS/NO Pathway and Alleviates Vascular Endothelium Injury by Iron Overload. Biol. Trace Elem. Res. 2020, 198, 87–97. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, Y.; Liu, M.; Yu, H.; Chen, Q.; Chen, Y.; Ruan, J.; Ding, Z.; Zhang, Y.; Wang, T. Citrus aurantium L. and Its Flavonoids Regulate TNBS-Induced Inflammatory Bowel Disease through Anti-Inflammation and Suppressing Isolated Jejunum Contraction. Int. J. Mol. Sci. 2018, 19, 3057. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Park, W.; Kang, T.W.; Cha, M.H.; Chun, J.M. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. J. Ethnopharmacol. 2018, 221, 151–159. [Google Scholar] [CrossRef]
- Nichols, L.A.; Jackson, D.E.; Manthey, J.A.; Shukla, S.D.; Holland, L.J. Citrus flavonoids repress the mRNA for stearoyl-CoA desaturase, a key enzyme in lipid synthesis and obesity control, in rat primary hepatocytes. Lipids Health Dis. 2011, 10, 36. [Google Scholar] [CrossRef]
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Drangova, M.; Huff, M.W. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr-/- mice. J. Lipid Res. 2018, 59, 1714–1728. [Google Scholar] [CrossRef]
- Chou, Y.C.; Ho, C.T.; Pan, M.H. Immature Citrus reticulata Extract Promotes Browning of Beige Adipocytes in High-Fat Diet-Induced C57BL/6 Mice. J. Agric. Food Chem. 2018, 66, 9697–9703. [Google Scholar] [CrossRef]
- Nguyen-Ngo, C.; Salomon, C.; Quak, S.; Lai, A.; Willcox, J.C.; Lappas, M. Nobiletin exerts anti-diabetic and anti-inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clin. Sci. 2020, 134, 571–592. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Parkar, N.A.; Bhattm, L.K.; Addepalli, V. Efficacy of nobiletin, a citrus flavonoid, in the treatment of the cardiovascular dysfunction of diabetes in rats. Food Funct. 2016, 7, 3121–3129. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Funamoto, M.; Suzuki, A.; Shimizu, K.; Sakurai, R.; Katanasaka, Y.; Miyazaki, Y.; Asakawa, T.; Kan, T.; Inagaki, J.; et al. A Novel Target Molecule of Nobiletin Derived from Citrus Peels has a Therapeutic Potency Against the Development of Heart Failure. Eur. Cardiol. 2017, 12, 105. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, R.; Liu, J.; Wang, H.; Wang, Z.; Liu, J.; Shan, Y.; Yu, H. The Effects of 5,6,7,8,3′,4′-Hexamethoxyflavone on Apoptosis of Cultured Human Choriocarcinoma Trophoblast Cells. Molecules 2020, 25, 946. [Google Scholar] [CrossRef] [PubMed]
- Goan, Y.G.; Wu, W.T.; Liu, C.I.; Neoh, C.A.; Wu, Y.J. Involvement of Mitochondrial Dysfunction, Endoplasmic Reticulum Stress, and the PI3K/AKT/mTOR Pathway in Nobiletin-Induced Apoptosis of Human Bladder Cancer Cells. Molecules 2019, 24, 2881. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.Y.; Joung, Y.H.; Park, J.H.; Kim, W.S.; Lee, H.K.; Song, K.D.; Park, Y.M.; Yang, Y.M. Nobiletin Inhibits Angiogenesis by Regulating Src/FAK/STAT3-Mediated Signaling through PXN in ER+; Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 935. [Google Scholar] [CrossRef] [PubMed]
- Nitta, A.; Itoh, A.; Hasegawa, T.; Nabeshima, T. beta-Amyloid protein-induced Alzheimer’s disease animal model. Neurosci. Lett. 1994, 170, 63–66. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring beta-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, O.V.; Sant’Angelo, A.; Costanzo, V.; Battaglia, F.; Arancio, O.; Shelanski, M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 13217–13221. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.K.; Lee, D.R.; Choi, B.K.; Le, B.; Yang, S.H. Ameliorating effect of Citrus aurantium extracts and nobiletin on β-amyloid (1-42)-induced memory impairment in mice. Mol. Med. Rep. 2019, 20, 3448–3455. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef]
- Bai, X.C.; Yan, C.; Yang, G.; Lu, P.; Ma, D.; Sun, L.; Zhou, R.; Scheres, S.; Shi, Y. An atomic structure of human γ-secretase. Nature 2015, 525, 212–217. [Google Scholar] [CrossRef]
- Augustinack, J.C.; Schneider, A.; Mandelkow, E.M.; Hyman, B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002, 103, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.W.; Ogino, K.; Shimabuku, A.; Taki, T.; Nakashima, H.; Ishihara, T.; Kitamoto, T. Amyloid precursor protein cytoplasmic domain with phospho-Thr668 accumulates in Alzheimer’s disease and its transgenic models: A role to mediate interaction of Abeta and tau. Acta Neuropathol. 2007, 113, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef]
- Gemma, C.; Vila, J.; Bachstetter, A.; Bickford, P.C. Oxidative stress and the aging brain: From theory to prevention. In Frontiers in Neuroscience. Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; CRC Press; American Publishing Group: Boca Raton, FL, USA, 2007; pp. 353–374. [Google Scholar]
- Hwang, J.H.; Lee, I.T.; Jeng, K.C.; Wang, M.F.; Hou, R.C.; Wu, S.M.; Chan, Y.C. Spirulina prevents memory dysfunction, reduces oxidative stress damage and augments antioxidant activity in senescence-accelerated mice. J. Nutr. Sci. Vitaminol. 2011, 57, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, T.; Akiguchi, I.; Yagi, H.; Irino, M.; Sugiyama, H.; Akiyama, H.; Shimada, A.; Takemura, M.; Ueno, M.; Kitabayashi, T.; et al. Neuropathological studies on strains of senescence-accelerated mice (SAM) with age-related deficits in learning and memory. Exp. Gerontol. 1997, 32, 161–169. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Shi, J.S. SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 75, 385–395. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Nguyen, T.T.; Shin, E.J.; Kim, H.C.; Yamada, S.; Nakai, T.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain. Res. 2013, 250, 351–360. [Google Scholar] [CrossRef]
- Bartus, R.T. On Neurodegenerative Diseases, Models, and Treatment Strategies: Lessons Learned and Lessons Forgotten a Generation Following the Cholinergic Hypothesis. Exp. Neurol. 2000, 163, 495–529. [Google Scholar] [CrossRef]
- Yamamoto, T.; Jin, J.; Watanabe, S. Characteristics of memory dysfunction in olfactory bulbectomized rats and the effects of cholinergic drugs. Behav. Brain Res. 1997, 83, 57–62. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Song, S.Y.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a Citrus Flavonoid That Improves Memory Impairment, Rescues Bulbectomy-Induced Cholinergic Neurodegeneration in Mice. J. Pharmacol. Sci. 2007, 105, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, P.; Layer, R.T.; Popik, P.; Nowak, G.; Paul, I.A.; Trullas, R. Adaptation of N-Methyl-D-Aspartate (NMDA) Receptors following Antidepressant Treatment: Implications for the Pharmacotherapy of Depression. Pharmacopsychiatry 1996, 29, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.C.; Kulkarni, S.K. Effects of MK-801 and ketamine on short-term memory deficits in passive avoidance step-down task paradigm in mice. Methods Find. Exp. Clin. Pharmacol. 1991, 13, 155–159. [Google Scholar]
- Malenfant, S.A.; O’Hearn, S.; Fleming, A.S. MK801, an NMDA antagonist, blocks acquisition of a spatial task but does not block maternal experience effects. Physiol. Behav. 1991, 49, 1129–1137. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamakuni, T.; Matsuzaki, K.; Nakata, N.; Onozuka, H.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Ohizumi, Y. Nobiletin, a Citrus Flavonoid, Reverses Learning Impairment Associated with N-Methyl-D-aspartate Receptor Antagonism by Activation of Extracellular Signal-Regulated Kinase Signaling. J. Pharmacol. Exp. Ther. 2007, 321, 784–790. [Google Scholar] [CrossRef]
- Alexander, G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar] [PubMed]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Esposito, E.; Di Matteo, V.; Benigno, A.; Pierucci, M.; Crescimanno, G.; Di Giovanni, G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007, 205, 295–312. [Google Scholar] [CrossRef]
- Kaakkola, S.; Teräväinen, H. Animal Models of Parkinsonism. Pharmacol. Toxicol. 1990, 67, 95–100. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Ohizumi, Y.; Yokosuka, A.; Mimaki, Y.; Fukunaga, K. Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice. Neuroscience 2014, 259, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Jeon, M.T.; Kim, H.D.; Jung, U.J.; Jang, M.C.; Chu, J.W.; Yang, S.J.; Choi, I.Y.; Choi, M.S.; Kim, S.R. Nobiletin protects dopaminergic neurons in the 1-methyl-4-phenylpyridinium-treated rat model of Parkinson’s disease. J. Med. Food. 2015, 18, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Flamm, E.S.; Demopoulos, H.B.; Seligman, M.L.; Poser, R.G.; Ransohoff, J. Free radicals in cerebral ischemia. Stroke 1978, 9, 445–447. [Google Scholar] [CrossRef]
- Mori, E. Impact of subcortical ischemic lesions on behavior and cognition. Ann. N. Y. Acad. Sci. 2002, 977, 141–148. [Google Scholar] [CrossRef]
- Watanabe, T.; Tanaka, M.; Watanabe, K.; Takamatsu, Y.; Tobe, A. Research and Development of the Free Radical Scavenger Edaravone as a Neuroprotectant. Yakugaku Zasshi 2004, 124, 99–111. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shioda, N.; Han, F.; Moriguchi, S.; Nakajima, A.; Yokosuka, A.; Mimaki, Y.; Sashida, Y.; Yamakuni, T.; Ohizumi, Y.; et al. Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMKII and CREB phosphorylation. Brain Res. 2009, 1295, 218–229. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Zhang, X.; Chen, L.; Zhao, X.; Bai, X.; Zhang, J. Nobiletin protects against cerebral ischemia via activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and ameliorating BBB permeability in rat. Brain Res Bull. 2013, 96, 45–53. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Zhang, C.; Bai, X.; Zhang, J.; Zhao, X.; Chen, L.; Wang, L.; Zhu, C.; Cui, L.; et al. Nobiletin promotes antioxidant and anti-inflammatory responses and elicits protection against ischemic stroke in vivo. Brain Res. 2016, 1636, 130–141. [Google Scholar] [CrossRef]
- Vexler, Z.S.; Roberts, T.P.; Bollen, A.W.; Derugin, N.; Arieff, A.I. Transient cerebral ischemia. Association of apoptosis induction with hypoperfusion. J. Clin. Investig. 1997, 99, 1453–1459. [Google Scholar] [CrossRef]
- Yasuda, N.; Ishii, T.; Oyama, D.; Fukuta, T.; Agato, Y.; Sato, A.; Shimizu, K.; Asai, T.; Asakawa, T.; Kan, T.; et al. Neuroprotective effect of nobiletin on cerebral ischemia–reperfusion injury in transient middle cerebral artery-occluded rats. Brain Res. 2014, 1559, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, F.; Yu, L.; Li, Z. Nobiletin alleviates cerebral ischemic-reperfusion injury via MAPK signaling pathway. Am. J. Transl. Res. 2019, 11, 5967–5977. [Google Scholar] [PubMed]
- Zheng, Y.; Bu, J.; Yu, L.; Chen, J.; Liu, H. Nobiletin improves propofol-induced neuroprotection via regulating Akt/mTOR and TLR 4/NF-κB signaling in ischemic brain injury in rats. Biomed. Pharmacother. 2017, 91, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.H.; Bigbee, M.J.; Boynton, G.E.; Wakeham, C.M.; Rosenheim, H.M.; Staral, C.J.; Morrissey, J.L.; Hund, A.K. Non-Steroidal Anti-Inflammatory Drugs in Alzheimer’s Disease and Parkinson’s Disease: Reconsidering the Role of Neuroinflammation. Pharmaceuticals 2010, 3, 1812–1841. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Mota, M.; Pizzi, M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim. Biophys. Acta 2016, 1862, 339–351. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin Protects against Systemic Inflammation-Stimulated Memory Impairment via MAPK and NF-κB Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, J.; Jung, S.C.; Park, D.B.; Maeng, Y.H.; Hong, J.Y.; Kim, S.J.; Lee, S.R.; Kim, S.J.; Kim, S.J.; et al. Anti-neuroinflammatory Activity of Nobiletin on Suppression of Microglial Activation. Biol. Pharm. Bull. 2010, 33, 1814–1821. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef]
- Nakajima, M.; Shimizu, R.; Furuta, K.; Sugino, M.; Watanabe, T.; Aoki, R.; Okuyama, S.; Furukawa, Y. Nobiletin Induces Oligodendrocyte Lineage Precursor Cells in a Cuprizone-Administered Demyelination Animal Model. J. Mult. Scler. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Ringman, J.M.; Liang, L.-J.; Zhou, Y.; Vangala, S.; Teng, E.; Kremen, S.; Wharton, D.; Goate, A.; Marcus, D.S.; Farlow, M.R.; et al. Early behavioural changes in familial Alzheimer’s disease in the Dominantly Inherited Alzheimer Network. Brain 2015, 138, 1036–1045. [Google Scholar] [CrossRef][Green Version]
- Novellino, F.; Saccà, V.; Donato, A.; Zaffino, P.; Spadea, M.F.; Vismara, M.; Arcidiacono, B.; Malara, N.; Presta, I.; Donato, G. Innate Immunity: A Common Denominator between Neurodegenerative and Neuropsychiatric Diseases. Int. J. Mol. Sci. 2020, 21, 1115. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Xu, H.L.; Feng, J.; Zhan, X.; Zhou, L.P.; Cui, C.C. Involvement of monoaminergic systems in the antidepressant-like effect of nobiletin. Physiol. Behav. 2011, 102, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Olanow, C.W.; Greenamyre, J.T.; Bezard, E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet 2014, 384, 545–555. [Google Scholar] [CrossRef]
- Nury, T.; Lizard, G.; Vejux, A. Lipids Nutrients in Parkinson and Alzheimer’s Diseases: Cell Death and Cytoprotection. Int. J. Mol. Sci. 2020, 21, 2501. [Google Scholar] [CrossRef]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Nemoto, K.; Ikeda, A.; Yoshida, C.; Kimura, J.; Mori, J.; Fujiwara, H.; Yokosuka, A.; Mimaki, Y.; Ohizumi, Y.; Degawa, M. Characteristics of nobiletin-mediated alteration of gene expression in cultured cell lines. Biochem. Biophys. Res. Commun. 2013, 431, 530–534. [Google Scholar] [CrossRef]
- Ikeda, A.; Nemoto, K.; Yoshida, C.; Miyata, S.; Mori, J.; Soejima, S.; Yokosuka, A.; Mimaki, Y.; Ohizumi, Y.; Degawa, M. Suppressive effect of nobiletin, a citrus polymethoxyflavonoid that downregulates thioredoxin-interacting protein expression, on tunicamycin-induced apoptosis in SK-N-SH human neuroblastoma cells. Neurosci. Lett. 2013, 549, 135–139. [Google Scholar] [CrossRef]
- Kennedy, M.B. Synaptic Signaling in Learning and Memory. Cold Spring Harb. Perspect. Biol. 2013, 8, a016824. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.; Obrietan, K.; Wong, S.T.; Poser, S.; Yano, S.; Wayman, G.; Deloulme, J.C.; Chan, G.; Storm, D.R. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 1998, 21, 869–883. [Google Scholar] [CrossRef]
- Mizuno, M.; Yamada, K.; Maekawa, N.; Saito, K.; Seishima, M.; Nabeshima, T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav. Brain Res. 2002, 133, 135–141. [Google Scholar] [CrossRef]
- Nagase, H.; Yamakuni, T.; Matsuzaki, K.; Maruyama, Y.; Kasahara, J.; Hinohara, Y.; Kondo, S.; Mimaki, Y.; Sashida, Y.; Tank, A.W.; et al. Mechanism of Neurotrophic Action of Nobiletin in PC12D Cells. Biochemistry 2005, 44, 13683–13691. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Yamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Biophys. Res. Commun. 2005, 337, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Yoshida, M.; Sun, W.; Nakajima, A.; Lai, Y.; Osaka, N.; Matsuzaki, K.; Yokosuka, A.; Mimaki, Y.; Naganuma, A.; et al. Potent activity of nobiletin-rich Citrus reticulata peel extract to facilitate cAMP/PKA/ERK/CREB signaling associated with learning and memory in cultured hippocampal neurons: Identification of the substances responsible for the pharmacological action. J. Neural Transm. 2013, 120, 1397–1409. [Google Scholar] [CrossRef]
- Al Rahim, M.; Nakajima, A.; Saigusa, D.; Tetsu, N.; Maruyama, Y.; Shibuya, M.; Yamakoshi, H.; Tomioka, Y.; Iwabuchi, Y.; Ohizumi, Y.; et al. 4′-Demethylnobiletin, a Bioactive Metabolite of Nobiletin Enhancing PKA/ERK/CREB Signaling, Rescues Learning Impairment Associated with NMDA Receptor Antagonism via Stimulation of the ERK Cascade. Biochemistry 2009, 48, 7713–7721. [Google Scholar] [CrossRef]
- Kessels, H.W.; Malinow, R. Synaptic AMPA Receptor Plasticity and Behavior. Neuron 2009, 61, 340–350. [Google Scholar] [CrossRef]
- Esteban, J.A.; Shi, S.-H.; Wilson, C.; Nuriya, M.; Huganir, R.L.; Malinow, R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 2003, 6, 136–143. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Miyazaki, K.; Sakai, S.; Yawo, H.; Nakata, N.; Moriguchi, S.; Fukunaga, K.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus. Eur. J. Pharmacol. 2008, 578, 194–200. [Google Scholar] [CrossRef]
- Sheng, M.; Cummings, J.; Roldan, L.A.; Jan, Y.N.; Jan, L.Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 1994, 368, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S.; Wang, Y.X.; Wenthold, R.J. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J. Neurosci. 1994, 14, 6102–6120. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Nemoto, K.; Degawa, M.; Yokosuka, A.; Mimaki, Y.; Shimizu, K.; Oku, N.; Ohizumi, Y. Upregulation of N-methyl-D-aspartate receptor subunits and c-Fos expressing genes in PC12D cells by nobiletin. Biol. Pharm. Bull. 2014, 37, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Yoshida, H. Progress in the Study of Muscarinic Acetylcholine Receptors; Establishment of the Subtypes or Subgroups. Nihon Yakurigaku Zasshi 1990, 95, 7–14. [Google Scholar] [CrossRef]
- Davies, P.; Verth, A.H. Regional distribution of muscarinic acetylcholine receptor in normal and Alzheimer’s-type dementia brains. Brain Res. 1977, 138, 385–392. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Kimura, J.; Shimizu, K.; Takito, J.; Nemoto, K.; Degawa, M.; Yokosuka, A.; Mimaki, Y.; Oku, N.; Ohizumi, Y. Upregulatory Effects of Nobiletin, a Citrus Flavonoid with Anti-dementia Activity, on the Gene Expression of mAChR, ChAT, and CBP. Planta Med. Lett. 2015, 2, e12–e14. [Google Scholar] [CrossRef][Green Version]
- Diaz, A.; Limon, D.; Chávez, R.; Zenteno, E.; Guevara, J. Aβ25-35 Injection into the Temporal Cortex Induces Chronic Inflammation that Contributes to Neurodegeneration and Spatial Memory Impairment in Rats. J. Alzheimers Dis. 2012, 30, 505–522. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Petre, B.A.; Hritcu, L. Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease. Antioxidants 2020, 9, 768. [Google Scholar] [CrossRef]
- Youn, K.; Lee, S.; Jun, M. Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity. Nutrients 2019, 11, 2648. [Google Scholar] [CrossRef]
- Iwata, N.; Tsubuki., S.; Takaki, Y.; Watanabe, K.; Sekiguchi, M.; Hosoki, E.; Kawashima-Morishima, M.; Lee, H.J.; Hama, E.; Sekine-Aizawa, Y.; et al. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat. Med. 2000, 6, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Shirotani, K.; Lu, B.; Gerard, N.P.; Gerard, C.; Hama, E.; Lee, H.-J.; Saido, T.C. Metabolic Regulation of Brain Abeta by Neprilysin. Science 2001, 292, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Takaki, Y.; Iwata, N.; Trojanowski, J.; Saido, T.C. Alzheimer’s disease, neuropeptides, neuropeptidase, and amyloid-beta peptide metabolism. Sci. Aging Knowl. Environ. 2003, 2003, PE1. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Kimura, J.; Sakamoto, M.; Yokosuka, A.; Mimaki, Y.; Murata, K.; Yamaguchi, K.; Ohizumi, Y. Nobiletin, a flavone from Citrus depressa, induces gene expression and increases the protein level and activity of neprilysin in SK-N-SH cells. Can. J. Physiol. Pharmacol. 2014, 92, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Shimizu, K.; Kajima, K.; Yokosuka, A.; Mimaki, Y.; Oku, N.; Ohizumi, Y. Nobiletin Reduces Intracellular and Extracellular β-Amyloid in iPS Cell-Derived Alzheimer’s Disease Model Neurons. Biol. Pharm. Bull. 2018, 41, 451–457. [Google Scholar] [CrossRef]
- Youn, K.; Yu, Y.; Lee, J.; Jeong, W.-S.; Ho, C.-T.; Jun, M. Polymethoxyflavones: Novel β-Secretase (BACE1) Inhibitors from Citrus Peels. Nutrients 2017, 9, 973. [Google Scholar] [CrossRef]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar] [CrossRef]
- Yang, J.S.; Wu, X.H.; Yu, H.G.; Teng, L.S. Tangeretin inhibits neurodegeneration and attenuates inflammatory responses and behavioural deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease dementia in rats. Inflammopharmacology 2017, 25, 471–484. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Scheper, W.; Hoozemans, J.J. The unfolded protein response in neurodegenerative diseases: A neuropathological perspective. Acta Neuropathol. 2015, 130, 315–331. [Google Scholar] [CrossRef]
- Hashida, K.; Kitao, Y.; Sudo, H.; Awa, Y.; Maeda, S.; Mori, K.; Takahashi, R.; Iinuma, M.; Hori, O. ATF6alpha Promotes Astroglial Activation and Neuronal Survival in a Chronic Mouse Model of Parkinson’s Disease. PLoS ONE 2012, 7, e47950. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Khanam, S.; Rahul, R.; Jyoti, S.; Naz, F.; Ali, F.; Siddique, Y.H. Protective effect of tangeritin in transgenic Drosophila model of Parkinson’s disease. Front. Biosci. 2017, 9, 44–53. [Google Scholar] [CrossRef]
- Fatima, A.; Siddique, Y.H. Role of tangeritin against cognitive impairments in transgenic Drosophila model of Parkinson’s disease. Neurosci. Lett. 2019, 705, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Feng, C.; Wang, D.; Qu, Y.; Yang, Y.; Wang, Y.; Sun, Z. Neuroprotective and Anti-inflammatory Effect of Tangeretin Against Cerebral Ischemia-Reperfusion Injury in Rats. Inflammation 2020, 43, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Estévez, V.; Hsieh, J. Human Brain Organoid Models of Developmental Epilepsies. Epilepsy Curr. 2020, 20, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Kuruba, R. Experimental Models of Status Epilepticus and Neuronal Injury for Evaluation of Therapeutic Interventions. Int. J. Mol. Sci. 2013, 14, 18284–18318. [Google Scholar] [CrossRef]
- Guo, X.Q.; Cao, Y.L.; Hao, F.; Yan, Z.R.; Wang, M.L.; Liu, X.W. Tangeretin alters neuronal apoptosis and ameliorates the severity of seizures in experimental epilepsy-induced rats by modulating apoptotic protein expressions, regulating matrix metalloproteinases, and activating the PI3K/Akt cell survival pathway. Adv. Med. Sci. 2017, 62, 246–253. [Google Scholar] [CrossRef]
- Kelly, D.M.; Rothwell, P.M. Prevention and treatment of stroke in patients with chronic kidney disease: An overview of evidence and current guidelines. Kidney Int. 2020, 97, 266–278. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Viggiano, D.; Wagner, C.A.; Martino, G.; Nedergaard, M.; Zoccali, C.; Unwin, R.J.; Capasso, G. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 2020, 16, 452–469. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.M.; Deng, Z.K. Tangeretin ameliorates renal failure via regulating oxidative stress, NF-κB-TNF-α/iNOS signalling and improves memory and cognitive deficits in 5/6 nephrectomized rats. Inflammopharmacology 2018, 26, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.J. A review of post-traumatic stress disorder. Part I: Historical development and classification. Injury 1998, 29, 87–91. [Google Scholar] [CrossRef]
- Richter-Levin, G.; Stork, O.; Schmidt, M.V. Animal models of PTSD: A challenge to be met. Mol. Psychiatry 2019, 24, 1135–1156. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. The polymethoxylated flavone, Tangeretin improves cognitive memory in rats experiencing a single episode of prolonged post-traumatic stress. Anim. Cells Syst. 2018, 22, 54–62. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Afshar, E.G. Tangeretin: A mechanistic review of its pharmacological and therapeutic effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, J.; Chen, Y.; Li, T.; Zhu, R.; Zhu, B.; Zhang, Y. Tangeretin protects human brain microvascular endothelial cells against oxygen-glucose deprivation-induced injury. J. Cell. Biochem. 2019, 120, 4883–4891. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Gao, H.; Wei, M.J. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol. Res. 2019, 145, 104253. [Google Scholar] [CrossRef]
- Shu, Z.; Yang, B.; Zhao, H.; Xu, B.; Jiao, W.; Wang, Q.; Wang, Z.; Kuang, H. Tangeretin exerts anti-neuroinflammatory effects via NF-κB modulation in lipopolysaccharide-stimulated microglial cells. Int. Immunopharmacol. 2014, 19, 275–282. [Google Scholar] [CrossRef]
- Ho, S.C.; Kuo, C.T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem. Toxicol. 2014, 71, 176–182. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, T.; Tu, J.X.; Li, G.; Zhou, Y. Tangeretin inhibits IL-1β induced proliferation of rheumatoid synovial fibroblasts and the production of COX-2, PGE2 and MMPs via modulation of p38 MAPK/ERK/JNK pathways. Bangladesh J. Pharmacol. 2015, 10, 714–725. [Google Scholar] [CrossRef][Green Version]
- Peng, S.; Zhang, Y.; Zhang, J.; Wang, H.; Ren, B. ERK in Learning and Memory: A Review of Recent Research. Int. J. Mol. Sci. 2010, 11, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Tsurumaki, H.; Aoki-Saito, H.; Sato, M.; Yatomi, M.; Takehara, K.; Hisada, T. Roles of Cyclic AMP Response Element Binding Activation in the ERK1/2 and p38 MAPK Signalling Pathway in Central Nervous System, Cardiovascular System, Osteoclast Differentiation and Mucin and Cytokine Production. Int. J. Mol. Sci. 2019, 20, 1346. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Shimada, N.; Kaji, M.; Morita, M.; Miyoshi, K.; Minami, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Watanabe, S.; et al. Heptamethoxyflavone, a citrus flavonoid, enhances brain-derived neurotrophic factor production and neurogenesis in the hippocampus following cerebral global ischemia in mice. Neurosci. Lett. 2012, 528, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Morita, M.; Miyoshi, K.; Nishigawa, Y.; Kaji, M.; Sawamoto, A.; Terugo, T.; Toyoda, N.; Makihata, N.; Amakura, Y.; et al. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid, on protection against memory impairment and neuronal cell death in a global cerebral ischemia mouse model. Neurochem. Int. 2014, 70, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Watanabe, S.; Fukata, T.; Nakajima, M.; Yoshimura, M.; Yoshida, T. Isolation and Characterization of Activators of ERK/MAPK from Citrus Plants. Int. J. Mol. Sci. 2012, 13, 1832–1845. [Google Scholar] [CrossRef]
- Okuyama, S.; Miyazaki, K.; Yamada, R.; Amakura, Y.; Yoshimura, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Permeation of Polymethoxyflavones into the Mouse Brain and Their Effect on MK-801-Induced Locomotive Hyperactivity. Int. J. Mol. Sci. 2017, 18, 489. [Google Scholar] [CrossRef]
- Okuyama, S.; Miyoshi, K.; Tsumura, Y.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Polymethoxylated Flavone, Attenuates Inflammation in the Mouse Hippocampus. Brain Sci. 2015, 5, 118–129. [Google Scholar] [CrossRef]
- Ihara, H.; Yamamoto, H.; Ida, T.; Tsutsuki, H.; Sakamoto, T.; Fujita, T.; Okada, T.; Kozaki, S. Inhibition of nitric oxide production and inducible nitric oxide synthase expression by a polymethoxyflavone from young fruits of Citrus unshiu in rat primary astrocytes. Biosci. Biotechnol. Biochem. 2012, 76, 1843–1848. [Google Scholar] [CrossRef]
- Sawamoto, A.; Okuyama, S.; Yamamoto, K.; Amakura, Y.; Yoshimura, M.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Flavonoid, Ameliorates Corticosterone-Induced Depression-like Behavior and Restores Brain-Derived Neurotrophic Factor Expression, Neurogenesis, and Neuroplasticity in the Hippocampus. Molecules 2016, 21, 541. [Google Scholar] [CrossRef]
- Sawamoto, A.; Okuyama, S.; Amakura, Y.; Yoshimura, M.; Yamada, T.; Yokogoshi, H.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone Ameliorates Depressive-Like Behavior and Hippocampal Neurochemical Changes in Chronic Unpredictable Mild Stressed Mice by Regulating the Brain-Derived Neurotrophic Factor: Requirement for ERK Activation. Int. J. Mol. Sci. 2017, 18, 2133. [Google Scholar] [CrossRef]
- Sawamoto, A.; Okuyama, S.; Nakajima, M.; Furukawa, Y. Citrus flavonoid 3,5,6,7,8,3′,4′-heptamethoxyflavone induces BDNF via cAMP/ERK/CREB signaling and reduces phosphodiesterase activity in C6 cells. Pharmacol. Rep. 2019, 71, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Wu, M.J.; Chen, P.Y.; Sheu, T.T.; Chiu, S.P.; Lin, M.H.; Ho, C.T.; Yen, J.H. Neurotrophic Effect of Citrus 5-Hydroxy-3,6,7,8,3′,4′-Hexamethoxyflavone: Promotion of Neurite Outgrowth via cAMP/PKA/CREB Pathway in PC12 Cells. PLoS ONE 2011, 6, e28280. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Kress, G.J.; Liao, F.; Dimitry, J.M.; Cedeno, M.R.; Fitzgerald, G.A.; Holtzman, D.M.; Musiek, E.S. Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med. 2018, 215, 1059–1068. [Google Scholar] [CrossRef]
- Nohara, K.; Nemkov, T.; D’Alessandro, A.; Yoo, S.-H.; Chen, Z. Coordinate Regulation of Cholesterol and Bile Acid Metabolism by the Clock Modifier Nobiletin in Metabolically Challenged Old Mice. Int. J. Mol. Sci. 2019, 20, 4281. [Google Scholar] [CrossRef]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Petrenko, V.; Gandasi, N.R.; Sage, D.; Tengholm, A.; Barg, S.; Dibner, C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2484–2495. [Google Scholar] [CrossRef]
- Shinozaki, A.; Misawa, K.; Ikeda, Y.; Haraguchi, A.; Kamagata, M.; Tahara, Y.; Shibata, S. Potent Effects of Flavonoid Nobiletin on Amplitude, Period, and Phase of the Circadian Clock Rhythm in PER2::LUCIFERASE Mouse Embryonic Fibroblasts. PLoS ONE 2017, 12, e0170904. [Google Scholar] [CrossRef]
- Gile, J.; Scott, B.; Eckle, T. The Period 2 Enhancer Nobiletin as Novel Therapy in Murine Models of Circadian Disruption Resembling Delirium. Crit. Care Med. 2018, 46, e600–e608. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, D.; Shibuya, M.; Jinno, D.; Yamakoshi, H.; Iwabuchi, Y.; Yokosuka, A.; Mimaki, Y.; Naganuma, A.; Ohizumi, Y.; Tomioka, Y.; et al. High-performance liquid chromatography with photodiode array detection for determination of nobiletin content in the brain and serum of mice administrated the natural compound. Anal. Bioanal. Chem. 2011, 400, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, M.; Matsumoto, T.; Watanabe, J. LC-MS/MS detection of citrus unshiu peel-derived flavonoids in the plasma and brain after oral administration of yokukansankachimpihange in rats. Xenobiotica 2019, 49, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Nemoto, K.; Ohizumi, Y. An evaluation of the genotoxicity and subchronic toxicity of the peel extract of Ponkan cultivar ‘Ohta ponkan’ (Citrus reticulata Blanco) that is rich in nobiletin and tangeretin with anti-dementia activity. Regul. Toxicol. Pharmacol. 2020, 114, 104670. [Google Scholar] [CrossRef]
- Vanhoecke, B.W.; Delporte, F.; Van Braeckel, E.; Heyerick, A.; Depypere, H.T.; Nuytinck, M.; De Keukeleire, D.; Bracke, M.E. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In Vivo 2005, 19, 103–107. [Google Scholar]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Liu, F.; Cui, Y.; Li, X. Dietary citrus and/or its extracts intake contributed to weight control: Evidence from a systematic review and meta-analysis of 13 randomized clinical trials. Phytother. Res. 2020, 34, 2006–2022. [Google Scholar] [CrossRef]
- Oben, J.; Enonchong, E.; Kothari, S.; Chambliss, W.; Garrison, R.; Dolnick, D. Phellodendron and Citrus extracts benefit cardiovascular health in osteoarthritis patients: A double-blind, placebo-controlled pilot study. Nutr. J. 2008, 7, 16. [Google Scholar] [CrossRef]
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I.; et al. Randomized Clinical Trial: Bergamot Citrus and Wild Cardoon Reduce Liver Steatosis and Body Weight in Non-diabetic Individuals Aged Over 50 Years. Front. Endocrinol. 2020, 11, 494. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, X.; Jiang, T.; Wu, K.; Ding, C.; Liu, Z.; Zhang, X.; Yu, T.; Song, C. Citrus aurantium Naringenin Prevents Osteosarcoma Progression and Recurrence in the Patients Who Underwent Osteosarcoma Surgery by Improving Antioxidant Capability. Oxidative Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Kamiya, T.; Furukawa, K.; Azumi, M.; Ishizuka, S.; Takayama, S.; Nagase, S.; Arai, H.; Yamakuni, T.; Yaegashi, N. Nobiletin-rich Citrus reticulata peels, a kampo medicine for Alzheimer’s disease: A case series. Geriatr. Gerontol. Int. 2013, 13, 236–238. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, K.; Ohizumi, Y. Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders. Nutrients 2021, 13, 145. https://doi.org/10.3390/nu13010145
Matsuzaki K, Ohizumi Y. Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders. Nutrients. 2021; 13(1):145. https://doi.org/10.3390/nu13010145
Chicago/Turabian StyleMatsuzaki, Kentaro, and Yasushi Ohizumi. 2021. "Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders" Nutrients 13, no. 1: 145. https://doi.org/10.3390/nu13010145
APA StyleMatsuzaki, K., & Ohizumi, Y. (2021). Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders. Nutrients, 13(1), 145. https://doi.org/10.3390/nu13010145





