Abstract
Gymnema inodorum (GI) is an indigenous medicinal plant and functional food in Thailand that has recently helped to reduce plasma glucose levels in healthy humans. It is renowned for the medicinal properties of gymnemic acid and its ability to suppress glucose absorption. However, the effects of gymnemic acids on adipogenesis that contribute to the accumulation of adipose tissues associated with obesity remain unknown. The present study aimed to determine the effects of gymnemic acids derived from GI tea on adipogenesis. We purified and identified GiA-7 and stephanosides C and B from GI tea that inhibited adipocyte differentiation in 3T3-L1 cells. These compounds also suppressed the expression of peroxisome proliferator-activated receptor gamma (Pparγ)-dependent genes, indicating that they inhibit lipid accumulation and the early stage of 3T3-L1 preadipocyte differentiation. Only GiA-7 induced the expression of uncoupling protein 1 (Ucp1) and pparγ coactivator 1 alpha (Pgc1α), suggesting that GiA-7 induces mitochondrial activity and beige-like adipocytes. This is the first finding of stephanosides C and B in Gymnema inodorum. Our results suggested that GiA-7 and stephanosides C and B from GI tea could help to prevent obesity.
1. Introduction
Gymnema sylvestre is a species of the genus Gymnema that is popular in India for reducing glucose levels, suppressing glucose absorption and preventing type 2 diabetes [1,2,3,4,5]. Gymnema inodorum (GI) is a species of same genus that is indigenous to Thailand, particularly in the northern region, where it is widely consumed. The effects of GI on glucose absorption and blood glucose levels have recently been investigated [6,7,8]. We previously found that extracts of GI leaves decreased blood glucose in alloxan-induced diabetic rats [9] that comprise a popular model with which to study type 1 diabetes mellitus. Alloxan selectively destroys insulin production in beta cells, which consequently results in high blood glucose levels [10]. However, about 90% of patients with diabetes have type 2 diabetes mellitus (DM) which is induced by a lack of exercise and inappropriate eating habits [11]. However, obesity is the leading risk factor for type 2 DM, and it also greatly increases the risk of fatty liver disease, atherosclerosis, metabolic diseases, insulin resistance and hypertension [12,13]. Obesity is characterized at the cellular level as being differentiated from preadipocytes. White adipose tissue (WAT) is specialized to store excess energy as triglycerides composed of fatty acids. Inhibiting preadipocyte differentiation can prevent the initiation and progression of obesity [14,15].
The differentiation of 3T3-L1 fibroblast-like cells into adipocyte-like cells stimulated by insulin and synthetic glucocorticoids is a popular model of adipogenesis and lipid metabolism in vitro [16,17]. Therefore, we applied the inhibition of 3T3-L1 cell differentiation to screen gymnemic acid extracted from GI tea. Gymnemic acid is an oleanane-type triterpene glycoside [16,17] that can exist as a single entity or as a mixture of several related compounds [18,19]. The major saponin fraction in Gymnema sylvestre is a gymnemic acid that comprises a complex mixture of at least nine similar glycosides and aglycone derivatives [20]. Moreover, only four gymnemic acids have been identified in GI, which renders the purification and identification of gymnemic acids difficult. Furthermore, current knowledge about these compounds purified from GI is limited. We isolated and purified GiA-7, stephanoside C and stephanoside B from GI tea that inhibited 3T3-L1 cell differentiation. We also determined the expression of the peroxisome proliferator-activated receptor gamma (Pparγ), CCAAT/enhancer-binding protein alpha (Cebpα), cluster of differentiation 36 (Cd36), fatty acid synthase (Fasn), pparγ coactivator 1 alpha (Pgc1α), lipin-1, adipose triglyceride lipase (Atgl), hormone-sensitive lipase (Hsl), sterol regulatory element-binding protein (Srebp)-1c, uncoupling protein 1 (Ucp1), glucose transporter type 4 (Glut4) and fatty acid binding protein 4 (Fabp4) genes to explain the signaling of adipogenesis inhibition in 3T3-L1 preadipocytes.
2. Materials and Methods
2.1. Extraction, Isolation and Purification
Fresh GI leaves (Development of Herbs and Fruit Products Community Enterprise (Chiang Mai, Thailand)) were powdered, washed, dried and then steamed for 3 min. The leaves were dried at 60 °C for 2 h, stir-fried to complete dryness and then stored in darkness.
After extracting GI tea powder with 98% methanol for 24 h, the extract was mixed with hexane in a separatory funnel. The lower solution was collected, evaporated to dryness and then the residue was washed with chloroform and methanol (2:1) to remove fat components. The washed, evaporated residue dissolved in methanol (crude gymnemic acid) was eluted through a Sep-Pak tC18 cartridge (Waters Corporation, Milford, MA, USA) with a gradient of 10–100% methanol and ethanol. Six active compounds were purified from the 90% methanol fraction by high-performance liquid chromatography (HPLC) using a Model CCPD computer-controlled pump (Tosoh, Tokyo, Japan) equipped with a Capcell PAK C18 5 µm, 20-mm inner diameter (i.d.), 250-mm column (Osaka Soda Co., Ltd., Osaka, Japan) and isocratic 80% methanol with 0.1% formic acid at a flow rate of 2.5 mL/min. The compounds were detected at 254 nm using a UV wavelength detector (JASCO International Co., Ltd., Tokyo, Japan).
2.2. Mass Spectrometry
Dried purified compounds were dissolved and diluted in dimethyl sulfoxide (Fujifilm Wako Pure Chemical Industries Ltd., Osaka, Japan) at 100 ppm. The accurate molecular formula was determined by Liquid Chromatography equipped with Quadrupole Time Of Flight Mass Spectrometry (LC/Q-TOF MS) using an Agilent 6530 Accurate-Mass Q-TOF LC/MS system (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with an electrospray ionization (ESI) interface. Compounds were separated by reversed-phase liquid chromatography using a photodiode array detector and monitored at a wavelength ranging from 210 to 600 nm at a flow rate of 0.4 mL/min using an ACQUITY UPLC BEH C18 column (50 × 2.1 mm i.d. and 1.7 μm particle size (Waters Corp.) at 40 °C). The mobile phase consisted of a linear gradient of 0.1% formic acid:acetonitrile (1:1) to 0.1% formic acid:acetonitrile (1:19) over 3 min. The high-resolution mass spectra (HRMS) conditions were: positive ion mode; desolvation gas, N2; temperature 350 °C, pressure, 40 psig; flow rate, 8 L/min and capillary, fragmentary and skimmer voltages of 3500, 100 and 65 V, respectively [18].
2.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
Dried compound 2 (10 mg) was exchanged into methanol-d4, 99.8 atom% D, containing 0.05% (v/v) Tetramethylsilane (TMS) (Cambridge Isotope Laboratories Inc., Andover, MA, USA). Dried compounds 5 and 6 (10 mg each) were exchanged into pyridine-d5, 99.5 atom% D (Cambridge Isotope Laboratories Inc.). Spectra were determined by one-dimensional (1H NMR, 13C NMR and dept-135) and two-dimensional COrrelated SpectroscopY (COSY), Heteronuclear Multiple Bond Correlation (HMBC) and Heteronuclear Multiple Quantum Coherence (HMQC) NMR using a Bruker 500 MHz NMR (Bruker Daltonics SPR, Hamburg, Germany).
2.4. Cell Culture
The mouse embryonic fibroblast cell line (3T3-L1 cell) was purchased from National Institutes of Biomedical Innovation, Health, and Nutrition (NIBIOHN), Osaka, Japan. These cells were cultured in low-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Fujifilm Wako Pure Chemical Corp.) containing 10% heat-inactivated fetal bovine serum (FBS; Biowest, Tokyo, Japan) at 37 °C under a humidified 5% CO2 atmosphere.
2.5. The Antiadipocyte Differentiation Activity
We seeded 3T3-L1 cells (1 × 105/mL in 200 µL) cultured as described above into collagen-coated 96-well plates in high-glucose DMEM (Fujifilm Wako Pure Chemical Corp.) under standard conditions for 24 h, then induced their differentiation into adipocytes using 10 µg/mL insulin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) and 1 µM water-soluble dexamethasone (Sigma-Aldrich Corp., St. Louis, MO, USA). After 1 h, the 3T3-L1 cells were incubated with samples for 7–10 days.
Cell proliferation was determined using CellTiter 96® AQueous One Solution Cell Proliferation Assays (Promega Corp., Madison, WI, USA), as described by the manufacturer. The absorbance of proliferating cells determined at 490 nm using an iMarkTM Microplate Reader (Bio-Rad Laboratories Inc., Hercules, CA, USA) was compared with that of untreated differentiated 3T3-L1 cells.
Intracellular lipid accumulation was determined using a Lipid Assay Kit (Cosmo Bio Co., Ltd., Tokyo, Japan), as described by the manufacturer. Differentiated 3T3-L1 cells were washed with phosphate-buffered saline (PBS), and fixed overnight with 4% formaldehyde at room temperature. The cells were then washed twice with distilled water, incubated with Oil red O at room temperature for 15 min and washed twice with distilled water. Oil red O extraction reagent was added into cells. Absorbance was read at 540 nm using the iMarkTM Microplate Reader. Absorption due to the intracellular lipid accumulation was determined and compared with that of control-differentiated 3T3-L1 cells.
2.6. Quantitative Real-Time PCR
We incubated 3T3-L1 cells (1 × 105/mL; 1 mL) seeded into collagen-coated 12-well plates in high-glucose DMEM under standard conditions for 3 days, then induced the cells to differentiate into adipocytes using 10 µg/mL insulin, 0.5 mM IBMX and 1 µM water-soluble dexamethasone for 1 h. The 3T3-L1 cells were then incubated with purified GiA-7 and stephanosides C and B from GI tea (100 µM each) for 8 days. The expression of genes associated with adipogenesis was analyzed using quantitative real-time PCR. Total RNA was extracted from the cells using RNAiso plus. Single-stranded cDNA was generated using PrimeScriptTM RT Master Mix. Quantitative real-time PCR was conducted using a SYBR® Premix Ex Taq™ II (Takara Bio. Inc., Otsu, Japan) and a LightCyclerTM (Roche Diagnostics, Mannheim, Germany). The sequences of all primers (Thermo Fisher Scientific Inc) are listed in Table 1 [19]. The PCR conditions were 95 °C for 10 s, followed by 45 cycles of 95 °C for 5 s, 58 °C for 10 s and at 72 °C for 10 s. The amount of target mRNA was normalized relative to the internal standard 36b4.

Table 1.
Primer sequences for real-time reverse transcription (RT)-PCR.
2.7. Statistical Analysis
Data were statistically assessed by one-way analyses of variance (ANOVAs) with Dunnett tests using EZR software version 1.52 (Jichi Medical University, Saitama, Japan), which is graphical user interface for R (The R Foundation for Statistical Computing) based on R commander [20]. Values are indicated as means ± SD. Significant differences are shown as p-values.
3. Results and Discussion
We measured the ability of the crude 10–100% methanol and ethanol fractions of gymnemic acid to inhibit 3T3-L1 cell differentiation. We found that the 90% methanol fraction was the most powerful inhibitor (Figure 1). We then found that compounds 2, 3, 5 and 6 among the six compounds separated by HPLC from this fraction (Figure 2) significantly inhibited 3T3-L1 cell differentiation (Figure 3). Compound 5 was the most powerful inhibitor. The inhibition of 3T3-L1 cell differentiation by compound 5 was concentration-dependent. Compound 6 also strongly inhibited 3T3-L1 cell differentiation. However, the yield of HPLC fraction 3 was very low. Therefore, HPLC fractions No. 2, 5 and 6 were further purified, and their structures were identified by NMR and mass spectrometry. The 13C NMR chemical shifts of compounds 2, 5 and 6 were compared with published 13C NMR chemical shifts of GiA-7 [6], stephanoside C and stephanoside B [21], respectively, and are shown in Table 2, Table 3, Table 4, Table 5 and Table 6, respectively. These findings showed that compounds 2, 5 and 6 were GiA-7, stephanoside C and stephanoside B, respectively.
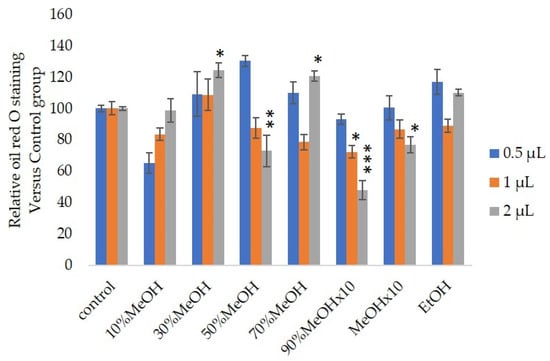
Figure 1.
Effects of Sep-PaktC18 fractions on 3T3-L1 cell differentiation. We assessed the abilities of 10%MeOH, 30%MeOH, 50%MeOH, 70%MeOH and EtOH fractions at the concentration of 10 mg/mL in ethanol and 90%MeOH and MeOH fractions at the concentration of 1 mg/mL in ethanol to inhibit 3T3-L1 cell differentiation. Values are shown as means ± SD (n = 4). * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. control (ANOVA with post hoc Dunnett tests).
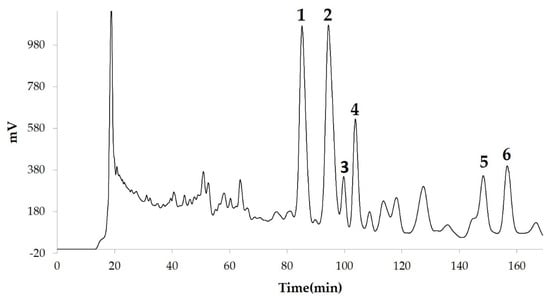
Figure 2.
Compounds separated by high-performance liquid chromatography (HPLC) from 90% methanol fraction.
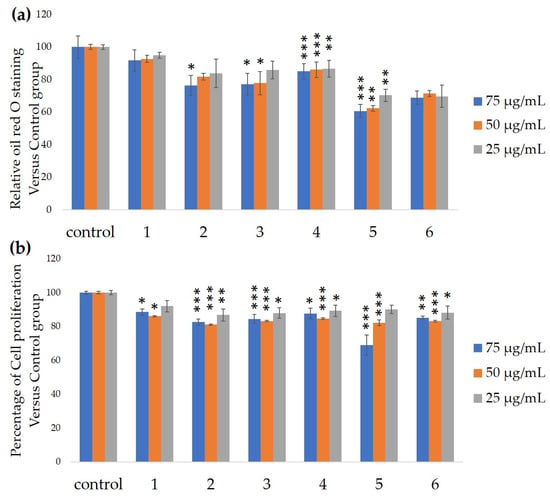
Figure 3.
Ability of HPLC fractions to inhibit 3T3-L1 cell differentiation. (a) Inhibition of adipogenesis. (b) Cell proliferation. Values are shown as means ± SD (n = 4). * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. control (ANOVA and post hoc Dunnett tests).

Table 2.
13C nuclear magnetic resonance (NMR) chemical shifts of GiA-7 and compound 2 (δ: ppm).

Table 3.
The 13C NMR chemical shifts of stephanoside C and compound 5 (δ: ppm).

Table 4.
The 13C NMR chemical shifts of sugar chains of stephanoside C and compound 5 (δ in ppm).

Table 5.
The 13C NMR chemical shifts of stephanoside B and compound 6 (δ: ppm).

Table 6.
The 13C NMR chemical shifts of sugar chains of stephanoside B and compound 6 (δ: ppm).
The molecular formulae of purified compounds 2, 5 and 6 were determined using Q-TOF LC/MS in the positive ion mode. The molecular formula of GiA-7 was C44H65NO12, according to the mass spectra (m/z 800.4580 (M + H)+, calcd. m/z 800.4582). Those of stephanoside C and stephanoside B were the same: C52H79NO18, calcd. 1006.5370. The accurate masses of stephanosides C and B were m/z 1006.5383 (M + H)+ and m/z 1006.5488 (M + H)+, respectively. The molecular formulae of stephanosides C and B are the same, but their sugar chains are d-thevetose and d-allomethylose, respectively. Figure S1 shows the structures of compounds 2, 5 and 6. The NMR and mass spectrometry data confirmed that compounds 2, 5 and 6 are GiA-7, stephanoside C and stephanoside B, respectively.
We assessed the ability of purified 25, 50 and 100-µM GiA-7, stephanoside C and stephanoside B extracted from GI tea to inhibit 3T3-L1 cell differentiation. After 10 days, intercellular lipid accumulation and viable cells were determined. Each of GiA-7, stephanoside C and stephanoside B at 100 µM reduced intercellular lipid accumulation (Figure 4). Stephanoside C was the most effective inhibitor, which is the lowest concentration of significantly inhibited 3T3-L1 cell differentiation. Moreover, the inhibition of 3T3-L1 cell differentiation by GiA-7, stephanoside C and stephanoside B was concentration-dependent.
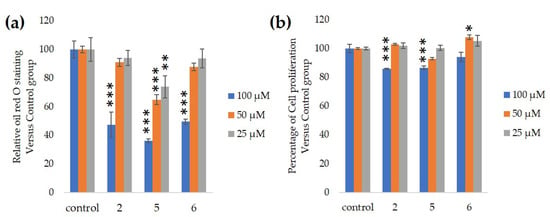
Figure 4.
Effects of compounds 2 (GiA-7), 5 (stephanoside C) and 6 (stephanoside B) on 3T3-L1 cell differentiation. (a) Inhibition of adipogenesis. (b) Cell proliferation. Values are shown as means ± SD (n = 4). * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. control (ANOVA and post hoc Dunnett tests).
Several markers associated with adipogenesis control 3T3-L1 cell differentiation [22]. We assessed the expression of the Pparγ, Cebpα, Fasn, Pgc1α, Cd36 and Fabp4 genes that are associated with differentiation into adipocytes to determine the effects of GiA-7, stephanoside C and stephanoside B on adipogenesis. Figure 5 shows that GiA-7, stephanoside C and stephanoside B at 100 µM significantly suppressed the expression of Pparγ, Cebpα, Fasn and Cd36. Stephanoside B and GiA-7 significantly suppressed Fabp4 expression. Stephanosides C and B also significantly suppressed Pgc1α expression. These results indicated that GiA-7, stephanoside C and stephanoside B inhibited the early stage of adipogenic differentiation by inhibiting of Pparγ-dependent mechanisms. Both Hsl and Atgl are phosphorylates upon appropriate physiological signaling to induce triacylglycerol (TG) lipolysis in adipocytes [23,24]. Figure 5 shows that stephanosides C, B and GiA-7 suppressed Hsl and Atgl gene expressions. These findings suggested that none of these compounds activated TG lipolysis. However, Pparγ directly regulates Hsl and Atgl gene expressions in adipocytes in vitro [25,26]. Our results suggested that these compounds downregulated Hsl and Atgl gene expressions by inhibiting Pparγ gene expression. Lipin-1 functions in lipid droplet biogenesis during adipocyte differentiation and generates diacylglycerol for lipid synthesis [27]. Lipin-1 is important for the process of TG accumulation during the early stage of adipogenesis. Lipin-1 is a key factor for adipocyte maturation and maintenance by regulating Pparγ and Cebpα [28]. Lipin-1 expression is required to induce the transcription of adipogenic genes, including Pparγ and Cebpα [29,30]. Figure 5 shows that stephanosides C and B and GiA-7 significantly suppressed lipin-1 expression. These findings suggest that these compounds inhibited Pparγ and Cebpα gene expressions by suppressing lipin-1 gene expression. These observations confirm that GiA-7, stephanoside C and stephanoside B inhibited the early stage of adipogenesis and prevented TG accumulation. The transcriptional cofactor, Pgc1α, is important for mitochondrial biogenesis. The regulation of Pgc1α expression enhances mitochondrial biogenesis through Srebp-1c upregulation [31,32]. The present study found that only GiA-7 induced Srebp-1c and Pgc1α. Srebp-1c is also a key regulator of adipocytes and is involved in lipid metabolism [33,34]. These findings suggest that GiA-7 regulates mitochondrial biogenesis through the Srebp-1c-dependent upregulation of Pgc1α. GiA-7 also inhibits lipid accumulation in 3T3-L1 preadipocytes by downregulating adipogenic transcription factors and genes associated with lipid accumulation. Both Pgc1α and Ucp1 are brown/beige cell-specific genes. Only GiA-7 induced the expression of Pgc1α and Ucp1. Beige adipocytes express low basal levels of Ucp1, whereas brown adipocytes constitutively express Ucp1. These findings suggest that GiA-7 inhibits the differentiation of white adipocytes and, also, induces beige-like adipocytes in 3T3-L1 mouse preadipocytes. Comprehensive profiles of gene expressions indicate that the characteristics of human brown and mouse beige adipocytes are compatible [33,34]. The activation of human brown adipocytes was recently examined as a possible novel therapeutic treatment for obesity [35]. Thus, GiA-7 might serve as a novel treatment for obesity in humans by inducing brown adipocytes.
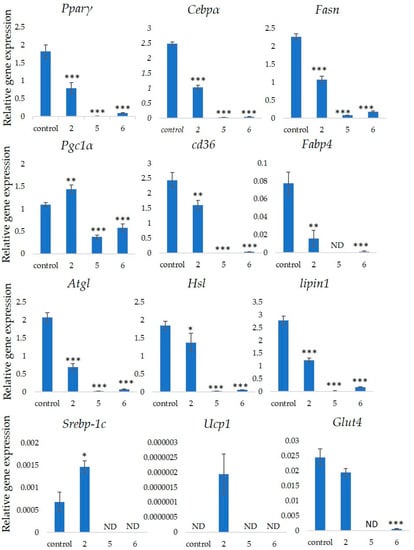
Figure 5.
Effects of stephanosides C and B and GiA-7 extracted from Gymnema inodorum (GI) tea on gene expressions at the initial stage of 3T3-L1 cell differentiation into adipocytes. The differentiation of 3T3-L1 cells was induced, and the cells were incubated with 100 µM GiA-7, stephanoside C and stephanoside B for 8 days; then, the gene expressions were measured. Values are shown as means ± SD (n = 4). * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. control (ANOVA with post hoc Dunnett tests. ND, not detected).
Gymnemic acid extracted from the leaves of Gymnema sylvestre comprises a mixture of triterpene glycosides that can reduce glucose levels and inhibit glucose absorption [36,37,38]. The aqueous extract of Gymnema sylvestre induces insulin secretion in MIN6 cells [39]. One study found that GiA-7 from GI leaves inhibits glucose absorption in the isolated intestinal tract and suppresses blood glucose in rats [6]. However, we found here that GiA-7 purified from gymnemic acid extracted from GI tea inhibited 3T3-L1 cell differentiation into adipocytes. Stephanoside C and stephanoside B isolated from the stems of Stephanotis lutchuensis var. japonica and Gongronema nepalense have ant-malarial activity [21,40]. This is the first report of stephanoside C and stephanoside B isolated from Gymnema inodorum inhibiting 3T3-L1 cell differentiation into adipocytes. As mentioned before, obesity is characterized at the cellular level as being differentiated from preadipocytes. GiA-7, Stephanoside C and stephanoside B present in GI tea inhibited preadipocyte differentiation by suppressing the Pparγ-dependent mechanisms. These findings suggest that consuming GI tea could play a role in the prevention of obesity.
4. Conclusions
Gymnema inodorum tea has been widely applied in Thailand to control high blood glucose. Here, we screened the ability of gymnemic acids extracted from GI tea to inhibit 3T3-L1 cell differentiation into adipocytes. We isolated and purified GiA-7, stephanoside C and stephanoside B from GI tea using column chromatography and C18 HPLC, respectively, then confirmed them using NMR and mass spectrometry. All three compounds inhibited 3T3-L1 cell differentiation into adipocytes. Moreover, we determined that these compounds inhibited the early stage of adipogenesis by suppressing the Lipin-1, Pparγ, Cebpα, Fasn, Cd36 and Fabp4 genes that are associated with adipogenesis. However, only GiA-7 induced Ucp1 and Pgc1α, suggesting that GiA-7 enhances mitochondrial activity and beige-like adipocytes among 3T3-L1 preadipocytes. Our findings suggest that the GiA-7, stephanoside C and stephanoside B from GI tea could help to prevent obesity.
5. Patents
Papawee Saiki and Yasuhiro Kawano, the methods of inhibiting fat synthesis, fat synthesis inhibitors and food and drink for suppressing fat synthesis, JP patent 2019-218481.
Supplementary Materials
The following is available online at https://www.mdpi.com/2072-6643/12/9/2851/s1, Figure S1: The structural formulae of compounds 2, 5 and 6.
Author Contributions
P.S. and P.K. (Prapaipat Klungsupya) conceptualization; P.S., Y.K. and T.O. methodology; P.S. and Y.K. validation; P.S. formal analysis; P.S. investigation; W.P., P.K. (Papitchaya Kongchinda), N.P. and T.M. resources; P.S., Y.K. and T.O. data curation; P.S. writing—original draft preparation; P.S., Y.K., T.O., P.K. (Prapaipat Klungsupya), T.M., W.P., P.K. (Papitchaya Kongchinda), N.P. and K.M. writing—review and editing; P.S. visualization; K.M. supervision; P.S. project administration; P.K. (Prapaipat Klungsupya) funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Thai Royal Government, Ministry of Higher Education, Science, Research and Innovation (MHESI) through the Thailand Institute of Scientific and Technological Research, TISTR (Grant Number: TISTR6334102122).
Acknowledgments
We thank Katsutaka Oishi and Tomoki Abe for their helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shanmugasundaram, E.; Rajeswari, G.; Baskaran, K.; Kumar, B.R.; Shanmugasundaram, K.R.; Ahmath, B.K. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J. Ethnopharmacol. 1990, 30, 281–294. [Google Scholar] [CrossRef]
- Shimizu, K.; Iino, A.; Nakajima, J.; Tanaka, K.; Nakajyo, S.; Urakawa, N.; Atsuchi, M.; Wada, T.; Yamashita, C. Suppression of glucose absorption by some fractions extracted from Gymnema sylvestre leaves. J. Vet. Med. Sci. 1997, 59, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Al-Romaiyan, A.; King, A.; Persaud, S.; Jones, P. A novel extract of Gymnema sylvestre improves glucose tolerance in vivo and stimulates insulin secretion and synthesis in vitro. Phytother. Res. 2013, 27, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Kathori, S.; Upadhyay, B. Effect of Gurmar (Gymnema sylvestre) powder intervention on the blood glucose levels among diabetics. Stud. Ethno-Med. 2009, 3, 133–135. [Google Scholar] [CrossRef]
- Kanetkar, P.; Singhal, R.; Kamat, M. Recent advances in indian herbal drug research guest editor: Thomas Paul Asir Devasagayam Gymnema sylvestre: A memoir. J. Clin. Biochem. Nutr. 2007, 41, 77–81. [Google Scholar] [CrossRef]
- Shimizu, K.; Ozeki, M.; Iino, A.; Nakajyo, S.; Urakawa, N.; Atsuchi, M. Structure-activity relationships of triterpenoid derivatives extracted from Gymnema inodorum leaves on glucose absorption. Jpn. J. Pharmacol. 2001, 86, 223–229. [Google Scholar] [CrossRef]
- Chiabchalard, A.; Tencomnao, T.; Santiyanont, R. Effect of Gymnema inodorum on postprandial peak plasma glucose levels in healthy human. Afr. J. Biotechnol. 2010, 9, 1079–1085. [Google Scholar]
- Shimizu, K.; Ozeki, M.; Tanaka, K.; Itoh, K.; Nakajyo, S.; Urakawa, N.; Atsuchi, M. Suppression of glucose absorption by extracts from the leaves of Gymnema inodorum. J. Vet. Med. Sci. 1997, 59, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Klungsupya, P.; Muangman, T.; Theangtrong, N.; Khayungarnnawee, A.; Phatvej, W.; Thisayakorn, K.; Rerk-Am, U.; Sematong, T.; Trangvacharakul, S.; Arunpairojana, V. Antioxidant and antihyperglycemic activities of Gymnema inodorum Dence. In Proceedings of the 8th NRCT-JSPS Joint Seminar Innovative Research in Natural Products for Sustainable Development, Bangkok, Thailand, 3–5 February 2009; pp. 207–209. [Google Scholar]
- Rohilla, A.; Ali, S. Alloxan induced diabetes: Mechanisms and effects. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 819–823. [Google Scholar]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef]
- Zeyda, M.; Stulnig, T.M. Obesity, inflammation, and insulin resistance—A mini-review. Gerontology 2009, 55, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Molecular determinants of brown adipocyte formation and function. Genes Dev. 2008, 22, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- Rubin, C.S.; Hirsch, A.; Fung, C.; Rosen, O.M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J. Biol. Chem. 1978, 253, 7570–7578. [Google Scholar]
- Taira, J.; Ogi, T. Induction of Antioxidant Protein HO-1 Through Nrf2-ARE Signaling Due to Pteryxin in Peucedanum Japonicum Thunb in RAW264. 7 Macrophage Cells. Antioxidants 2019, 8, 621. [Google Scholar] [CrossRef]
- Oishi, K.; Ohyama, S.; Higo-Yamamoto, S. Chronic sleep disorder induced by psychophysiological stress induces glucose intolerance without adipose inflammation in mice. Biochem. Biophys. Res. Commun. 2018, 495, 2616–2621. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Okada, N.; Kann, Y.; Arihara, S. Steroidal glycosides from the fresh stem of Stephanotis lutchuensis var. japonica (Asclepiadaceae). Chemical structures of stephanosides AJ. Chem. Pharm. Bull. 1996, 44, 1790–1796. [Google Scholar] [CrossRef][Green Version]
- Drolet, R.; Richard, C.; Sniderman, A.D.; Mailloux, J.; Fortier, M.; Huot, C.; Rheaume, C.; Tchernof, A. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int. J. Obes. (Lond.) 2008, 32, 283–291. [Google Scholar] [CrossRef]
- Xie, M.; Roy, R. AMP-Activated Kinase Regulates Lipid Droplet Localization and Stability of Adipose Triglyceride Lipase in C. elegans Dauer Larvae. PLoS ONE 2015, 10, e0130480. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, D.; Zhuang, X.; Wang, Z.; Ni, Y.; Chen, S.; Sun, F. Berberine increases adipose triglyceride lipase in 3T3-L1 adipocytes through the AMPK pathway. Lipids Health Dis. 2016, 15, 214. [Google Scholar] [CrossRef]
- Deng, T.; Shan, S.; Li, P.P.; Shen, Z.F.; Lu, X.P.; Cheng, J.; Ning, Z.Q. Peroxisome proliferator-activated receptor-gamma transcriptionally up-regulates hormone-sensitive lipase via the involvement of specificity protein-1. Endocrinology 2006, 147, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Schupp, M.; Guan, H.-P.; Gardner, N.P.; Lazar, M.A.; Flier, J.S. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1736–E1745. [Google Scholar] [CrossRef]
- Sembongi, H.; Miranda, M.; Han, G.S.; Fakas, S.; Grimsey, N.; Vendrell, J.; Carman, G.M.; Siniossoglou, S. Distinct roles of the phosphatidate phosphatases lipin 1 and 2 during adipogenesis and lipid droplet biogenesis in 3T3-L1 cells. J. Biol. Chem. 2013, 288, 34502–34513. [Google Scholar] [CrossRef]
- Kim, H.E.; Bae, E.; Jeong, D.-Y.; Kim, M.-J.; Jin, W.-J.; Park, S.-W.; Han, G.-S.; Carman, G.M.; Koh, E.; Kim, K.-S. Lipin1 regulates PPARγ transcriptional activity. Biochem. J. 2013, 453, 49–60. [Google Scholar] [CrossRef]
- Zhang, P.; Takeuchi, K.; Csaki, L.S.; Reue, K. Lipin-1 phosphatidic phosphatase activity modulates phosphatidate levels to promote peroxisome proliferator-activated receptor gamma (PPARgamma) gene expression during adipogenesis. J. Biol. Chem. 2012, 287, 3485–3494. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Peterfy, M.; Reue, K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J. Biol. Chem. 2004, 279, 29558–29564. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fujii, N.; Narita, T.; Higami, Y. SREBP-1c-Dependent Metabolic Remodeling of White Adipose Tissue by Caloric Restriction. Int. J. Mol. Sci. 2018, 19, 3335. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Neither brown nor white. Nature 2012, 488, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, Y.; Nojima, H.; Matsuda, H.; Murakami, T.; Yoshikawa, M.; Kimura, I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J. Asian Nat. Prod. Res. 2000, 2, 321–327. [Google Scholar] [CrossRef]
- Wang, L.; Luo, H.; Miyoshi, M.; Imoto, T.; Hiji, Y.; Sasaki, T. Inhibitory effect of gymnemic acid on intestinal absorption of oleic acid in rats. Can. J. Physiol. Pharmacol. 1998, 76, 1017–1023. [Google Scholar] [CrossRef]
- Liu, H.-M.; Kiuchi, F.; Tsuda, Y. Isolation and structure elucidation of gymnemic acids, antisweet principles of Gymnema sylvestre. Chem. Pharm. Bull. 1992, 40, 1366–1375. [Google Scholar] [CrossRef]
- Asare-Anane, H.; Huang, G.; Amiel, S.; Jones, P.; Persaud, S. Stimulation of insulin secretion by an aqueous extract of Gymnema sylvestre: Role of intracellular calcium. In Proceedings of the 196th Meeting of the Society for Endocrinology and Society for Endocrinology Joint Endocrinology and Diabetes Day, London, UK, 7–9 November 2005. [Google Scholar]
- Libman, A.; Zhang, H.; Ma, C.; Southavong, B.; Sydara, K.; Bouamanivong, S.; Tan, G.T.; Fong, H.H.; Soejarto, D.D. A first new antimalarial pregnane glycoside from Gongronema napalense. Asian J. Tradit. Med. 2008, 3, 203. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).