Effects of Nutrient Intake on Diagnostic Measures of Sarcopenia among Arab Men: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
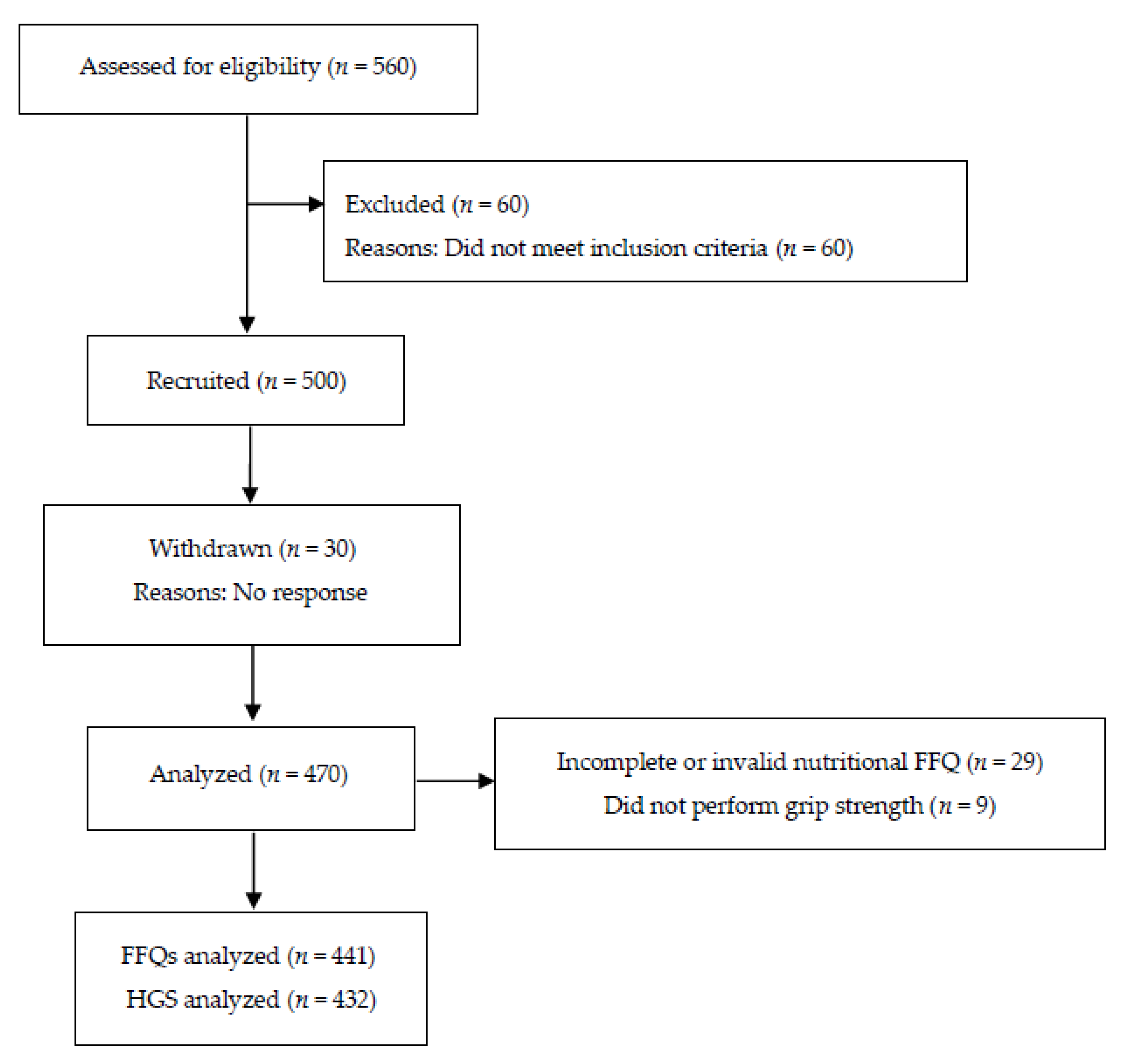
2.1. Participants
2.2. Study Design
2.3. Measurements
2.3.1. Anthropometry
2.3.2. Body Composition
2.3.3. Diagnostic Measures of Sarcopenia
Muscle Mass
Muscle Strength
2.3.4. Nutrient Intake Assessments
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Between-Group Differences
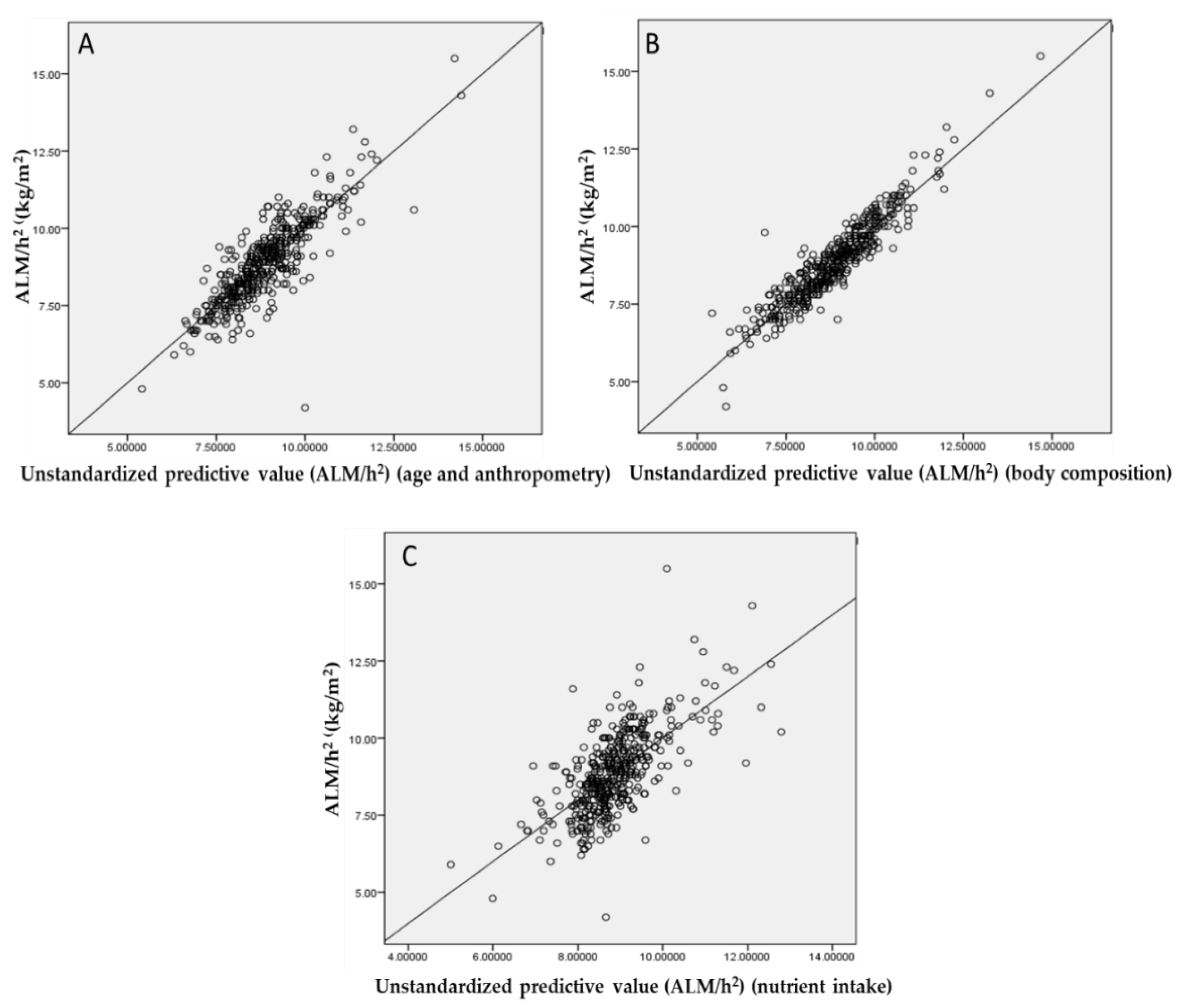
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prentice, A.M.; Jebb, S.A. Beyond body mass index. Obes. Rev. 2001, 2, 141–147. [Google Scholar] [CrossRef]
- Buffa, R.; Floris, G.U.; Putzu, P.F.; Marini, E. Body composition variations in ageing. Coll. Antropol. 2011, 35, 259–265. [Google Scholar]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A. European Working Group on Sarcopenia in Older People: Sarcopenia: European consensus on definition and diagnosis. Report of the European Workign Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, K.-I.; Paik, N.-J.; Kim, K.-W.; Jang, H.C.; Lim, J.-Y. Muscle strength: A better index of low physical performance than muscle mass in older adults. Geriatr. Gerontol. Int. 2016, 16, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; May, C.; Patel, H.P.; Baxter, M.; Sayer, A.A.; Roberts, H. A feasibility study of implementing grip strength measurement into routine hospital practice (GRImP): Study protocol. Pilot Feasibility Stud. 2016, 2, 27. [Google Scholar] [CrossRef]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 1199–1204. [Google Scholar] [CrossRef]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R.; Shardell, M.D.; Alley, D.E.; Cawthon, P.M.; Fragala, M.S.; Harris, T.B.; Kenny, A.M.; Peters, K.W.; Ferrucci, L.; Guralnik, J.M.; et al. Criteria for Clinically Relevant Weakness and Low Lean Mass and Their Longitudinal Association With Incident Mobility Impairment and Mortality: The Foundation for the National Institutes of Health (FNIH) Sarcopenia Project. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The Healthcare Costs of Sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Topinková, E.; Michel, J.-P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef]
- Montero-Fernández, N.; Serra-Rexach, J.A. Role of exercise on sarcopenia in the elderly. Eur. J. Phys. Rehabil. Med. 2013, 49, 131–143. [Google Scholar]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef]
- Deer, R.R.; Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 248–253. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein intake and muscle health in old age: From biological plausibility to clinical evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil–derived n−3 PUFA therapy increases muscle mass and function in healthy older adults1. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Park, S.; Ham, J.-O.; Lee, B.-K. A positive association of vitamin D deficiency and sarcopenia in 50 year old women, but not men. Clin. Nutr. 2014, 33, 900–905. [Google Scholar] [CrossRef]
- Kim, M.K.; Baek, K.H.; Song, K.-H.; Il Kang, M.; Park, C.Y.; Lee, W.Y.; Oh, K.W. Vitamin D Deficiency Is Associated with Sarcopenia in Older Koreans, Regardless of Obesity: The Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J. Clin. Endocrinol. Metab. 2011, 96, 3250–3256. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Wilson, J.M.; Lee, S.-R. Dietary implications on mechanisms of sarcopenia: Roles of protein, amino acids and antioxidants. J. Nutr. Biochem. 2010, 21, 1–13. [Google Scholar] [CrossRef]
- Khor, S.C.; Abdul Karim, N.; Wan Ngah, W.Z.; Mohd Yusof, Y.A.; Makpol, S. Vitamin E in Sarcopenia: Current Evidences on Its Role in Prevention and Treatment. Oxid. Med. Cell. Longev. 2014, 2014, 914853. [Google Scholar] [CrossRef] [PubMed]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e13. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M.; Lauretani, F.; Bandinelli, S.; Bos, A.; Corsi, A.M.; Simonsick, E.M.; Ferrucci, L. Magnesium and muscle performance in older persons: The InCHIANTI study. Am. J. Clin. Nutr. 2006, 84, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.-Y.; Bruyère, O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin. Exp. Res. 2019, 31, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Blizzard, L.; Fell, J.; Giles, G.; Jones, G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: The Tasmanian Older Adult Cohort study. J. Am. Geriatr. Soc. 2010, 58, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human kinetics books Champaign: Champaign, IL, USA, 1988; Volume 177. [Google Scholar]
- Heymsfield, S.B.; Smith, R.; Aulet, M.; Bensen, B.; Lichtman, S.; Wang, J.; Pierson, R.N., Jr. Appendicular skeletal muscle mass: Measurement by dual-photon absorptiometry. Am. J. Clin. Nutr. 1990, 52, 214–218. [Google Scholar] [CrossRef]
- Alkahtani, S.A. A cross-sectional study on sarcopenia using different methods: Reference values for healthy Saudi young men. BMC Musculoskelet. Disord. 2017, 18, 119. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Gosadi, I.M.; Alatar, A.A.; Otayf, M.M.; AlJahani, D.M.; Ghabbani, H.M.; AlRajban, W.A.; Alrsheed, A.M.; Al-Nasser, K.A. Development of a Saudi Food Frequency Questionnaire and testing its reliability and validity. Saudi Med. J. 2017, 38, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 38–53. [Google Scholar] [PubMed]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Bradlee, M.L.; Mustafa, J.; Singer, M.R.; Moore, L.L. High-Protein Foods and Physical Activity Protect Against Age-Related Muscle Loss and Functional Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 88–94. [Google Scholar] [CrossRef]
- Rossato, L.T.; de Branco, F.M.S.; Azeredo, C.M.; Rinaldi, A.E.M.; de Oliveira, E.P. Association between omega-3 fatty acids intake and muscle strength in older adults: A study from National Health and Nutrition Examination Survey (NHANES) 1999–2002. Clin. Nutr. 2020, 39, 3434–3441. [Google Scholar] [CrossRef]
- Semba, R.D.; Blaum, C.; Guralnik, J.M.; Moncrief, D.T.; Ricks, M.O.; Fried, L.P. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin. Exp. Res. 2003, 15, 482–487. [Google Scholar] [CrossRef]
- Zou, K.H.; Tuncali, K.; Silverman, S.G. Correlation and Simple Linear Regression. Radiology 2003, 227, 617–628. [Google Scholar] [CrossRef]
- Cameron, J.D.; Sigal, R.J.; Kenny, G.P.; Alberga, A.S.; Prud’homme, D.; Phillips, P.; Doucette, S.; Goldfield, G. Body composition and energy intake—skeletal muscle mass is the strongest predictor of food intake in obese adolescents: The HEARTY trial. Appl. Physiol. Nutr. Metab. 2016, 41, 611–617. [Google Scholar] [CrossRef]
- Okamura, T.; Miki, A.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort. J. Diabetes 2019, 11, 477–483. [Google Scholar] [CrossRef]
- Newman, A.B.; Lee, J.S.; Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Nevitt, M.; Harris, T.B. Weight change and the conservation of lean mass in old age: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005, 82, 872–878. [Google Scholar] [CrossRef]
- Risonar, M.G.D.; Rayco-Solon, P.; Ribaya-Mercado, J.D.; Solon, J.A.A.; Cabalda, A.B.; Tengco, L.W.; Solon, F.S. Physical activity, energy requirements, and adequacy of dietary intakes of older persons in a rural Filipino community. Nutr. J. 2009, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Jungjohann, S.M.; Lührmann, P.M.; Bender, R.; Blettner, M.; Neuhäuser-Berthold, M. Eight-year trends in food, energy and macronutrient intake in a sample of elderly German subjects. Br. J. Nutr. 2005, 93, 361–378. [Google Scholar] [CrossRef] [PubMed]
- De Groot, C.; Van Staveren, W.; De Graaf, C. Determinants of macronutrient intake in elderly people. Eur. J. Clin. Nutr. 2000, 54, S70–S76. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Anorexia of aging: Physiologic and pathologic. Am. J. Clin. Nutr. 1997, 66, 760–773. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- ter Borg, S.; de Groot, L.C.P.G.M.; Mijnarends, D.M.; de Vries, J.H.M.; Verlaan, S.; Meijboom, S.; Luiking, Y.C.; Schols, J.M.G.A. Differences in Nutrient Intake and Biochemical Nutrient Status Between Sarcopenic and Nonsarcopenic Older Adults—Results From the Maastricht Sarcopenia Study. J. Am. Med. Dir. Assoc. 2016, 17, 393–401. [Google Scholar] [CrossRef]
- Dupuy, C.; Lauwers-Cances, V.; Van Kan, G.A.; Gillette, S.; Schott, A.-M.; Beauchet, O.; Annweiler, C.; Vellas, B.; Rolland, Y. Dietary vitamin D intake and muscle mass in older women. Results from a cross-sectional analysis of the EPIDOS study. J. Nutr. Health Aging 2013, 17, 119–124. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; De Groot, L.; Van Staveren, W. Dietary assessment in elderly people: Experiences gained from studies in the Netherlands. Eur. J. Clin. Nutr. 2009, 63, S69–S74. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Akishita, M.; Chen, L.-K. Growing research on sarcopenia in Asia. Geriatr. Gerontol. Int. 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Total (n = 441) |
|---|---|
| Age (year) | 46.80 ± 15.98 |
| Height (cm) | 168.40 ± 6.90 |
| Body weight (kg) | 81.12 ± 15.55 |
| BMI (kg/m2) | 28.59 ± 5.08 |
| WC (cm) | 92.59 ± 20.46 |
| Body fat (%) | 27.41 ± 7.45 |
| Fat mass (kg) | 23.41 ± 9.81 |
| Muscle mass (kg) | 55.08 ± 8.00 |
| ALM/h2 (kg/m2) | 8.86 ± 1.32 |
| HGS (kg) | 39.31 ± 8.91 |
| Energy (kcal/day) | 2327.84 ± 679.59 |
| CHO (g/day) | 293.03 ± 80.57 |
| CHO (energy%) | 51.47 ± 11.46 |
| Protein (g/day) | 140.14 ± 104.64 |
| Protein (energy%) | 22.62 ± 11.07 |
| Fat (g/day) | 66.31 ± 31.79 |
| Fat (energy%) | 25.90 ± 10.09 |
| Total omega-3 fatty acids (g/day) | 0.10 ± 0.07 |
| Vitamin D (ug/day) | 2.89 ± 2.04 |
| Vitamin E (mg/day) | 3.53 ± 2.22 |
| Ca (mg/day) | 393.67 ± 175.80 |
| Mg (mg/day) | 66.72 ± 37.77 |
| Parameters | EWGSOP2 | −1 SD < Reference Values Arab Men | ||||
|---|---|---|---|---|---|---|
| Normal (ALM/h2 ≥ 7.0 kg/m2) | Low (ALM/h2 < 7.0 kg/m2) | p-Value 2 | Normal (ALM/h2 ≥ 8.68 kg/m2) | Low (ALM/h2 < 8.68 kg/m2) | p-Value 2 | |
| n (441) | 420 | 21 | 238 | 203 | ||
| Age (year) | 49 (32–60) | 62 (47–67.5) | 0.002 | 42.91 ± 14.46 | 51.37 ± 16.49 | <0.001 |
| Height (cm) | 168.45 ± 6.92 | 167.39 ± 6.51 | 0.493 | 169.21 ± 6.98 | 167.45 ± 6.70 | 0.008 |
| Body weight (kg) | 81.98 ± 15.22 | 63.93 ± 11.79 | <0.001 | 89.04 ± 14.89 | 71.84 ± 10.30 | <0.001 |
| BMI (kg/m2) | 28.87 ± 4.94 | 22.90 ± 4.37 | <0.001 | 31.08 ± 4.77 | 25.66 ± 3.68 | <0.001 |
| WC (cm) | 95.5 (85–105) | 92 (76–98) | 0.090 | 95.81 ± 20.86 | 88.78 ± 19.26 | <0.001 |
| Body fat (%) | 27.41 ± 7.18 | 27.48 ± 11.85 | 0.979 | 28.46 ± 6.86 | 26.18 ± 7.93 | <0.001 |
| Fat mass (kg) | 23.36 ± 9.69 | 18.75 ± 11.44 | 0.035 | 26.31 ± 10.15 | 19.42 ± 7.95 | <0.001 |
| Muscle mass (kg) | 55.69 ± 7.65 | 42.74 ± 3.74 | <0.001 | 59.73 ± 6.33 | 49.62 ± 6.08 | <0.001 |
| HGS (kg) | 39.75 ± 8.76 | 30.57 ± 7.29 | 0.003 | 42.35 ± 8.15 | 35.68 ± 8.41 | <0.001 |
| Energy (kcal/day) | 2322.71 ± 680.47 | 2430.39 ± 669.55 | 0.479 | 2415.16 ± 713.87 | 2225.46 ± 623.35 | 0.003 |
| CHO (g/day) | 291.82 ± 80.37 | 317.23 ± 82.84 | 0.159 | 290.16 ± 79.52 | 296.40 ± 81.85 | 0.418 |
| CHO (energy%) | 51.39 ± 11.48 | 53.14 ± 11.11 | 0.493 | 49.27 ± 11.35 | 54.05 ± 11.06 | <0.001 |
| Protein (g/day) | 139.60 ± 104.32 | 150.94 ± 113.12 | 0.629 | 151.63 ± 113.82 | 126.67 ± 91.19 | 0.011 |
| Protein (energy%) | 22.62 ± 11.09 | 22.67 ± 11.52 | 0.985 | 23.58 ± 11.71 | 21.50 ± 10.24 | 0.047 |
| Fat (g/day) | 66.33 ± 32.05 | 61.97 ± 26.47 | 0.540 | 72.00 ± 34.80 | 59.24 ± 26.32 | <0.001 |
| Fat (energy%) | 25 (18–32) | 19 (16–28) | 0.214 | 27.11 ± 10.29 | 24.47 ± 9.71 | 0.006 |
| Total omega-3 fatty acids (g/day) | 0.10 ± 0.07 | 0.10 ± 0.05 | 0.899 | 0.10 ± 0.07 | 0.10 ± 0.07 | 0.336 |
| Vitamin D (ug/day) | 2.86 ± 2.02 | 3.39 ± 2.41 | 0.247 | 2.80 ± 2.04 | 2.99 ± 2.04 | 0.314 |
| Vitamin E (mg/day) | 3.25 (1.66–4.89) | 3.48 (2.15–4.69) | 0.427 | 3.37 ± 2.30 | 3.73 ± 2.1 | 0.093 |
| Ca (mg/day) | 396.57 ± 177.51 | 335.76 ± 126.77 | 0.122 | 399.37 ± 184.58 | 386.99 ± 165.12 | 0.462 |
| Mg (mg/day) | 55.09 (41.24–81.17) | 45.67 (40.33–97.38) | 0.725 | 68.09 ± 38.12 | 65.11 ± 37.38 | 0.410 |
| Parameters | HGS (kg) | p-Value 2 | ||
|---|---|---|---|---|
| <27 kg | 27–42 kg | >42 kg | ||
| n (432) | 35 | 237 | 160 | |
| Age (year) | 63.49 ± 14.76(ab) 3 | 48.84 ± 15.51(ac) | 40.11 ± 13.12(bc) | <0.001 |
| Height (cm) | 164.06 ± 5.80(a) | 166.64 ± 6.37(b) | 172.01 ± 6.25(ab) | <0.001 |
| Body weight (kg) | 71.83 ± 12.69(ab) | 78.33 ± 13.81(ac) | 87.28 ± 16.34(bc) | <0.001 |
| BMI (kg/m2) | 88.06 ± 25.02 | 93.06 ± 19.55 | 93.06 ± 20.76 | 0.384 |
| WC (cm) | 26.79 ± 5.04(a) | 28.21 ± 4.72(b) | 29.51 ± 5.37(ab) | 0.004 |
| Body fat (%) | 27.96 ± 8.80 | 27.90 ± 7.15 | 26.44 ± 7.44 | 0.139 |
| Fat mass (kg) | 20.79 ± 9.40 | 22.61 ± 9.03 | 24.33 ± 10.72 | 0.079 |
| Muscle mass (kg) | 48.86 ± 7.34(ab) | 52.96 ± 7.22(ac) | 59.67 ± 7.05(bc) | <0.001 |
| ALM/h2 (kg/m2) | 7.73 ± 1.16(ab) | 8.6578 ± 1.22(ac) | 9.4188 ± 1.27(bc) | <0.001 |
| Energy (kcal/day) | 2259.76 ± 535.90 | 2252.57 ± 612.69(a) | 2467.23 ± 787.04(a) | <0.001 |
| CHO (g/day) | 291.42 ± 81.12 | 290.74 ± 77.68 | 297.69 ± 84.45 | 0.693 |
| CHO (energy%) | 52.17 ± 11.56 | 52.69 ± 11.12(a) | 49.49 ± 11.71(a) | 0.022 |
| Protein (g/day) | 130.84 ± 93.82 | 130.29 ± 92.48(a) | 159.16 ± 122.36(a) | 0.023 |
| Protein (energy%) | 21.77 ± 10.50 | 22.03 ± 10.87 | 23.89 ± 11.56 | 0.230 |
| Fat (g/day) | 63.41 ± 27.57 | 63.16 ± 32.67(a) | 71.09± 31.26(a) | 0.046 |
| Fat (energy%) | 26.14 ± 11.07 | 25.27 ± 10.22 | 26.57 ± 9.52 | 0.442 |
| Total omega-3 fatty acids (g/day) | 0.13 ± 0.14(ab) | 0.01 ± 0.06(ac) | 0.10 ± 0.06(bc) | 0.018 |
| Vitamin D (ug/day) | 3.33 ± 2.09 | 3.01 ± 2.15 | 2.69 ± 1.87 | 0.141 |
| Vitamin E (mg/day) | 3.56 ± 1.82 | 3.39 ± 2.03 | 3.71 ± 2.57 | 0.383 |
| Ca (mg/day) | 424.21 ± 155.74 | 395.38 ± 169.80 | 383.66 ± 183.80 | 0.444 |
| Mg (mg/day) | 67.68 ± 40.75 | 65.67 ± 37.46 | 67.40 ± 37.33 | 0.886 |
| Parameters | ALM/h2 (n = 441) | HGS (kg) (n = 432) | ||
|---|---|---|---|---|
| Pearson Correlation | p-Value 1 | Pearson Correlation | p-Value | |
| Age (year) | −0.31 | <0.001 | −0.45 | <0.001 |
| Height (cm) | 0.11 | 0.018 | 0.44 | <0.001 |
| Body weight (kg) | 0.74 | <0.001 | 0.37 | <0.001 |
| BMI (kg/m2) | 0.28 | <0.001 | 0.05 | 0.338 |
| WC (cm) | 0.74 | <0.001 | 0.19 | <0.001 |
| Body fat (%) | 0.23 | <0.001 | −0.12 | 0.016 |
| Fat mass (kg) | 0.50 | <0.001 | 0.12 | 0.017 |
| Muscle mass (kg) | 0.80 | <0.001 | 0.53 | <0.001 |
| Energy (kcal/day) | 0.17 | <0.001 | 0.12 | 0.014 |
| CHO (g/day) | −0.04 | 0.419 | 0.01 | 0.829 |
| CHO (energy%) | −0.24 | <0.001 | −0.13 | 0.007 |
| Protein (g/day) | 0.18 | <0.001 | 0.12 | 0.009 |
| Protein (energy%) | 0.17 | <0.001 | 0.09 | 0.040 |
| Fat (g/day) | 0.18 | <0.001 | 0.09 | 0.075 |
| Fat (energy%) | 0.07 | 0.117 | 0.04 | 0.445 |
| Total omega-3 fatty acids (g/day) | −0.02 | 0.622 | −0.09 | 0.041 |
| Vitamin D (ug/day) | −0.05 | 0.258 | −0.12 | 0.015 |
| Vitamin E (mg/day) | −0.08 | 0.102 | 0.07 | 0.177 |
| Ca (mg/day) | 0.03 | 0.565 | −0.04 | 0.388 |
| Mg (mg/day) | 0.05 | 0.323 | 0.00 | 0.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhussain, M.H.; Alkahtani, S.; Aljuhani, O.; Habib, S.S. Effects of Nutrient Intake on Diagnostic Measures of Sarcopenia among Arab Men: A Cross-Sectional Study. Nutrients 2021, 13, 114. https://doi.org/10.3390/nu13010114
Alhussain MH, Alkahtani S, Aljuhani O, Habib SS. Effects of Nutrient Intake on Diagnostic Measures of Sarcopenia among Arab Men: A Cross-Sectional Study. Nutrients. 2021; 13(1):114. https://doi.org/10.3390/nu13010114
Chicago/Turabian StyleAlhussain, Maha H., Shaea Alkahtani, Osama Aljuhani, and Syed Shahid Habib. 2021. "Effects of Nutrient Intake on Diagnostic Measures of Sarcopenia among Arab Men: A Cross-Sectional Study" Nutrients 13, no. 1: 114. https://doi.org/10.3390/nu13010114
APA StyleAlhussain, M. H., Alkahtani, S., Aljuhani, O., & Habib, S. S. (2021). Effects of Nutrient Intake on Diagnostic Measures of Sarcopenia among Arab Men: A Cross-Sectional Study. Nutrients, 13(1), 114. https://doi.org/10.3390/nu13010114





