Carrot Pomace Polysaccharide (CPP) Improves Influenza Vaccine Efficacy in Immunosuppressed Mice via Dendritic Cell Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Influenza Vaccine
2.3. Immunization
2.4. Preparation of Food-Derived Polysaccharides
2.5. Bone Marrow-Derived Dendritic Cell (BMDC) Culture
2.6. Mixed Lymphocyte Reaction
2.7. Cytokine Beads Array
2.8. Cell Staining and Flow Cytometry
2.9. Influenza Antigen-Specific Antibody Titer Measurement
2.10. Statistical Analysis
3. Results
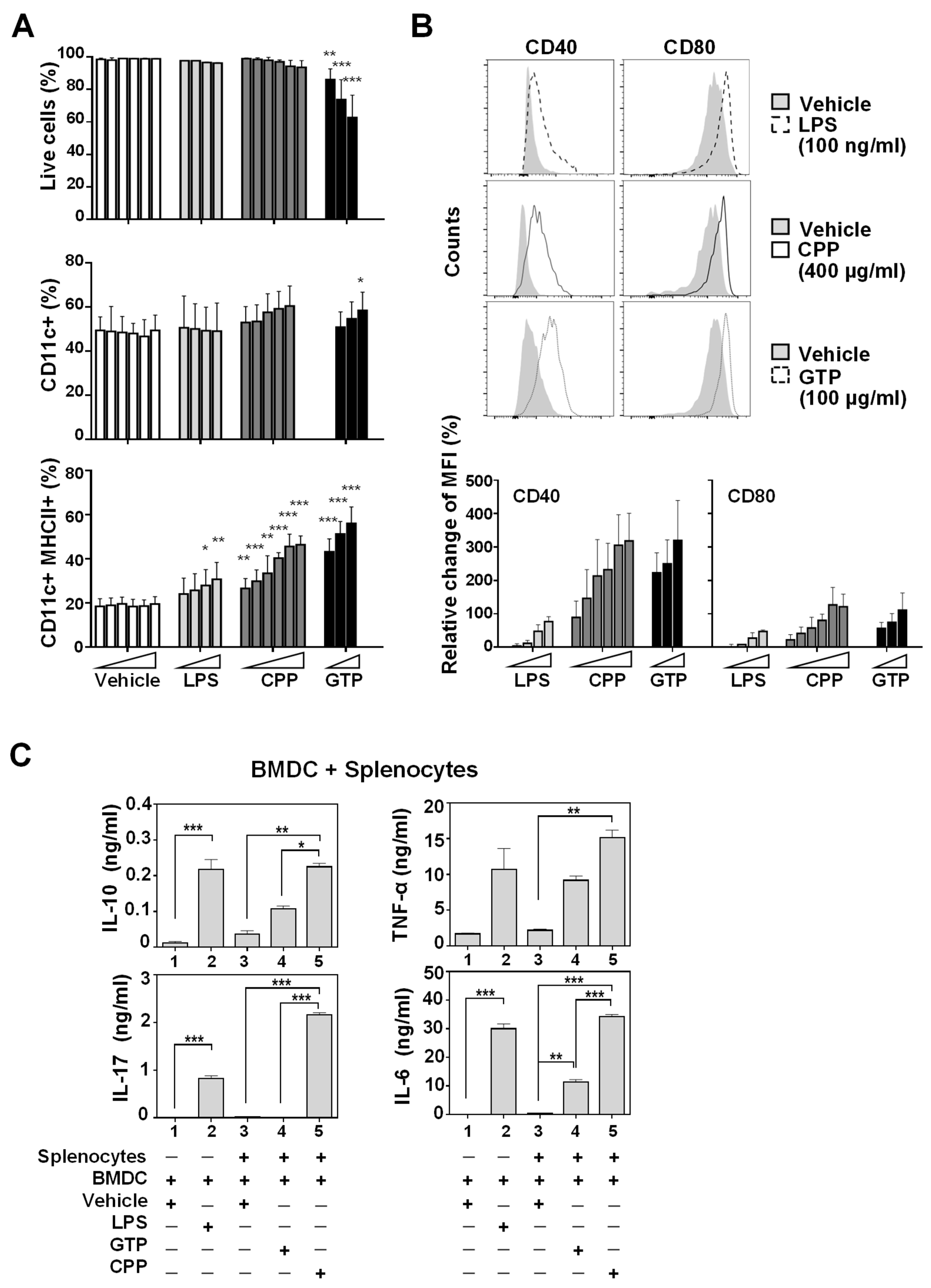
3.1. Bone Marrow-Derived Dendritic Cell (BMDC) Maturation and Activation In Vitro Was Promoted by Food-Derived Polysaccharides
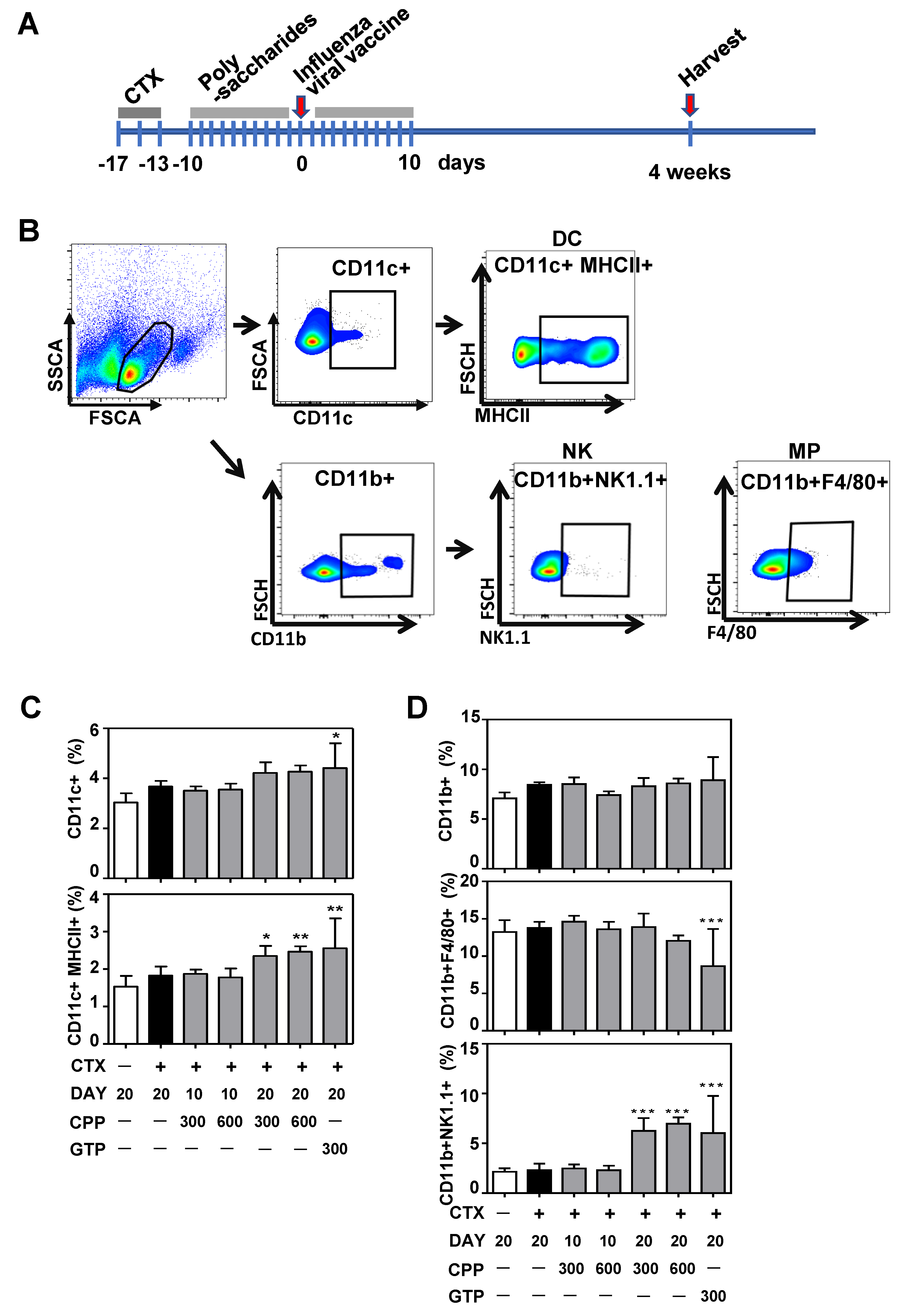
3.2. CPP Increases Dendritic Cell and Natural Killer(NK) Cell Population in Mouse Splenocytes
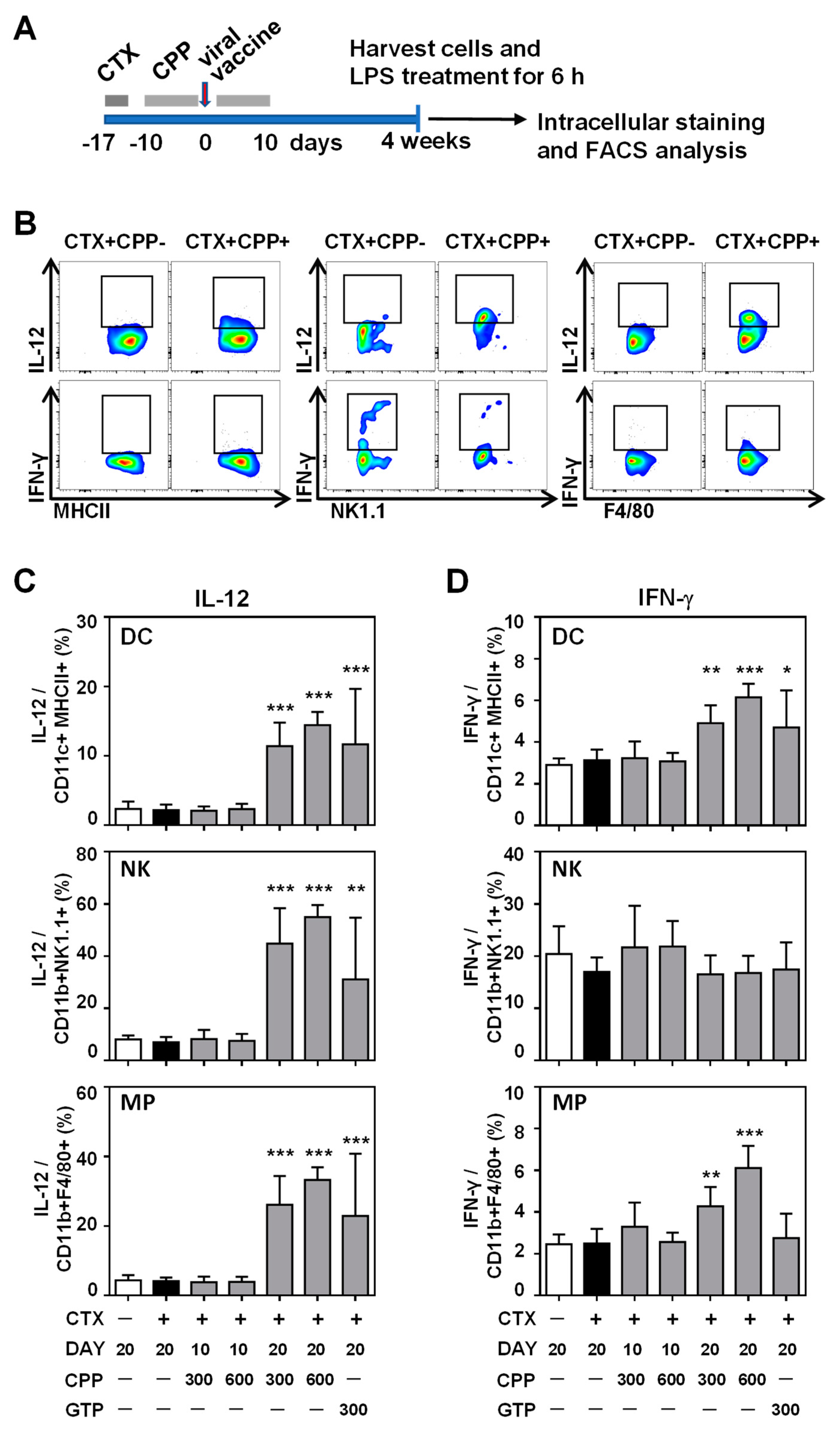
3.3. CPP Enhances the IL-12 Production Ability of Innate Immune Cells
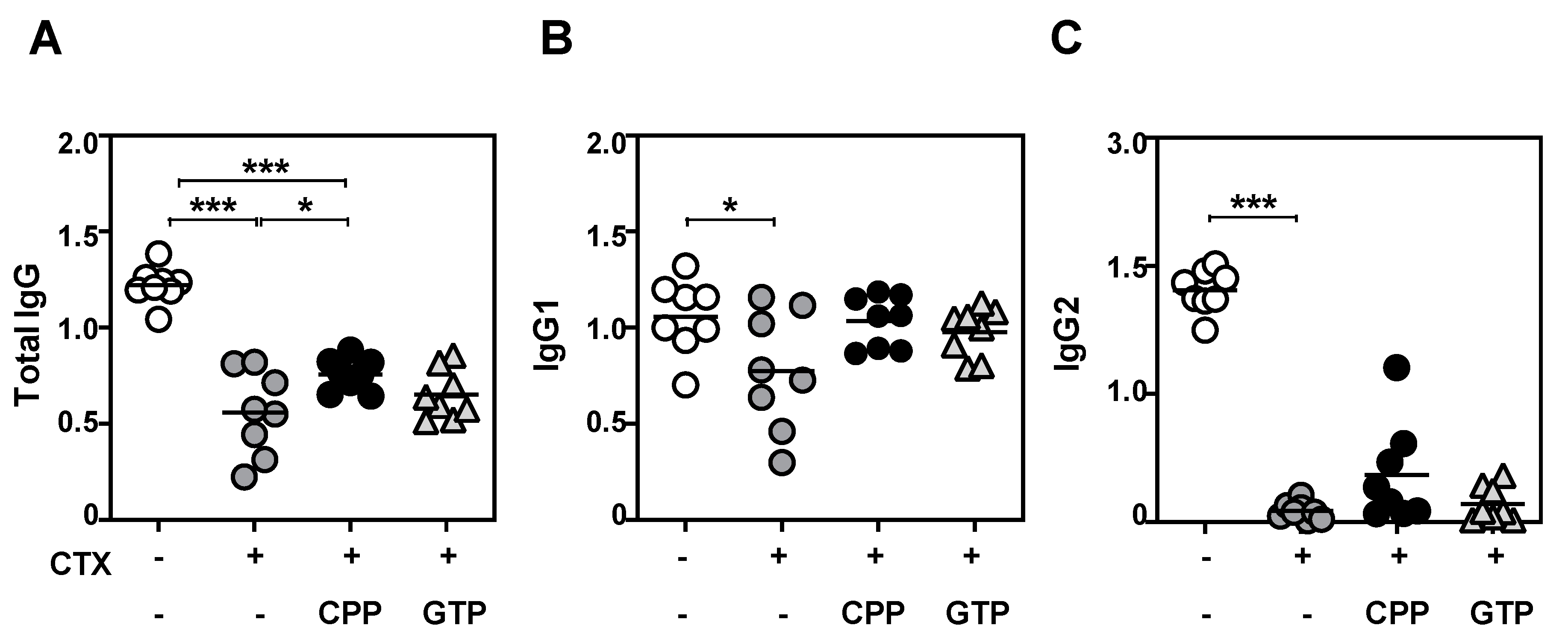
3.4. CPP Treatment Enhanced Antibody Production to Vaccine Challenge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef] [PubMed]
- Zangwill, K.M.; Belshe, R.B. Safety and efficacy of trivalent inactivated influenza vaccine in young children: A summary for the new era of routine vaccination. Pediatric Infect. Dis. J. 2004, 23, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B.; Herndler-Brandstetter, D.; Schwanninger, A.; Weiskopf, D.; Grubeck-Loebenstein, B. Biology of immune responses to vaccines in elderly persons. Clin. Infect. Dis. 2008, 46, 1078–1084. [Google Scholar] [CrossRef]
- Zbinden, D.; Manuel, O. Influenza vaccination in immunocompromised patients: Efficacy and safety. Immunotherapy 2014, 6, 131–139. [Google Scholar] [CrossRef]
- Leroux-Roels, I.; Borkowski, A.; Vanwolleghem, T.; Dramé, M.; Clement, F.; Hons, E.; Devaster, J.-M.; Leroux-Roels, G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: A randomised controlled trial. Lancet 2007, 370, 580–589. [Google Scholar] [CrossRef]
- Petrovsky, N. Freeing vaccine adjuvants from dangerous immunological dogma. Expert Rev. Vaccines 2008, 7, 7–10. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2018; pp. 14–21. [Google Scholar]
- Sun, B.; Yu, S.; Zhao, D.; Guo, S.; Wang, X.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef]
- Yoo, D.-G.; Kim, M.-C.; Park, M.-K.; Park, K.-M.; Quan, F.-S.; Song, J.-M.; Wee, J.J.; Wang, B.-Z.; Cho, Y.-K.; Compans, R.W. Protective effect of ginseng polysaccharides on influenza viral infection. PLoS ONE 2012, 7, e33678. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Y.; Wan, C.; Wang, H.; Hou, L.; Chang, J.; Fan, K.; Xie, X. Immunomodulatory activity and protective effects of polysaccharide from Eupatorium adenophorum leaf extract on highly pathogenic H5N1 influenza infection. Evid.-Based Complementary Altern. Med. 2013, 2013, 194976. [Google Scholar] [CrossRef]
- Proudfoot, O.; Esparon, S.; Tang, C.-K.; Laurie, K.; Barr, I.; Pietersz, G. Mannan adjuvants intranasally administered inactivated influenza virus in mice rendering low doses inductive of strong serum IgG and IgA in the lung. BMC Infect. Dis. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soema, P.C.; Willems, G.-J.; van Twillert, K.; van de Wijdeven, G.; Boog, C.J.; Kersten, G.F.; Amorij, J.-P. Solid bioneedle-delivered influenza vaccines are highly thermostable and induce both humoral and cellular immune responses. PLoS ONE 2014, 9, e92806. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Buttxs, M.S.; Qayyum, M.M.N.; Suleria, H.A.R. Immunity: Plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antitumor activity of polysaccharides: An overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef]
- Dearman, R.J.; Cumberbatch, M.; Maxwell, G.; Basketter, D.A.; Kimber, I. Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology 2009, 126, 475–484. [Google Scholar] [CrossRef]
- Park, H.-R.; Lee, S.J.; Im, S.-B.; Shin, M.-S.; Choi, H.-J.; Park, H.-Y.; Shin, K.-S. Signaling pathway and structural features of macrophage-activating pectic polysaccharide from Korean citrus, Cheongkyool peels. Int. J. Biol. Macromol. 2019, 137, 657–665. [Google Scholar] [CrossRef]
- Sistigu, A.; Viaud, S.; Chaput, N.; Bracci, L.; Proietti, E.; Zitvogel, L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 369–383. [Google Scholar]
- Monteiro, J.M.; Harvey, C.; Trinchieri, G. Role of interleukin-12 in primary influenza virus infection. J. Virol. 1998, 72, 4825–4831. [Google Scholar] [CrossRef]
- Grohmann, U.; Belladonna, M.L.; Bianchi, R.; Orabona, C.; Ayroldi, E.; Fioretti, M.C.; Puccetti, P. IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity 1998, 9, 315–323. [Google Scholar] [CrossRef]
- Wagstaffe, H.R.; Nielsen, C.M.; Riley, E.M.; Goodier, M.R. IL-15 promotes polyfunctional NK cell responses to influenza by boosting IL-12 production. J. Immunol. 2018, 200, 2738–2747. [Google Scholar] [CrossRef]
- Macatonia, S.E.; Hosken, N.A.; Litton, M.; Vieira, P.; Hsieh, C.-S.; Culpepper, J.A.; Wysocka, M.; Trinchieri, G.; Murphy, K.M.; O’Garra, A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995, 154, 5071–5079. [Google Scholar] [PubMed]
- Kang, S.; Brown, H.M.; Hwang, S. Direct antiviral mechanisms of interferon-gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.P.; Cole, G.A.; Nathanson, N. Virus-specific immunologic depression in mice following combined immunization and cyclophosphamide-induced immunosuppression. J. Immunol. 1971, 106, 427–430. [Google Scholar] [PubMed]
- Abou-Donia, H.; Jennings, R.; Potter, C. The spread and persistence of influenza viruses in normal and cyclophosphamide-treated mice. J. Med. Virol. 1981, 7, 251–262. [Google Scholar] [CrossRef]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30. [Google Scholar] [CrossRef]
- Jain, A.; Pasare, C. Innate control of adaptive immunity: Beyond the three-signal paradigm. J. Immunol. 2017, 198, 3791–3800. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.; Bergami-Santos, P.C.; Barbuto, J.A. Human dendritic cells: Their heterogeneity and clinical application potential in cancer immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef]
- Dudek, A.M.; Martin, S.; Garg, A.D.; Agostinis, P. Immature, semi-mature, and fully mature dendritic cells: Toward a DC-cancer cells interface that augments anticancer immunity. Front. Immunol. 2013, 4, 438. [Google Scholar] [CrossRef]
- Durai, V.; Murphy, K.M. Functions of murine dendritic cells. Immunity 2016, 45, 719–736. [Google Scholar] [CrossRef]
- Saade, F.; Honda-Okubo, Y.; Trec, S.; Petrovsky, N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 2013, 31, 1999–2007. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, M.K.; Yun, Y.-P.; Kim, Y.; Kim, J.S.; Kim, Y.S.; Kim, K.; Han, S.S.; Lee, C.-K. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int. Immunopharmacol. 2001, 1, 1275–1284. [Google Scholar] [CrossRef]
- Shao, P.; Zhao, L.-H.; Pan, J.-P. Regulation on maturation and function of dendritic cells by Astragalus mongholicus polysaccharides. Int. Immunopharmacol. 2006, 6, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Law, H.K.W.; Lin, Z.-B.; Lau, Y.L.; Chan, G.C.-F. Response of human dendritic cells to different immunomodulatory polysaccharides derived from mushroom and barley. Int. Immunol. 2007, 19, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Rossol, S.; Marinos, G.; Carucci, P.; Singer, M.V.; Williams, R.; Naoumov, N.V. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J. Clin. Investig. 1997, 99, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, G.; Pack, M.; Thomas, D.; Paludan, C.; Schmid, D.; Strowig, T.; Bougras, G.; Muller, W.A.; Moretta, L.; Münz, C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. USA 2004, 101, 16606–16611. [Google Scholar] [CrossRef]
- O’Garra, A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 1998, 8, 275–283. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Goodarzi, M.; Drayton, D.L.; von Andrian, U.H. T cell–and B cell–independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006, 7, 507–516. [Google Scholar] [CrossRef]
- Paust, S.; Gill, H.S.; Wang, B.-Z.; Flynn, M.P.; Moseman, E.A.; Senman, B.; Szczepanik, M.; Telenti, A.; Askenase, P.W.; Compans, R.W. Critical role for the chemokine receptor CXCR6 in NK cell–mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010, 11, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Gillard, G.O.; Bivas-Benita, M.; Hovav, A.-H.; Grandpre, L.E.; Panas, M.W.; Seaman, M.S.; Haynes, B.F.; Letvin, N.L. Thy1+ NK cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog 2011, 7, e1002141. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Palgen, J.-L.; Tchitchek, N.; Elhmouzi-Younes, J.; Delandre, S.; Namet, I.; Rosenbaum, P.; Dereuddre-Bosquet, N.; Martinon, F.; Cosma, A.; Lévy, Y. Prime and boost vaccination elicit a distinct innate myeloid cell immune response. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Bekkering, S.; Arts, R.J.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.D.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T. Metabolic induction of trained immunity through the mevalonate pathway. Cell 2018, 172, 135–146.e139. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Kim, Y.; Lee, H.; Kim, J.; Han, B.K.; Go, E.; Kwon, S.; Kang, J.-G.; You, S.; Kwon, J. Carrot Pomace Polysaccharide (CPP) Improves Influenza Vaccine Efficacy in Immunosuppressed Mice via Dendritic Cell Activation. Nutrients 2020, 12, 2740. https://doi.org/10.3390/nu12092740
Sun P, Kim Y, Lee H, Kim J, Han BK, Go E, Kwon S, Kang J-G, You S, Kwon J. Carrot Pomace Polysaccharide (CPP) Improves Influenza Vaccine Efficacy in Immunosuppressed Mice via Dendritic Cell Activation. Nutrients. 2020; 12(9):2740. https://doi.org/10.3390/nu12092740
Chicago/Turabian StyleSun, Pureum, Yeeun Kim, Hoyoung Lee, Jihyun Kim, Bok Kyung Han, Eunbyeol Go, Somin Kwon, Ju-Gyeong Kang, Sooseong You, and Jaeyul Kwon. 2020. "Carrot Pomace Polysaccharide (CPP) Improves Influenza Vaccine Efficacy in Immunosuppressed Mice via Dendritic Cell Activation" Nutrients 12, no. 9: 2740. https://doi.org/10.3390/nu12092740
APA StyleSun, P., Kim, Y., Lee, H., Kim, J., Han, B. K., Go, E., Kwon, S., Kang, J.-G., You, S., & Kwon, J. (2020). Carrot Pomace Polysaccharide (CPP) Improves Influenza Vaccine Efficacy in Immunosuppressed Mice via Dendritic Cell Activation. Nutrients, 12(9), 2740. https://doi.org/10.3390/nu12092740





