1. Introduction
Hypercholesterolemia is a metabolic disorder that mainly results in an elevated concentration of plasma low density lipoprotein (LDL) cholesterol [
1]. Hypercholesterolemia has been associated with many cardiovascular diseases, including atherosclerosis, stroke, cerebral paralysis, myocardial infarction [
2] and also inflammation and cancer [
3]. One of the bigger challenges in modern medicine is the identification of a cure for hypercholesterolemia which does not confer side effects. In recent times, plant-sourced products have been considered to be possible novel therapeutic agents as these are considerably less toxic, cost-effective and most importantly, they produce no or relatively lesser side effects as compared to their synthetic counterparts.
Mikania micrantha Kunth originates from the tropical central and southern part of America and is extensively spread in the Pacific region and Southeast Asian countries.
M. micrantha is traditionally used to treat stomach aches, jaundice, respiratory diseases, dysentery and rheumatism. This perennial creeping vine is also consumed as a juice as an alternative medicine for the treatment of diabetes, hypertension and hypercholesterolemia [
4,
5].
M. micrantha were previously reported to possess many health benefits such as antioxidant [
6], anti-diabetic [
7], anti-cancer [
8,
9], antiproliferative [
10], anti-dermatophytic [
11], anti-inflammatory [
12] and antibacterial [
5] activities. These beneficial effects are related to the richness of chemical constituents such as terpenoids, flavonoids, alkaloids and vitamins [
5,
6,
11,
13].
Despite its traditional use, scientific findings to prove the traditional claims of the anti-hypercholesterolemia properties of M. micrantha are limited. For that reason, this study aimed to determine the hypocholesterolemic potential of an ethyl acetate extract of M. micrantha stems (EAMMS) on male high-cholesterol-fed rats by determining the serum lipid profile [TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoproteins cholesterol; LDL-C, low-density lipoproteins cholesterol], lipid peroxidation, enzymatic activities and histopathological evaluation.
2. Materials and Methods
2.1. Reagents and Chemicals
Ethyl acetate (HmbG Chemical, Hamburg, Germany), ethanol (R&M Chemicals), haematoxylin (Sigma-Aldrich, St Louis, MO, USA), simvastatin (Pharmaniaga Logistics (M) Sdn. Bhd, Malaysia), 10% formalin (R&M Chemicals), xylene (R&M Chemicals) and eosin (Leica Biosystems Richmond Inc., Richmond, IL, USA) were used in this study. Pierce BCA Protein Assay Kits for protein quantification were purchased from Thermo Fisher Scientific (Rockford, IL, USA). Malondialdehyde (MDA) assay kits for lipid peroxidation assays were purchased from Elabscience Biotechnology Inc. (Houston, TX, USA). ELISA Kits (HMGCR and ACAT2) were purchased from Sunlong Biotech Co. Ltd. (Zhejiang, China). High cholesterol diet (1%) was purchased from EnvigoTeklad (Cambridgeshire, UK).
2.2. Sample Preparation
Mikania micrantha was collected in August 2017 from Negeri Sembilan, Malaysia (GPS: 2.695652,102.160987) and a plant sample was deposited in the Forest Research Institute Malaysia (FRIM), Kepong, Selangor, Malaysia for taxonomic identification with a voucher specimen number of SBID 051/15. Fresh stems of
M. micrantha was selected and washed and then dried at 35 °C for 72 h in a ventilated drying oven.
M. micrantha powdered stems were then extracted using ethyl acetate and evaporated at 48 °C [
6].
2.3. Animals
Sprague Dawley rats (male, 150–200 g weight) were purchased in this study. The rats were given tap water ad libitum and acclimatized under standardized laboratory circumstances (temperature 22 ± 2 °C; humidity 60 ± 4%; 12 h light-dark cycle). All animals used had received approval from the Universiti Putra Malaysia’s Institutional Animal Care and Use Committee (UPM/IACUC/AUP-R081/2017).
2.4. Animal Experimental Design
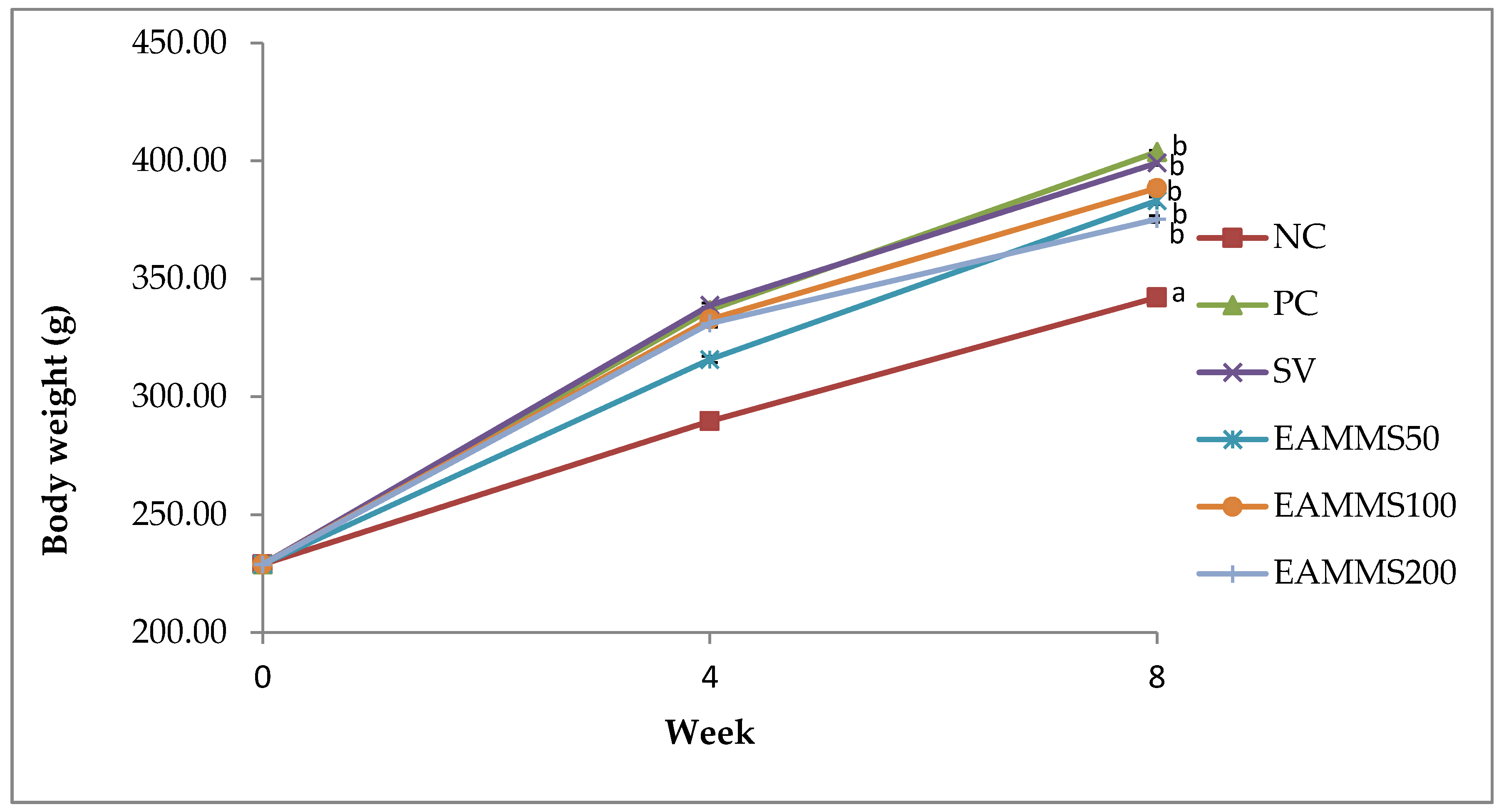
The rats were divided into six groups (n = 6). Group 1 as normal control (NC) was fed with a normal diet for 8 weeks. Group 2 to 6 were orally administered with high cholesterol diet (1%) throughout the study for 8 weeks. After the 4th week of the induction period, Group 2 was served as cholesterol-induced rats (PC). Group 3 was treated by orally administered via gavage with an aqueous suspension of simvastatin, SV (10 mg/kg) and groups of 4, 5 and 6 were orally administered via gavage with EAMMS at the dosage of 50, 100 and 200 mg/kg, respectively, during the treatment periods. The rats were treated for 4 weeks. Upon the administration of the last treatment dose, the animals were left to fast for 18 h. Blood samples were then acquired through the cardiac puncture approach with subjects under anaesthesia (ketamine/xylazine). Then, the blood samples were centrifuged at 3000 rpm at 4 °C for 10 min and the serum was stored at a temperature of −80 °C until the point of the assay. The liver and kidney were excised, weight and also stored at a temperature of −80 °C until the point of analysis.
2.5. Liver, Kidney and Haematogram Profile Analysis
Analysis of both liver and kidney profile including aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea and creatinine were analyzed using a fully automated BiOLiS 24i Premium clinical analyzer (Hitachi, Bolton, UK). The total number of red blood cells (RBC), haemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular haemoglobin volume (MCHC), white blood cell (WBC), icterus index, and plasma protein concentration were analysed.
2.6. Lipid Profile Analysis
The parameters that were analysed included total cholesterol (TC), triglycerides (TG), low density lipoproteins (LDL-C) and high density lipoproteins (HDL-C). The analyses were conducted using a fully automated clinical analyser (BiOLiS 24i Premium, Hitachi).
2.7. Serum Lipid Peroxidation
MDA level was quantified in the blood serum using a rat malondialdehydes (MDA) ELISA kit from Elabscience Biotechnology Inc., (Houston, TX, USA). All the procedures were conducted carefully according to the manufacturer’s instructions. The absorbance was measured at 450 nm.
2.8. Histopathological Examination
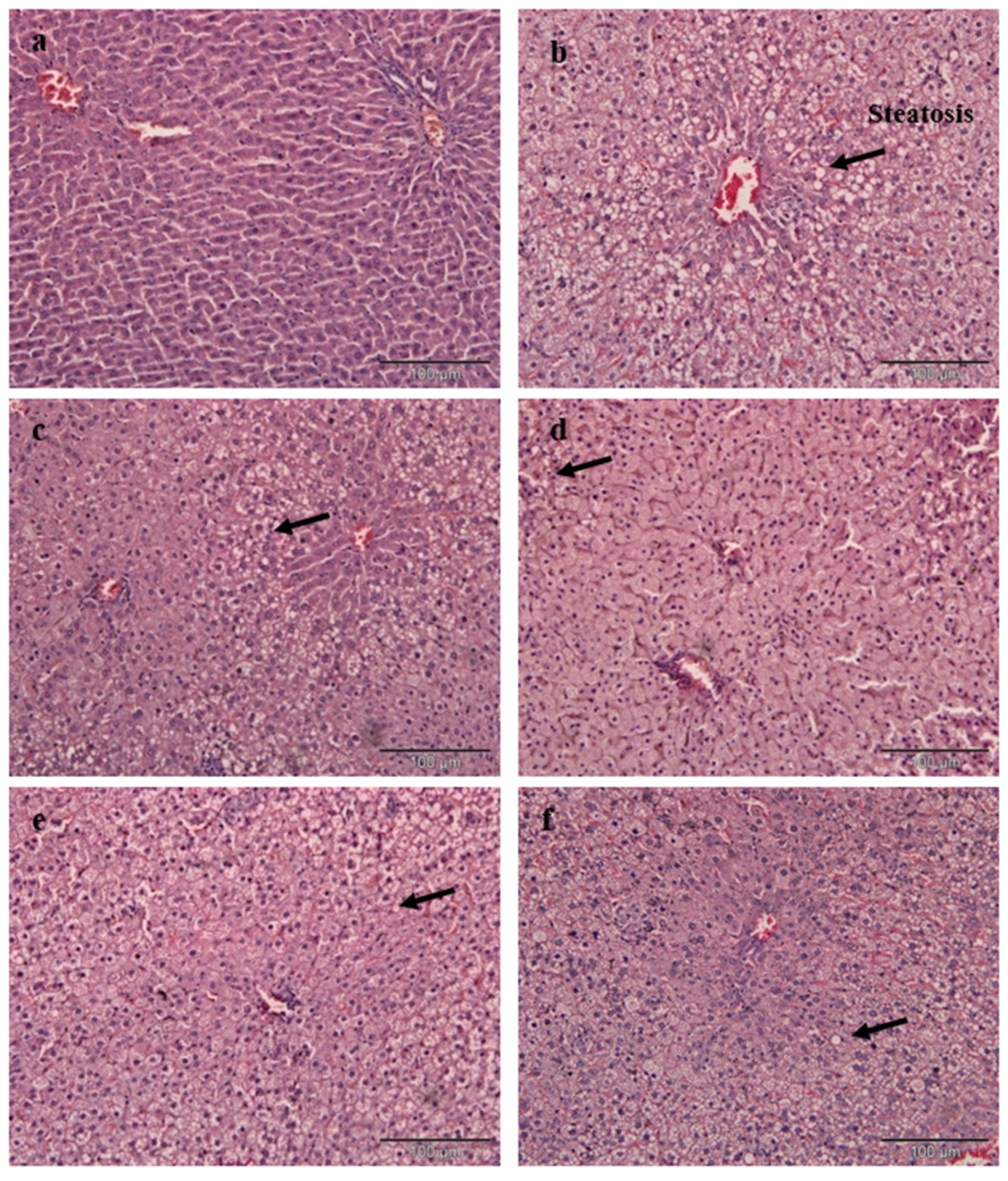
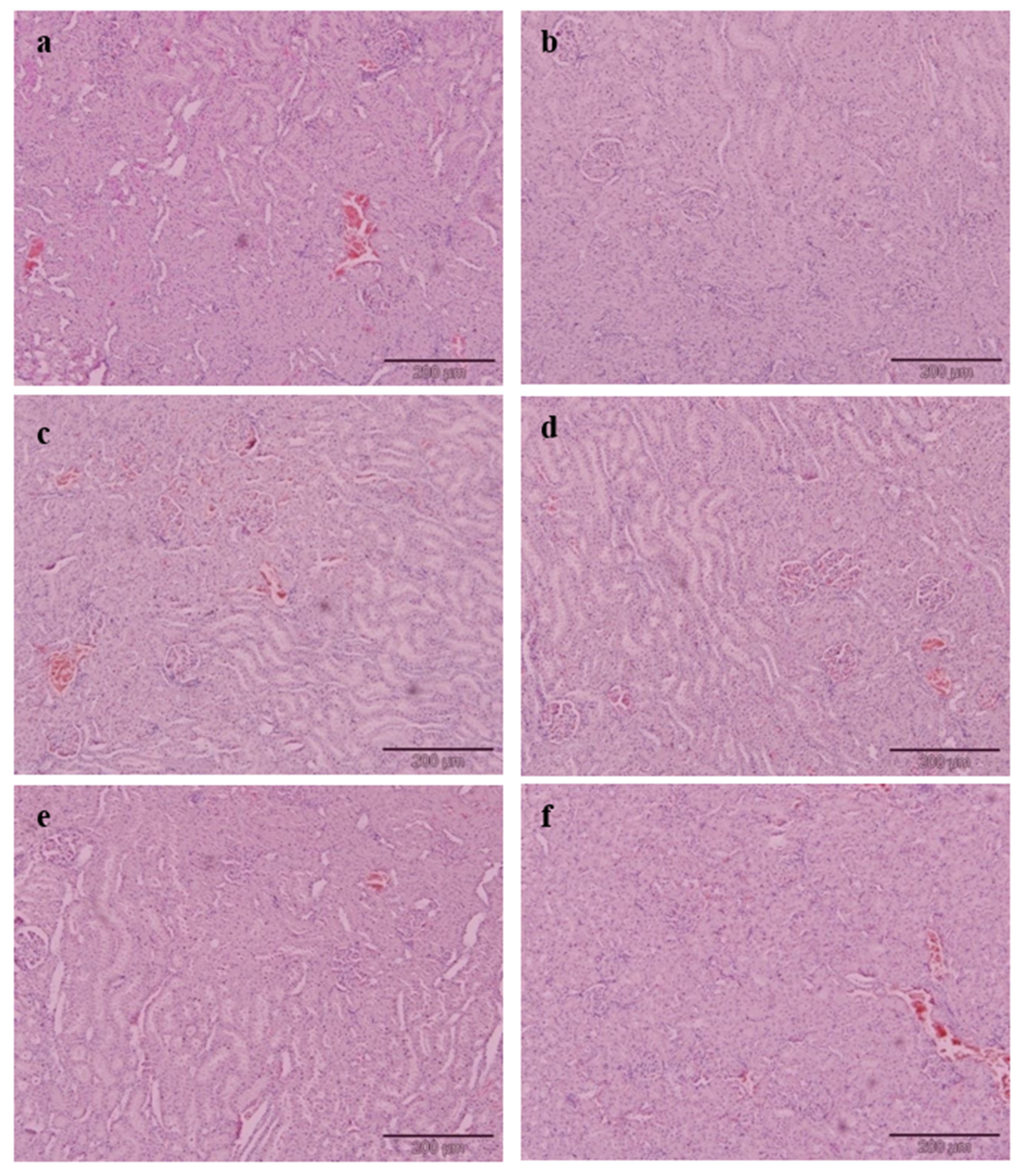
Briefly, 10% formalin buffer solution was used to fix the liver and kidney. Upon fixing, the tissues were subjected to paraffin embedding and being stained with haematoxylin and eosin (H&E) dye. The tissues of interest which have been stained were viewed and analysed using an image analyser. The histology of selected tissues was evaluated qualitatively and quantitatively. The microscopic analysis of all tissue samples was evaluated as a blind study by a pathologist and other researchers. Grading was conducted on the severity of steatosis which afflicted liver tissues and this was done according to the approach as described by Brunt et al. [
14].
2.9. HMGCR and ACAT2 Activity Assays
Protein from liver tissue was homogenized in phosphate buffered saline (PBS, pH 7.4) at 4 °C. The supernatant was then centrifuged at 3500× g with 4 °C for 10 min and quantified with a BCA protein assay kit according to kit instructions. The levels of HMG-CoA reductase (HMGCR) and acetyl-CoA acetyltransferase 2 (ACAT2) enzymes were measured according to the ELISA protocol provided by the manufacturer.
2.10. Statistical Analysis
The data were expressed as mean ± standard error of the mean (SEM) for body and organ weight, haematological parameters, liver and kidney profile, lipid profile, lipid peroxidation and enzymatic analysis. All data were analysed using one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests (p < 0.05).
4. Discussion
Feeding with a cholesterol-enriched diet is one of the most commonly used methods for the induction of hypercholesterolemia in rats [
15]. Administration of a high cholesterol diet to rats produces a marked increase of serum TC, TG and LDL-C as well as body weight when compared to a normal diet. A previous study revealed that a 1% increase in cholesterol intake by rats led to hypercholesterolemia, as evidenced by significant increases in serum TC, TG and LDL-C levels [
16]. These observed changes are akin to those which could be expected when there is an excessive cholesterol load reaching the liver; a load that exceeds normal physiological limits. This will cause the inability of the liver to metabolize lipids thereby resulting in a relatively higher return of cholesterol into the blood circulation [
17]. The relatively high levels of LDL which were found in rats which were fed with the cholesterol-enriched diet may be associated with the downward regulation of low density lipoprotein receptors (LDLR) by saturated fatty acids and dietary cholesterol [
18]. According to Fungwe et al. [
19], the elevation of triglycerides is caused by the cholesterol that was present in the diet which had been shown to diminish the oxidation of fatty acids and in the process, increase triglyceride and hepatic function levels.
Treatment with EAMMS extracts at all dosages showed marked decreases in serum TC, TG, and LDL-C levels and increases of HDL-C compared to the rats in the PC group after 4 weeks of supplementation. This phenomenon was explained by Adaramoye et al. [
20] who reasoned that the decreased levels of cholesterol and triglycerides upon treatment are attributable to the lowering of the biosynthesis of hepatic triglycerides and the redeployment of cholesterol molecules between the molecules of lipoproteins. The effect of EAMMS extracts is also related to the abundance of chemical constituents’ presence in EAMMS extracts such as terpenoids, flavonoids, alkaloids and vitamins [
5,
6,
11]. Terpenoids (particularly sesquiterpene lactones) are the major compounds found in the ethyl acetate extracts of the flowers, leaves and the whole part of
M. micrantha [
11]. The chemical profile of
M. micrantha led to the identification of terpenoids such as stigmasterol, stigmasteryl-β-D-glucopyranoside, acetyl β-amyrin and lupeol [
10]. The structural similarity of stigmasterol or plant sterol with cholesterol makes plant sterols some of the best substances in reducing cholesterol levels in the blood.
Several mechanisms have been postulated to clarify the process of cholesterol reduction by phytosterols or plant sterols. Plant sterols compete with biliary and also dietary cholesterol as an inhibitor to bind with mixed micelles for solubilization of micellar in the upper intestinal lumen that leads to the general reduction in the ability of the intestinal lining in absorbing cholesterol [
21,
22]. This hypothesis is consistent with the postulation byBrufau et al. [
23] which showed that dietary plant sterols can diminish TC and LDL-C in animal and human models. Plant sterol and stanol ester increase LDLR mRNA and ex vivo LDLR protein expression in the monocytes as well as T-lymphocytes of humans and this changes correlated negatively with the changes of concentration of LDL in the blood, it may be postulated that upregulating LDLR expression leads to decreased LDL formation along the apolipoprotein B cascade [
24].
One of the more important methods for diagnosing the cause of disease and the health status of rats is the assessment of haematological parameters. In this study, no significant changes were detected in all haematological values excluding MCV between EAMMS extract, simvastatin and the control groups. However, the administration of the EAMMS caused mild microcytic anemia due to the level of MCV in rat’s blood. MCV represents the average volume of the red blood cells. This abnormal blood condition could be caused by the presence of chemical constituents in plant extracts such as flavonoids and alkaloids saponins. Alkaloids have been shown to cause liver cirrhosis, liver megalocytosis and nodular hyperplasia [
25] while terpenoids increase membrane permeability to divalent and monovalent ions [
25].
Modern toxicology often involves the utilisation of blood sera or tissues as markers to assess damage to organs and cells besides the induction, activation and inhibition of enzymes. The kidneys and liver are organs which play major roles in the detoxification of metabolic substances [
26]. AST and ALT are markers which are normally used to detect and assess injuries onto hepatocytes. It is noted that both these markers are introduced into the bloodstream following incidences where cell damage or necrosis occurs [
27]. From the findings of this study, PC rats exhibited significant increases in AST and ALT levels as compared to NC rats. A similar elevation of AST and ALT levels was observed in hypercholesterolemic rats by the findings of Souza et al. [
28]. In the broader sense, hypercholesterolemia is often associated with toxicity due to heightened levels of liver enzymes as well as the peroxidation of lipids that produces a lot of free radicals in blood sera and tissues [
29,
30].
Rats which were treated with simvastatin recorded significant elevations in AST and ALT levels. It is noteworthy that prominent adverse effects associated with the administration of the statin group are asymptomatic increases in liver transaminases as well as myopathy [
31]. According to Castro et al. [
32], the frequency of liver enzymes increased in a small proportion of those taking statins (2.5%). On the contrary, rats which were supplemented with EAMMS showed no differences in AST and ALT levels as compared to those in the NC group. It can, therefore, be deduced that the EAMMS treatment may suppress the level of AST and ALT in the blood and possibly help in the healing of the hepatic tissue damage, suggesting that plant extract can stabilize the plasma membrane as well as cures the damage of hepatic tissues [
33].
It has been determined that one of the vital mechanisms of cellular damage that are caused by free radicals such as reactive oxygen species (ROS) is lipid peroxidation. One of the products of the peroxidation of lipid is malondialdehyde (MDA) and this is used as an index to indicate oxygen-free radicals’ levels. MDA constitutes the main fraction of aldehydes that are produced upon the metabolism of lipid hydroperoxides and is extensively used to quantify and for the determination of lipid peroxidation. Elevation of serum MDA and TC in the hypercholesterolemic rats without any treatment suggested that the occurrence of lipid peroxidation eventually caused hypercholesterolemia [
34]. According to Yokozawa et al. [
35], decreases in lipid peroxidation could result in a reduction in the probability of hypercholesterolemia. In this present study, treatment with EAMMS extract was found to confer protection against lipid peroxidation in hypercholesterolemia-induced rats. EAMMS-treated rats were used to reduce MDA concentration nearly normal level compared to the rats with high cholesterol diet, therefore suggesting that EAMMS might possess an antioxidant activity owing to the presence of its phenol, alkane hydrocarbons, flavonoids and phytosterol [
6], thus suggesting that EAMMS leads to beneficial response on oxidative stress in hypercholesterolemic rats. Many previous studies have shown that plant polyphenols, flavonoids, carotenoids, vitamins can effectively lower the level of TC, TG, LDL and MDA in hyper-cholesterolemic rodents [
36,
37,
38,
39]. The present results were following the findings of Musolino et al. [
40,
41] that demonstrated that bergamot polyphenolic fraction (BPF) prevented the alteration of lipid profile in hypercholesterolemic rats, counteracting oxidative stress markers and also ameliorate the dysregulation of the lipoprotein metabolism; suggesting that the richness of antioxidant properties may play an important role in improving dyslipidemia.
Cholesterol-enriched diets resulted in a remarkable change of liver histology in the PC group. A large accumulation of lipid droplets within hepatocytes of the liver is known as steatosis and has been observed microscopically. The histopathological result of liver tissue in this study is consistent with the postulation by Zheng et al. [
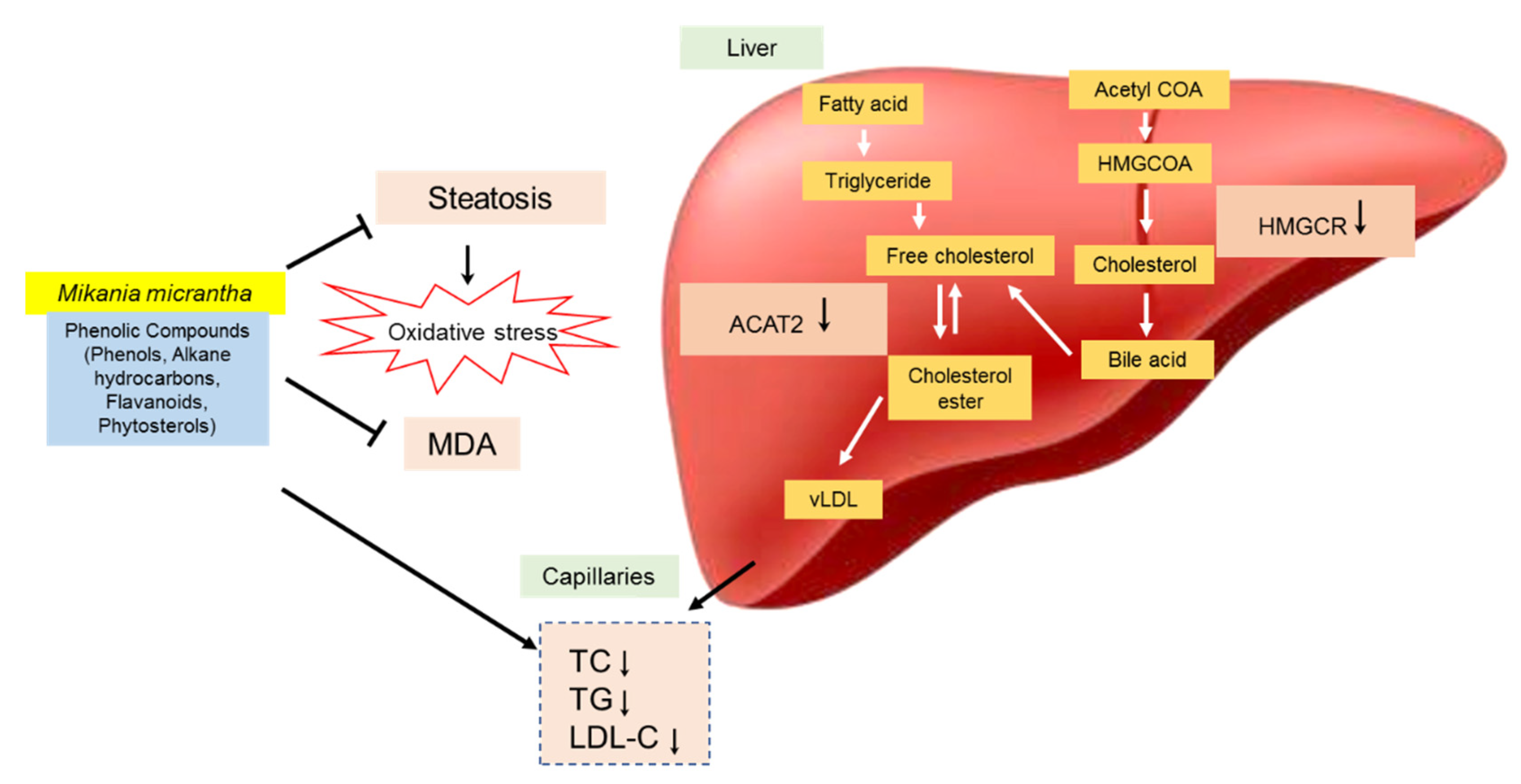
42], who found the same hepatic architecture with the presence of large fat vacuoles in high cholesterol rats. The administration of EAMMS extracts at all dosages and simvastatin (10 mg/kg) ameliorated and reduced the hepatic lipid droplets in hepatocytes of the liver. No histological changes have been observed in the kidney tissues in all the experimental rats. These facts indicated that the EAMMS were able to inhibit the accumulation of fat in the liver due to some flavonoids in the plant extract which down-regulates the enzyme HMG-CoA reductase, the key enzyme in the process of cholesterol biosynthesis. The interaction of bioactive compounds in the plant extract with enzyme-substrate complex caused the changes of the active site of the enzyme, thus prevents the formation of cholesterol. The bioactive compounds in
M. micrantha may help to suppress the HMGCR activity and reduce cholesterol biosynthesis in the mevalonate pathway [
43]. In agreement, the previous study was done by Gliozzi et al. [
44], who also reported that the flavonoids and phenolic compounds in BPF inhibit the endogenous biosynthesis of cholesterol that mediated by HMG-CoA reductase enzyme on the mechanistic action of glycosylated polyphenols (bruteridin and melitidin), which is bind to the catalytic site of HMG-CoA reductase as an endogenous HMG-CoA substrate and causing inhibition of cholesterol synthesis.
The presence of caffeic acid ester, also known as chlorogenic acid such as 3,5-di-O-caffeoylquinic acid n-butyl ester in
M. micrantha is reported [
45,
46]. The present findings suggested that these active compounds also accountable for the cholesterol-lowering activity, possibly mediated by down-regulation of HMGCR and up-regulation of LDL receptor in addition to down-regulating of ACAT2. Karthikesan et al. [
47] also reported that the administration of chlorogenic acid for 45 days strongly reduced the activity of HMGCR and ACAT of lipid metabolism in rats. ACAT2 has important roles in cholesterol esterification, intestinal cholesterol absorption and ApoB-containing lipoprotein release [
48]. It was reported that the inhibition of ACAT2 can effectively inhibit cholesterol absorption and reduce fat levels [
49]. Phytosterols such as stigmasterol and sitosterol showed significantly reduced mRNA expression of ACAT2 activity in rodents [
50]. The mechanism of the hypocholesterolemic action of
M. micrantha extracts is summarized in
Figure 4.