Diet Quality Is Associated with Serum Antioxidant Capacity in Women with Breast Cancer: A Cross Sectional Study
Abstract
1. Introduction
2. Materials and Methods
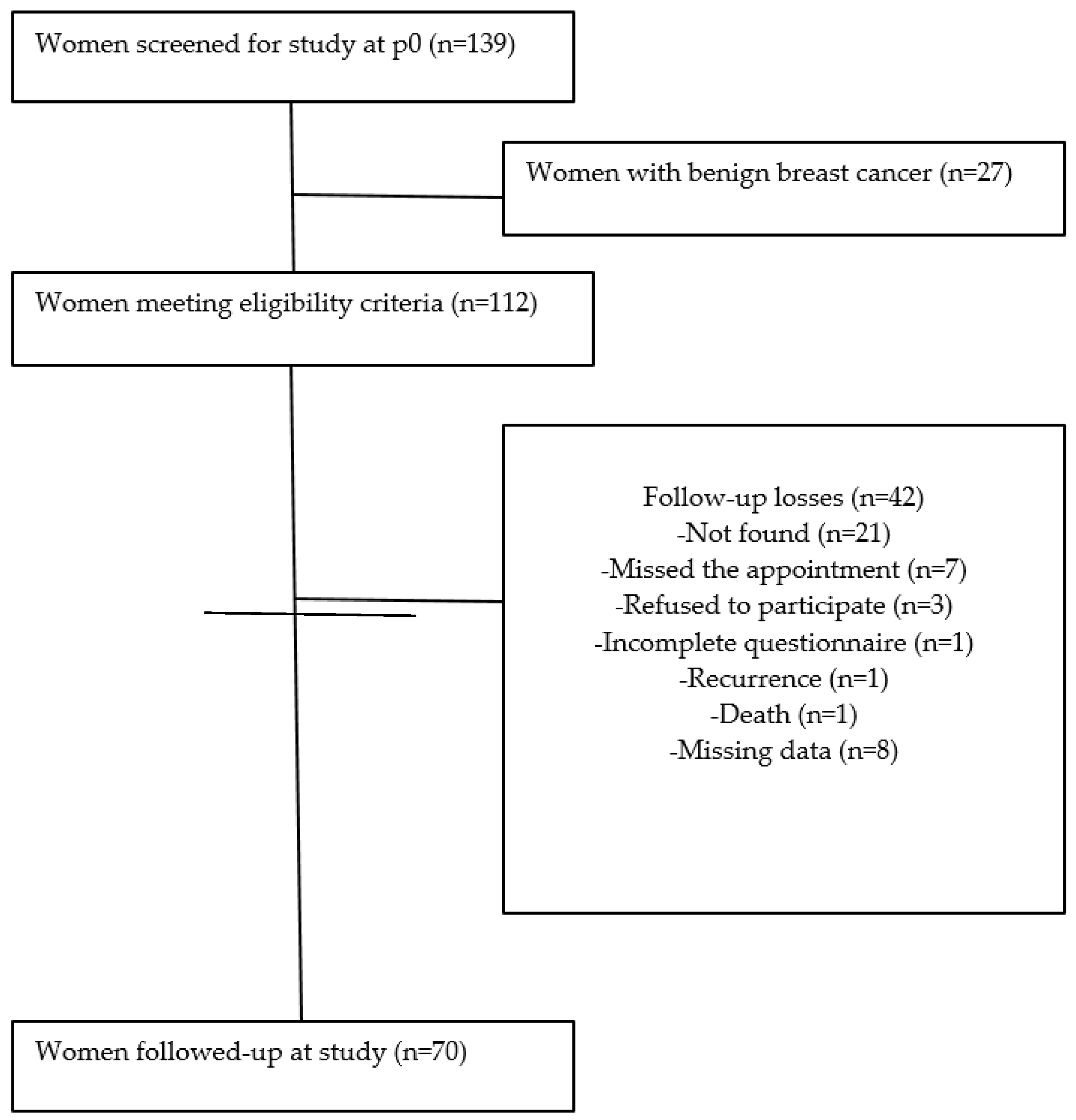
2.1. Study Design and Sampling
2.2. Oxidative Stress Analysis
2.3. Diet Quality Assessment
2.4. Other Assessments
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017, 16, 151. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Instituto Nacional de Câncer. In Estimativa 2018: Incidência de Câncer no Brasil; Instituto Nacional de Câncer: Rio de Janeiro, Brazil, 2018. Available online: http://www1.inca.gov.br/rbc/n_64/v01/pdf/15-resenha-estimativa-2018-incidencia-de-cancer-no-brasil.pdf (accessed on 20 April 2020).
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, E.E.; Abba, M.C.; Lacunza, E.; Fernánde, E.; Guerci, A.M. Polymorphic variants in oxidative stress genes and acute toxicity in breast cancer patients receiving radiotherapy. Cancer Res. Treat. 2016, 3, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Galvan, D.; Di Pietro, P.F.; Vieira, F.G.K.; Ambrosi, C.; Cesa, C.; Cardoso, A.L.; Cavalcante, L.D.S.; Crippa, C.G.; Da Silva, E.L. Increased Body Weight and Blood Oxidative Stress in Breast Cancer Patients after Adjuvant Chemotherapy. Breast J. 2013, 19, 555–557. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Lapenna, S.; Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566. [Google Scholar] [CrossRef]
- Potentas, E.; Witkowska, A.M.; Zujko, E. Mediterranean diet for breast cancer prevention and treatment in postmenopausal women. Prz. Menopauzalny 2015, 14, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean diet and cancer: Epidemiological evidence and mechanism of selected aspects. BMC Surg. 2013, 13, S14. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.F.C.J.; Júnior, A.L.G.; De Alencar, M.V.O.B.; Tabrez, S.; Islam, T.; Jabir, N.R.; Oves, M.; Alam, M.Z.; Asghar, M.N.; Ali, E.S.; et al. Effect of Diets, Familial History, and Alternative Therapies on Genomic Instability of Breast Cancer Patients. Appl. Biochem. Biotechnol. 2018, 188, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Subar, A.F. 90th Anniversary Commentary: Diet Quality Indexes in Nutritional Epidemiology Inform Dietary Guidance and Public Health. J. Nutr. 2018, 148, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, C.; Zhou, C.; Zhuang, J.; Tang, S.; Yu, J.; Tian, J.; Feng, F.; Liu, L.; Zhang, T.; et al. Meta-analysis of the association between the dietary inflammatory index (DII) and breast cancer risk. Eur. J. Clin. Nutr. 2018, 73, 509–517. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Irwin, M.L.; Smith, A.W.; Neuhouser, M.L.; Reedy, J.; McTiernan, A.; Alfano, C.M.; Bernstein, L.; Ulrich, C.M.; Baumgartner, K.B.; et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 2011, 22, 589–598. [Google Scholar] [CrossRef]
- Kim, E.; Willett, W.; Fung, T.; Rosner, B.; Holmes, M. Diet Quality Indices and Postmenopausal Breast Cancer Survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef]
- Izano, M.A.; Fung, T.T.; Chiuve, S.S.; Hu, F.B.; Holmes, M.D. Are Diet Quality Scores After Breast Cancer Diagnosis Associated with Improved Breast Cancer Survival? Nutr. Cancer 2013, 65, 820–826. [Google Scholar] [CrossRef]
- Ceccatto, V.; Di Pietro, P.F.; Previdelli, A.N.; Vieira, F.G.K.; Schiavon, C.C.; Engel, R.; Cardoso, A.L.; De Assis, M.A.A.; Crippa, C.G.; González-Chica, D.A. Brazilian healthy eating index revised (BHEI-R) of women before and during adjuvant treatment for breast cancer. Nutr. Hosp. 2014, 30, 1101–1109. [Google Scholar]
- Custódio, I.D.D.; Marinho, E.D.C.; Gontijo, C.A.; Pereira, T.S.S.; Paiva, C.E.; Maia, Y.C.P. Impact of Chemotherapy on Diet and Nutritional Status of Women with Breast Cancer: A Prospective Study. PLoS ONE 2016, 11, e0157113. [Google Scholar] [CrossRef]
- Vieira, F.G.K. Características Sociodemográficas, Reprodutivas, Clínicas, Nutricionais e de Estresse Oxidativo de Mulheres Com Câncer de Mama. Master’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2008. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–890. [Google Scholar] [PubMed]
- Esterbauer, H.; Cheeseman, K. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Method Enzym. 1990, 186, 407–421. [Google Scholar]
- Nourooz-zadeh, J.; Tajaddini-sarmadi, J.; Wolff, S.P. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal. Biochem 1994, 220, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzym. 1990, 186, 464–478. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sichieri, R.; Everhart, J.E. Validity of a Brazilian food frequency questionnaire against dietary recalls and estimated energy intake. Nutr. Res. 1998, 18, 1649–1659. [Google Scholar] [CrossRef]
- Pinheiro, A.B. Tabela Para Avaliação de Consumo Alimentar em Medidas Caseiras, 5th ed.; Atheneu: São Paulo, Brazil, 2008; 131p. [Google Scholar]
- NEPA—Núcleo de Estudos e Pesquisas em Alimentação. Tabela Brasileira de Composição de Alimentos, 4th ed.; UNICAMP: Campinas, Brazil, 2011. [Google Scholar]
- USDA. Department of Agriculture.Agricultural Research Service. National Nutrient Database for Standard Reference, Release 18. Nutrient Data Laboratory. 2005. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 1 February 2019).
- Previdelli, A.N.; Andrade, S.C.; Pires, M.M.; Ferreira, S.R.G.; Fisberg, R.M. Índice de Qualidade da Dieta Revisado para população brasileira. Rev. Saúde Pública 2011, 45, 794–798. [Google Scholar] [CrossRef]
- Andrade, S.C.; Previdelli, A.N.; Marchioni, D.M.L.; Fisberg, R.M. Evaluation of the reliability and validity of the Brazilian Healthy Eating Index Revised. Rev. Saude Publica 2013, 47, 675–683. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. In Guia Alimentar Para a População Brasileira, 2nd ed.; Departamento de Atenção Básica: Brasília, Brazil, 2014; 156p. [Google Scholar]
- Di Pietro, P.F.; Medeiros, N.I.; Vieira, F.G.; Fausto, M.A.; Belló-Klein, A. Breast cancer in southern Brazil: Association with past dietary intake. Nutr. Hosp. 2007, 22, 565–572. [Google Scholar]
- Petroski, E.L. Antropometria: Técnicas e PadronizaçÕes; Palotti: Porto Alegre, Brazil, 1999. [Google Scholar]
- WHO—World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; WHO Technical Report Series 854; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- WHO—World Health Organization. Waist Circumference and Waist-Hip Ratio; Report of WHO Expert Consultation; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- IOM—Institute of Medicine of the National Academies. Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005.
- Prescha, A.; Zablocka-Slowinska, K.; Płaczkowska, S.; Gorczyca, D.; Łuczak, A.; Majewska, M.; Grajeta, H. Diet Quality and Its Relationship with Antioxidant Status in Patients with Rheumatoid Arthritis. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Browner, W.S.; Newman, T.B.; Hulley, S.B. Estimating the sample size and the statistical power: Applications and examples. In Outlining Clinical Research: An. Epidemiological Approach; Hulley, S.B., Ed.; Artmed: Porto Alegre, Brazil, 2008; pp. 83–112. [Google Scholar]
- Sateesh, R.; Bitla, A.R.R.; Bugudu, S.R.; Mutheeswariah, Y.; Narendra, H.; Phaneedra, B.V.; Lakshmi, A.Y. Oxidative stress in relation to obesity in breast cancer. Indian J. Cancer 2019, 56, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, C.C.; Vieira, F.G.; Ceccatto, V.; De Liz, S.; Cardoso, A.L.; Sabel, C.; González-Chica, D.A.; Da Silva, E.L.; Galvan, D.; Crippa, C.G.; et al. Nutrition Education Intervention for Women With Breast Cancer: Effect on Nutritional Factors and Oxidative Stress. J. Nutr. Educ. Behav. 2015, 47, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Rajendran, R.; Kuroda, K.; Isogai, E.; Krstic-Demonacos, M.; Demonacos, C. Oxidative stress and breast cancer biomarkers: The case of the cytochrome P450 2E1. J. Cancer Metastasis Treat. 2016, 2, 268–276. [Google Scholar] [CrossRef][Green Version]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, S5–S24. [Google Scholar] [CrossRef]
- Rockenbach, G.; Di Pietro, P.F.; Ambrosi, C.; Boaventura, B.C.B.; Vieira, F.G.K.; Crippa, C.G.; Da Silva, E.L.; Fausto, M.A. Dietary intake and oxidative stress in breast cancer: Before and after treatments. Nutr. Hosp. 2011, 26, 737–744. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Tsatsakis, A.M.; Kouretas, D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones 2012, 17, 11–21. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Ray, G.; Batra, S.; Shukla, N.K.; Deo, S.; Raina, V.; Ashok, S.; Husain, S.A. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res. Treat. 2000, 59, 163–170. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.A.; Pagliarani, S.; Benedetti, S.; Canestrari, F.; Zhu, W.; Qin, W.; Sauter, E.R. The 8-epimer of prostaglandin F(2alpha), a marker of lipid peroxidation and oxidative stress, is decreased in the nipple aspirate fluid of women with breast cancer. Int. J. Cancer 2007, 120, 1971–1976. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.A.; Medda, V. Protein oxidation in breast microenvironment: Nipple aspirate fluid collected from breast cancer women contains increased protein carbonyl concentration. Anal. Cell Pathol. 2009, 31, 383–392. [Google Scholar]
- Rossner, P.; Terry, M.B.; Gammon, M.D.; Agrawal, M.; Zhang, F.F.; Ferris, J.S.; Teitelbaum, S.L.; Eng, S.M.; Gaudet, M.M.; Neugut, A.I.; et al. Plasma protein carbonyl levels and breast cancer risk. J. Cell. Mol. Med. 2007, 11, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Tesarova, P.; Kalousova, M.; Trnkova, B.; Soukupova, J.A.; Argalasova, S.; Petruzelka, L.; Zima, T. Carbonyl and oxidative stress in patients with breast cancer: Is there a relation to the stage of the disease? Neoplasma 2007, 54, 219–224. [Google Scholar] [PubMed]
- Pande, D.; Negi, R.; Karki, K.; Khanna, S.; Khanna, R.S.; Khanna, H.D. Oxidative damage markers as possible discriminatory biomarkers in breast carcinoma. Transl. Res. 2012, 160, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.Y.; Suh, Y.J.; Kim, S.W.; Baik, H.W.; Sung, C.J.; Kim, H.S. Evaluation of dietary factors in relation to the biomarkers of oxidative stress and inflammation in breast cancer risk. Nutrition 2011, 27, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Giuliano, A.R.; Shaw, J.W.; Rock, C.L.; Ritenbaugh, K.; Hakim, I.A.; Hollenbach, K.A.; Alberts, D.S.; Pierce, J.P. Diet and Biomarkers of Oxidative Damage in Women Previously Treated for Breast Cancer. Nutr. Cancer 2005, 51, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Murphy, E.A.; Hurley, T.G.; Hébert, J.R. Effect of Cruciferous Vegetable Intake on Oxidative Stress Biomarkers: Differences by Breast Cancer Status. Cancer Investig. 2017, 35, 277–287. [Google Scholar] [CrossRef] [PubMed]
- De Vries, Y.C.; Berg, M.M.G.A.V.D.; De Vries, J.H.M.; Boesveldt, S.; De Kruif, J.T.C.M.; Buist, N.; Haringhuizen, A.; Los, M.; Sommeijer, D.W.; Timmer-Bonte, J.H.N.; et al. Differences in dietary intake during chemotherapy in breast cancer patients compared to women without cancer. Support. Care Cancer 2017, 25, 2581–2591. [Google Scholar] [CrossRef]
- Boltong, A.; Keast, R. The influence of chemotherapy on taste perception and food hedonics: A systematic review. Cancer Treat. Rev. 2012, 38, 152–163. [Google Scholar] [CrossRef]
- Steinbach, S.; Hummel, T.; Böhner, C.; Berktold, S.; Hundt, W.; Kriner, M.; Heinrich, P.; Sommer, H.; Hanusch, C.; Prechtl, A.; et al. Qualitative and Quantitative Assessment of Taste and Smell Changes in Patients Undergoing Chemotherapy for Breast Cancer or Gynecologic Malignancies. J. Clin. Oncol. 2009, 27, 1899–1905. [Google Scholar] [CrossRef]
- Tartari, R.F.; Busnello, F.M.; Nunes, C.H.A. Perfil Nutricional de Pacientes em Tratamento Quimioterápico em um Ambulatório Especializado em Quimioterapia. Rev. Bras. Cancerol. 2010, 56, 43–50. [Google Scholar]
- Patterson, R.E.; Cadmus, L.A.; Emond, J.A.; Pierce, J.P. Physical activity, diet, adiposity and female breast cancer prognosis: A review of the epidemiologic literature. Maturitas 2010, 66, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Rock, C.L.; Thompson, P.A.; Caan, B.J.; Cussler, E.; Flatt, S.W.; Pierce, J.P. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: A secondary analysis from the Women’s Healthy Eating and Living Study. Breast Cancer Res. Treat. 2010, 125, 519–527. [Google Scholar] [CrossRef]
- WCRF/AICR—World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity, and Breast Cancer Survivors. Continuous Update Project Report. 2014. Available online: https://www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf (accessed on 2 February 2019).
- Yancik, R.; Wesley, M.N.; Ries, L.A.; Havlik, R.J.; Edwards, B.K.; Yates, J.W. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 2011, 285, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Ogle, K.S.; Swanson, G.M.; Woods, N.; Azzouz, F. Cancer and comorbidity: Redefining chronic diseases. Cancer 2000, 88, 653–663. [Google Scholar] [CrossRef]
- Braithwaite, D.; Tammemagi, C.M.; Moore, D.H.; Ozanne, E.M.; Hiatt, R.A.; Belkora, J.; West, D.W.; Satariano, W.A.; Liebman, M.; Esserman, L. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int. J. Cancer 2009, 124, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Jessri, M.; Houshiar-Rad, A.; Mirzaei, H.R.; Rashidkhani, B. Dietary patterns and breast cancer risk among women. Public Health Nutr. 2014, 17, 1098–1106. [Google Scholar] [CrossRef]
- Hartman, T.J.; Gapstur, S.M.; Gaudet, M.M.; Shah, R.; Flanders, W.D.; Wang, Y.; McCullough, M.L. Dietary Energy Density and Postmenopausal Breast Cancer Incidence in the Cancer Prevention Study II Nutrition Cohort. J. Nutr. 2016, 146, 2045–2050. [Google Scholar] [CrossRef]
- Pantavos, A.; Ruiter, R.; Feskens, E.F.; De Keyser, C.E.; Hofman, A.; Stricker, B.H.; Franco, O.H.; Jong, J.C.K.-D. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: The Rotterdam study. Int. J. Cancer 2015, 136, 2178–2186. [Google Scholar] [CrossRef]
| BHEI-R Tertiles (p0) | p | |||
|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | ||
| Age a (years) | 48.54 (10.54) | 51.96 (9.44) | 56.35 (11.16) | 0.012 * |
| Weight b (kg) | 68.00 (57.75, 81.50) | 69.50 (59.60, 78.80) | 65.90 (56.50, 73.00) | 0.470 ** |
| Waist circumference (cm) | 82.25 (76.25, 98.50) | 89.50 (83.00, 98.00) | 84.0 (81.50, 95.00) | 0.301 ** |
| Smoking, n (%) | 0.745 # | |||
| Yes | 6 (25.0) | 4 (17.4) | 6 (26.1) | |
| No | 18 (75.0) | 19 (82.6) | 17 (73.9) | |
| Alcohol, n (%) | 0.793 # | |||
| Yes | 2 (8.3) | 1 (4.4) | 1 (4.3) | |
| No | 22 (91.7) | 22 (95.6) | 22 (95.7) | |
| Race, n (%) | 0.354 # | |||
| White | 22 (91.7) | 21 (91.3) | 23 (100.0) | |
| Brown | 2 (8.3) | 2 (8.7) | 0 (0.0) | |
| Education | 0.077 # | |||
| <8 years | 17 (70.8) | 15 (65.2) | 17 (73.9) | |
| 9–11 years | 6 (25.0) | 3 (13.1) | 6 (26.1) | |
| >12 years | 1 (4.2) | 5 (21.7) | 0 (0.0) | |
| BMI (kg/m2), n (%) | 0.208 # | |||
| <24.9 kg/m2 | 11 (45.8) | 5 (21.7) | 9 (39.1) | |
| ≥25 kg/m2 | 13 (54.2) | 18 (78.3) | 14 (60.9) | |
| Tumor classification, n (%) | 0.236 # | |||
| Invasive carcinoma | 21 (32.3) | 21 (32.3) | 23 (35.4) | |
| Carcinoma in situ | 3 (60.0) | 2 (40.0) | 0 (0.0) | |
| Tumor stage, n (%) | 0.604 # | |||
| 0 | 0 (0.0) | 2 (8.7) | 0 (0.0) | |
| I | 9 (37.5) | 7 (30.4) | 7 (30.4) | |
| II | 10 (41.7) | 9 (39.1) | 10 (43.5) | |
| III | 5 (20.8) | 5 (21.7) | 6 (26.1) | |
| Treatment type, n (%) | 0.622 # | |||
| Radiotherapy | 6 (37.5) | 5 (31.2) | 5 (31.3) | |
| Chemotherapy | 6 (31.6) | 5 (26.3) | 8 (42.1) | |
| Radiotherapy in association with chemotherapy | 12 (40.0) | 10 (33.3) | 8 (26.7) | |
| No | 0 (0.0) | 3 (60.0) | 2 (40.0) | |
| Hormone therapy, n (%) | 0.138 # | |||
| Tamoxifen | 21 (87.6) | 15 (65.2) | 15 (65.2) | |
| Aromatase inhibitor | 3 (12.5) | 8 (34.8) | 8 (34.8) | |
| Physical Activity Level (PAL) b | 1.36 (1.30–1.40) | 1.32 (1.30–1.34) | 1.33 (1.29–1.36) | 0.520 ** |
| Surgery type, n (%) | 0.576 # | |||
| Partial mastectomy | 9 (37.5) | 4 (17.4) | 5 (21.7) | |
| Radical mastectomy | 10 (41.7) | 13 (56.5) | 13 (56.5) | |
| Sectorectomy | 5 (20.8) | 6 (26.1) | 5 (21.7) |
| p0 | p1 | p | |
|---|---|---|---|
| Total BHEI-R b | 76.23 (71.21–83.17) | 76.47 (73.01–81.21) | 0.684 * |
| Total Fruits (0–5) b | 4.98 (3.68–5) | 5 (3.16–5) | 0.325 * |
| Whole Fruits (0–5) b | 5 (4.49–5) | 5 (5–5) | 0.042 * |
| Total Vegetables (0–5) b | 4.92 (2.93–5) | 3.53 (2.34–5) | 0.042 * |
| Dark Green and Orange Vegetables and Legumes (0–5) b | 5 (4.51–5) | 5 (3.63–5) | 0.084 * |
| Total Grains (0–5) b | 5 (5–5) | 5 (5–5) | 0.718 * |
| Whole Grains (0–5) b | 0 (0–0.51) | 0 (0–0.52) | 0.718 * |
| Milk and dairy products (0–10) a | 5.05 (0.34) | 4.11 (0.27) | 0.005 ** |
| Meat, eggs and legumes (0–10) b | 8.8. (6.83–10) | 6.79 (4.72–10) | <0.001 * |
| Oils (0–10) b | 10 (10–10) | 10 (10–10) | 0.421 * |
| Saturated Fat (0–10) b | 6.9 (4.33–9.06) | 8.75 (7–10) | <0.001 * |
| Sodium (0–10) b | 8.73 (7.05–9.67) | 9.81 (8.58–10) | <0.001 * |
| Calories from SoFAAS (0–20) b | 17.75 (13.52–20) | 17.75 (14.21–20) | 0.678 * |
| TBARS b | 4.81 (4.13–5.68) | 8.69 (4.3–13.16) | <0.001 * |
| LH b | 3.81 (2.84–6.06) | 5.59 (1.80–9.75) | 0.046 * |
| Carbonyl b | 0.74 (0.57–1.14) | 0.94 (0.86–1;07) | 0.023 * |
| GSH b | 76.02 (64.62–91.72) | 79.65 (56.58–92.29) | 0.932 * |
| FRAP a | 629.47 (19.09) | 573.75 (22.66) | 0.051 ** |
| Oxidative Stress Biomarkers | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BHEI-R | TBARS-log * | LH-log † | Carbonyl-log ‡ | GSH § | FRAP § | ||||||||||
| Tertiles | β | (CI95%) | p | β | (CI95%) | p | β | (CI95%) | p | β | (CI95%) | p | β | (CI95%) | p |
| 1st tertile | Ref | Ref | Ref | Ref | Ref | ||||||||||
| 2nd tertile | 0.076 | (−0.189; 0.341) | 0.569 | −0.098 | (−0.544; 0.348) | 0.661 | −0.004 | (−0.263; 0.255) | 0.977 | −8.296 | (−20.947; 4.355) | 0.195 | 70.083 | (−17.512; 157.678) | 0.115 |
| 3rd tertile | 0.040 | (−0.235; 0.314) | 0.775 | 0.123 | (−0.328; 0.575) | 0.587 | −0.270 | (−0.542; 0.001) | 0.051 | −5.383 | (−18.166; 7.399) | 0.403 | 106.781 | (18.273; 195.289) | 0.019 |
| BHEI-R | Increased Biomarkers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TBARS-log * | LH-log † | Carbonyl-log ‡ | GSH § | FRAP ǁ | ||||||
| OR (CI95%) | p | OR (CI95%) | p | OR (CI95%) | p | OR (CI95%) | p | OR (CI95%) | p | |
| Reduction | 1 | 1 | 1 | 1 | 1 | |||||
| Increase | 0.36 | 0.086 | 0.88 | 0.815 | 0.86 | 0.797 | 1.12 | 0.820 | 0.56 | 0.260 |
| (0.11–1.15) | (0.32–2.47) | (028–2.67) | (0.43–2.92) | (0.20–1.55) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitz, L.K.; Baptista, S.d.L.; Santos, E.d.S.; Hinnig, P.F.; Rockenbach, G.; Vieira, F.G.K.; de Assis, M.A.A.; da Silva, E.L.; Boaventura, B.C.B.; Pietro, P.F.D. Diet Quality Is Associated with Serum Antioxidant Capacity in Women with Breast Cancer: A Cross Sectional Study. Nutrients 2021, 13, 115. https://doi.org/10.3390/nu13010115
Reitz LK, Baptista SdL, Santos EdS, Hinnig PF, Rockenbach G, Vieira FGK, de Assis MAA, da Silva EL, Boaventura BCB, Pietro PFD. Diet Quality Is Associated with Serum Antioxidant Capacity in Women with Breast Cancer: A Cross Sectional Study. Nutrients. 2021; 13(1):115. https://doi.org/10.3390/nu13010115
Chicago/Turabian StyleReitz, Luiza K., Sheyla de L. Baptista, Elaine da S. Santos, Patrícia F. Hinnig, Gabriele Rockenbach, Francilene G. K. Vieira, Maria A. A. de Assis, Edson L. da Silva, Brunna C. B. Boaventura, and Patrícia F. Di Pietro. 2021. "Diet Quality Is Associated with Serum Antioxidant Capacity in Women with Breast Cancer: A Cross Sectional Study" Nutrients 13, no. 1: 115. https://doi.org/10.3390/nu13010115
APA StyleReitz, L. K., Baptista, S. d. L., Santos, E. d. S., Hinnig, P. F., Rockenbach, G., Vieira, F. G. K., de Assis, M. A. A., da Silva, E. L., Boaventura, B. C. B., & Pietro, P. F. D. (2021). Diet Quality Is Associated with Serum Antioxidant Capacity in Women with Breast Cancer: A Cross Sectional Study. Nutrients, 13(1), 115. https://doi.org/10.3390/nu13010115






