Antitumoral Properties of the Nutritional Supplement Ocoxin Oral Solution: A Comprehensive Review
Abstract
:1. Introduction
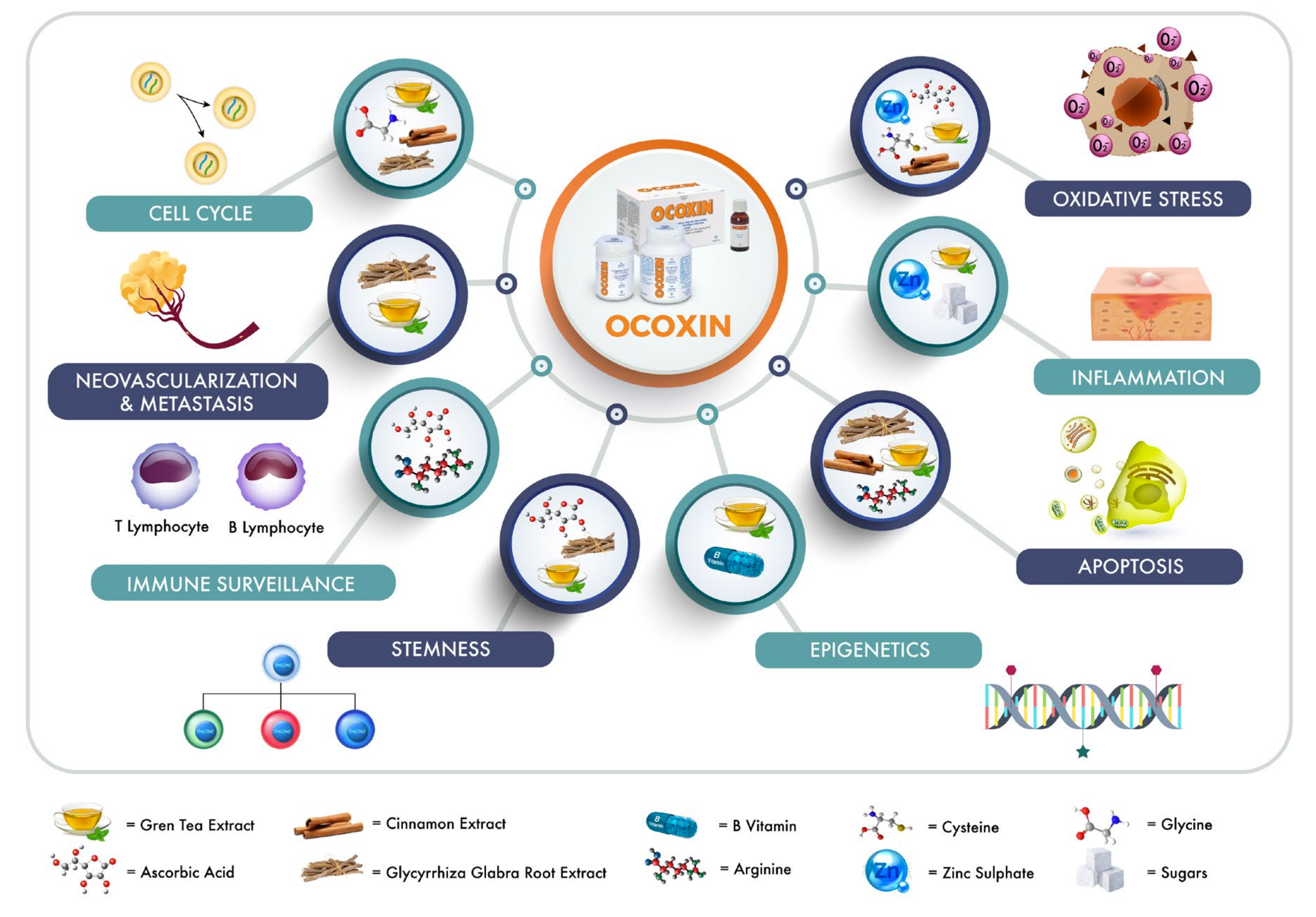
2. OOS Components
2.1. Plant Extracts
2.1.1. Green Tea Extract
2.1.2. Cinnamon Extract
2.1.3. Glycyrrhiza Glabra Root Extract
2.2. Vitamins
2.2.1. Ascorbic Acid
2.2.2. B Vitamins
2.2.3. Amino Acids
2.2.4. Glycine
2.2.5. Arginine
2.2.6. Cysteine
2.3. Sugars
Glucosamine and Sucralose
2.4. Other Components of OOS
3. OOS in Preclinical Models
3.1. Breast Cancer
3.2. Leukemias
3.3. Digestive Tract Neoplasias
3.4. Lung Cancer
3.5. Glioblastoma
4. Clinical Activity of OOS
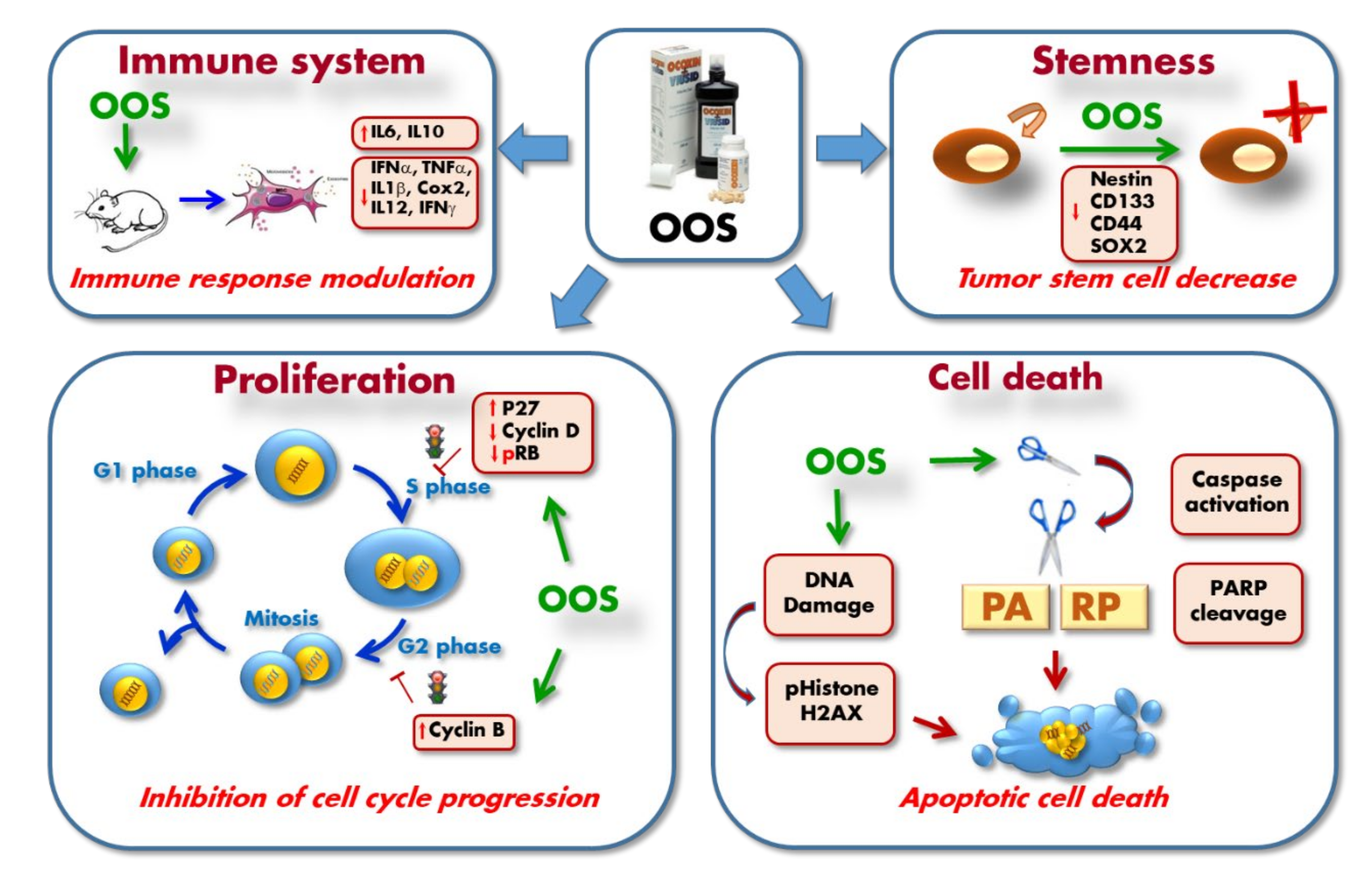
5. Mechanism of Action of OOS
6. Concluding Remarks
7. Methodology of the Review
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin—Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Khalil, A.A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Pharmacological Activities of Epigallocatechin-3-gallate in Signaling Pathways: An Update on Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, P.-L.; Lin, C.-C. Green tea constituent (-)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J. Biomed. Sci. 2003, 10, 219–227. [Google Scholar] [PubMed]
- Umeda, D.; Tachibana, H.; Yamada, K. Epigallocatechin-3-O-gallate disrupts stress fibers and the contractile ring by reducing myosin regulatory light chain phosphorylation mediated through the target molecule 67 kDa laminin receptor. Biochem. Biophys. Res. Commun. 2005, 333, 628–635. [Google Scholar] [CrossRef]
- Shimizu, M.; Adachi, S.; Masuda, M.; Kozawa, O.; Moriwaki, H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol. Nutr. Food Res. 2011, 55, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Yasuda, Y.; Kubota, M.; Adachi, S.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (-)-Epigallocatechin gallate inhibits growth and activation of the VEGF/VEGFR axis in human colorectal cancer cells. Chem. Interact. 2010, 185, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Na, H.-K.; Chun, K.-S.; Kim, Y.-K.; Lee, S.J.; Lee, S.S.; Lee, O.-S.; Sim, Y.-C.; Surh, Y.-J. Inhibition of phorbol ester-induced COX-2 expression by epigallocatechin gallate in mouse skin and cultured human mammary epithelial cells. J. Nutr. 2003, 133 (Suppl. 1), 3805S–3810S. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M.; Deguchi, A.; Lim, J.T.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef] [Green Version]
- Esparís-Ogando, A.; Montero, J.C.; Arribas, J.; Ocana, A.; Pandiella, A. Targeting the EGF/HER Ligand-Receptor System in Cancer. Curr. Pharm. Des. 2016, 22, 5887–5898. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, B.; Song, Z.; Han, S.; Wang, M. Estrogen receptor-alpha36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J. Pharmacol. Sci. 2016, 130, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/beta-catenin pathway mediates (-)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar]
- Nandakumar, V.; Vaid, M.; Katiyar, S.K. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis 2011, 32, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, W.; Fang, S.; Chen, T.; Fang, J.; Mo, Y.; Li, D.; Xiong, S.; Liu, W.; Song, L.; Shen, J.; et al. A novel role of HuR in -Epigallocatechin-3-gallate (EGCG) induces tumour cells apoptosis. J. Cell. Mol. Med. 2019, 23, 3767–3771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, R.O. Historical overview of the cinnamon industry. CRC Crit. Rev. Food Sci. Nutr. 1978, 10, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.-B.; Han, K.-T.; Cho, K.-S.; Ha, J.; Park, H.-J.; Nam, J.-H.; Kil, U.-H.; Lee, K.-T. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Manda, M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol. Int. 2011, 35, 607–615. [Google Scholar] [CrossRef]
- Pal, D.; Banerjee, S.; Mukherjee, S.; Roy, A.; Panda, C.K.; Das, S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: Downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J. Dermatol. Sci. 2010, 59, 31–39. [Google Scholar] [CrossRef]
- Hussain, A.; Brahmbhatt, K.; Priyani, A.; Ahmed, M.; Rizvi, T.A.; Sharma, C. Eugenol enhances the chemotherapeutic potential of gemcitabine and induces anticarcinogenic and anti-inflammatory activity in human cervical cancer cells. Cancer Biother. Radiopharm. 2011, 26, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ka, H.; Park, H.-J.; Jung, H.-J.; Choi, J.-W.; Cho, K.-S.; Ha, J.; Lee, K.-T. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 2003, 196, 143–152. [Google Scholar] [CrossRef]
- Wu, S.-J.; Ng, L.-T.; Lin, C.-C.; Ng, L.-T. Cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells through activation of the proapoptotic Bcl-2 family proteins and MAPK pathway. Life Sci. 2005, 77, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lee, S.H.; Lee, J.W.; Ban, J.O.; Lee, S.Y.; Yoo, H.S.; Jung, J.-K.; Moon, N.C.; Oh, K.W.; Hong, J.T. 2-hydroxycinnamaldehyde inhibits SW620 colon cancer cell growth through AP-1 inactivation. J. Pharmacol. Sci. 2007, 104, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.-A.; Han, D.C.; Son, K.-H.; Han, M.Y.; Lim, J.-S.; Ha, J.-H.; Lee, C.W.; Kim, H.M.; Kim, H.-C.; Kwon, B.-M. Antitumor effect of the cinnamaldehyde derivative CB403 through the arrest of cell cycle progression in the G2/M phase. Biochem. Pharmacol. 2003, 65, 1343–1350. [Google Scholar] [CrossRef]
- Wong, J.H.; Sze, S.C.W.; Ng, T.B.; Cheung, R.C.F.; Tam, C.; Zhang, K.Y.; Dan, X.; Chan, Y.S.; Cho, W.C.S.; Ng, C.C.W.; et al. Apoptosis and Anti-cancer Drug Discovery: The Power of Medicinal Fungi and Plants. Curr. Med. Chem. 2019, 25, 5613–5630. [Google Scholar] [CrossRef]
- Cai, S.; Bi, Z.; Bai, Y.; Zhang, H.; Zhai, D.; Xiao, C.; Tang, Y.; Yang, L.; Zhang, X.; Li, K.; et al. Glycyrrhizic Acid-Induced Differentiation Repressed Stemness in Hepatocellular Carcinoma by Targeting c-Jun N-Terminal Kinase 1. Front. Oncol. 2020, 9, 1431. [Google Scholar] [CrossRef]
- Li, Y.-W.; Yang, F.-C.; Lu, H.-Q.; Zhang, J.-S. Hepatocellular carcinoma and hepatitis B surface protein. World J. Gastroenterol. 2016, 22, 1943–1952. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Chen, S.-Y.; Wu, C.-H.; Lu, C.-C.; Yen, G. Glycyrrhizin Attenuates the Process of Epithelial-to-Mesenchymal Transition by Modulating HMGB1 Initiated Novel Signaling Pathway in Prostate Cancer Cells. J. Agric. Food Chem. 2019, 67, 3323–3332. [Google Scholar] [CrossRef]
- Qiu, M.; Huang, K.; Liu, Y.; Yang, Y.; Tang, H.; Liu, X.; Wang, C.; Chen, H.; Xiong, Y.; Zhang, J.; et al. Modulation of intestinal microbiota by glycyrrhizic acid prevents high-fat diet-enhanced pre-metastatic niche formation and metastasis. Mucosal Immunol. 2019, 12, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, N.J.; Samuel, S.M.; Büsselberg, D. Combination Therapy with Vitamin C Could Eradicate Cancer Stem Cells. Biomolecules 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roa, F.J.; Peña, E.; Gatica, M.; Escobar-Acuña, K.; Saavedra, P.; Maldonado, M.; Cuevas, M.E.; Moraga-Cid, G.; Rivas, C.I.; Muñoz-Montesino, C. Therapeutic Use of Vitamin C in Cancer: Physiological Considerations. Front. Pharmacol. 2020, 11, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Sadzuka, Y. In Vitro Anticancer Activities of B6 Vitamers: A Mini-review. Anticancer Res. 2019, 39, 3429–3432. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vacchelli, E.; Michels, J.; Garcia, P.; Kepp, O.; Senovilla, L.; Vitale, I.; Kroemer, G. Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene 2013, 32, 4995–5004. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Mukherjee, S.; Bhattacharya, A.; Basak, U.; Chakraborty, S.; Paul, S.; Khan, P.; Jana, K.; Hazra, T.K.; Das, T. Pyridoxine enhances chemo-responsiveness of breast cancer stem cells via redox reconditioning. Free. Radic. Biol. Med. 2020, 152, 152–165. [Google Scholar] [CrossRef]
- McGuire, J.J. Anticancer antifolates: Current status and future directions. Curr. Pharm. Des. 2003, 9, 2593–2613. [Google Scholar] [CrossRef]
- Koury, M.J.; Ponka, P. New insights into erythropoiesis: The roles of folate, vitamin B12, and iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef]
- Battezzati, A.; Riso, P. Amino acids: Fuel, building blocks for proteins, and signals. Nutrition 2002, 18, 773–774. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.-L. Amino acid signaling in TOR activation. Annu. Rev. Biochem. 2011, 80, 1001–1032. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Sharma, L.P.; Narayanaswamy, J.C. Investigating the Role of Glutamate in Obsessive-Compulsive Disorder: Current Perspectives. Neuropsychiatr. Dis. Treat. 2020, 16, 1003–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Rusyn, I.; Rose, M.L.; Bojes, H.K.; Thurman, R.G. Novel role of oxidants in the molecular mechanism of action of peroxisome proliferators. Antioxid. Redox Signal 2000, 2, 607–621. [Google Scholar] [CrossRef] [Green Version]
- Rose, M.L.; Madren, J.; Bunzendahl, H.; Thurman, R.G. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis 1999, 20, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Maddocks, O.D.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; Broek, N.J.F.V.D.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.M.; Kruiswijk, F.; et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017, 544, 372–376. [Google Scholar] [CrossRef]
- Al-Koussa, H.; El Mais, N.; Maalouf, H.; Abi-Habib, R.; El-Sibai, M. Arginine deprivation: A potential therapeutic for cancer cell metastasis? A review. Cancer Cell Int. 2020, 20, 150. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Pinzon-Guzman, C.; Barbul, A.; Albaugh, V.L. Arginine-Dual roles as an onconutrient and immunonutrient. J. Surg. Oncol. 2016, 115, 273–280. [Google Scholar] [CrossRef]
- Stechmiller, J.K.; Childress, M.B.; Porter, B.T. Arginine immunonutrition in critically ill patients: A clinical dilemma. Am. J. Crit. Care 2004, 13, 17–23. [Google Scholar] [CrossRef]
- Lind, D.S. Arginine and cancer. J. Nutr. 2004, 134 (Suppl. 10), 2837S–2841S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibbs, J.B., Jr.; Taintor, R.R.; Vavrin, Z. Macrophage cytotoxicity: Role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 1987, 235, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Albina, J.E.; Reichner, J.S. Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev. 1998, 17, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Ezeriņa, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Boil. 2018, 25, 447–459.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, S.L.; White, E.; Kantor, E.D.; Zhang, Y.; Rho, J.; Song, X.; Milne, G.; Lampe, P.D.; Lampe, J.W. Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PLoS ONE 2015, 10, e0117534. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Sanz, G.; Díez-Villanueva, A.; Vilorio-Marqués, L.; Gracia, E.; Aragones, N.; Olmedo-Requena, R.; Llorca, J.; Vidán, J.; Amiano, P.; Nos, P.; et al. Possible role of chondroitin sulphate and glucosamine for primary prevention of colorectal cancer. Results from the MCC-Spain study. Sci. Rep. 2018, 8, 2040. [Google Scholar] [CrossRef] [Green Version]
- Brasky, T.M.; Lampe, J.W.; Slatore, C.G.; White, E. Use of glucosamine and chondroitin and lung cancer risk in the VITamins And Lifestyle (VITAL) cohort. Cancer Causes Control. 2011, 22, 1333–1342. [Google Scholar] [CrossRef] [Green Version]
- Cen, X.; Liu, Y.; Wang, S.; Yang, X.; Shi, Z.; Liang, X. Glucosamine oral administration as an adjunct to hyaluronic acid injection in treating temporomandibular joint osteoarthritis. Oral Dis. 2017, 24, 404–411. [Google Scholar] [CrossRef]
- Chappell, G.; Borghoff, S.; Pham, L.; Doepker, C.; Wikoff, D. Lack of potential carcinogenicity for sucralose—Systematic evaluation and integration of mechanistic data into the totality of the evidence. Food Chem. Toxicol. 2019, 135, 110898. [Google Scholar] [CrossRef]
- Bessler, H.; Djaldetti, M. The impact of three commercial sweeteners on cytokine expression by mononuclears impelled by colon carcinoma cells. Int. J. Food Sci. Nutr. 2019, 70, 970–976. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.J.; Snell, D.C.; Kucuk, O. Zinc in cancer prevention. Nutr. Cancer 2009, 61, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants (Basel) 2019, 8, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangthawan, D.; Phungrassami, T.; Sinkitjarurnchai, W. Effects of zinc sulfate supplementation on cell-mediated immune response in head and neck cancer patients treated with radiation therapy. Nutr. Cancer 2015, 67, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Russell, I.J.; Michalek, J.E.; Flechas, J.D.; Abraham, G.E. Treatment of fibromyalgia syndrome with Super Malic: A randomized, double blind, placebo controlled, crossover pilot study. J. Rheumatol. 1995, 22, 953–958. [Google Scholar]
- Hernandez-Garcia, S.; González, V.; Sanz, E.; Pandiella, A. Effect of Oncoxin Oral Solution in HER2-Overexpressing Breast Cancer. Nutr. Cancer 2015, 67, 1159–1169. [Google Scholar] [CrossRef]
- Pérez-Peña, J.; Díaz-Rodríguez, E.; Sanz, E.; Pandiella, A. Central Role of Cell Cycle Regulation in the Antitumoral Action of Ocoxin. Nutrients 2019, 11, 1068. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Rodriguez, E.; Hernandez-Garcia, S.; Sanz, E.; Pandiella, A. Antitumoral effect of Ocoxin on acute myeloid leukemia. Oncotarget 2016, 7, 6231–6242. [Google Scholar] [CrossRef] [Green Version]
- Márquez, J.; Mena, J.; Hernandez-Unzueta, I.; Benedicto, A.; Sanz, E.; Arteta, B.; Olaso, E. Ocoxin(R) oral solution slows down tumor growth in an experimental model of colorectal cancer metastasis to the liver in Balb/c mice. Oncol. Rep. 2016, 35, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Unzueta, I.; Benedicto, A.; Olaso, E.; Sanz, E.; Viera, C.; Arteta, B.; Márquez, J. Ocoxin oral solution((R)) as a complement to irinotecan chemotherapy in the metastatic progression of colorectal cancer to the liver. Oncol. Lett. 2017, 13, 4002–4012. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Rodriguez, E.; El-Mallah, A.-M.; Sanz, E.; Pandiella, A. Antitumoral effect of Ocoxin in hepatocellular carcinoma. Oncol. Lett. 2017, 14, 1950–1958. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Unzueta, I.; Benedicto, A.; Romayor, I.; Herrero, A.; Sanz, E.; Arteta, B.; Olaso, E.; Márquez, J. Ocoxin Oral Solution Exerts an Antitumoral Effect in Pancreatic Cancer and Reduces the Stromal-Mediated Chemoresistance. Pancreas 2019, 48, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, E.; Sanz, E.; Pandiella, A. Antitumoral effect of Ocoxin, a natural compound-containing nutritional supplement, in small cell lung cancer. Int. J. Oncol. 2018, 53, 113–123. [Google Scholar] [PubMed]
- Hernández-SanMiguel, E.; Gargini, R.; Cejalvo, T.; Segura-Collar, B.; Núñez-Hervada, P.; Hortigüela, R.; Sepúlveda-Sánchez, J.M.; Hernández-Laín, A.; Pérez-Núñez, A.; Sanz, E.; et al. Ocoxin Modulates Cancer Stem Cells and M2 Macrophage Polarization in Glioblastoma. Oxidative Med. Cell. Longev. 2019, 2019, 9719730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, I.C.; Silva, J.A.; Alfonso, G.; Candanedo, H.; Cuervo, Y.; Mestre, B.; Cabello, J.R.M.; Lence, J.; Lugoyo, M.; Sanz, E. Oncoxin-Viusid with radiotherapy and chemotherapy in patients with head and neck cancer: Results from a phase II, randomised, double-blind study. J. Cancer Sci. Ther. 2018, 10, 317–327. [Google Scholar] [CrossRef]
- Kaidarova, D.R.; Kopp, M.V.; Pokrovsky, V.S.; Dzhugashvili, М.; Akimzhanova, Z.M.; Abdrakhmanov, R.Z.; Babich, E.N.; Bilan, E.V.; Byakhov, A.V.; Gurov, S.N.; et al. Multicomponent nutritional supplement Oncoxin and its influence on quality of life and therapy toxicity in patients receiving adjuvant chemotherapy. Oncol. Lett. 2019, 18, 5644–5652. [Google Scholar] [CrossRef] [Green Version]
- Shumsky, A.; Bilan, E.; Sanz, E.; Petrovskiy, F.I. Oncoxin nutritional supplement in the management of chemotherapy- and/or radiotherapy-associated oral mucositis. Mol. Clin. Oncol. 2019, 10, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Lorente, R.R.; Durán, D.H.; Viamontes, J.G.; Anta, J.L.; Reyes, R.O.; Navares, E.S. Efficacy of Oncoxin-Viusid on the Reduction of Adverse Reactions to Chemotherapy and Radiotherapy in Patients Diagnosed with Cervical Cancer and Endometrial Adenocarcinoma. J. Cancer Ther. 2020, 11, 276–295. [Google Scholar] [CrossRef]
- Dzhugashvili, M.; Pokrovsky, V.S.; Snegovoy, A.V. Novel approaches for the correction of micronutrient deficiency in patients with malignant tumors. Malig. Tumours 2016, 2, 55–65. [Google Scholar]
- Al-Mahtab, M.; Akbar, S.M.F.; Khan, M.S.I.; Rahman, S. Increased survival of patients with end-stage hepatocellular carcinoma due to intake of ONCOXIN(R), a dietary supplement. Indian J. Cancer 2015, 52, 443–446. [Google Scholar]
- Lovio, O.R.G.; Daniel, A.A.; Yánez, L.A.G.; Rodríguez, M.O.; González, C.V.; Anta, J.J.L.; Sanz, E. Efficacy and safety of Oncoxin-Viusid, a nutritional supplement, in twenty patients with stage IIB-III of cutaneous melanoma: An open-label proof of concept study. J. Cancer Sci. Ther. 2019, 11, 263–268. [Google Scholar]
- Uddin, D.; Islam, M.; Mahmood, I.; Ghosh, A.; Khatun, R.; Kundu, S. Findings of the 3-month supportive treatment with Oncoxin solution beside the standard modalities of patients with different neoplastic diseases. J. Teach. Assoc. 2009, 22, 172–175. [Google Scholar] [CrossRef] [Green Version]
| Components | Quantity |
|---|---|
| Plant extracts | |
| Glycyrrhiza glabra extract | 200 mg |
| Green tea extract (EGC) | 25 mg |
| Cinnamon extract | 3 mg |
| Vitamins | |
| Ascorbic acid (Vit. C) | 120 mg |
| Pyridoxine (Vit. B6) | 4 mg |
| Cyanocobalamin (Vit. B12) | 2 μg |
| Folic acid (Vit. B9) | 400 μg |
| Calcium pantothenate (Vit. B5) | 12 mg |
| Amino acids | |
| Glycine | 2000 mg |
| Arginine | 640 mg |
| Cysteine | 204 mg |
| Sugars | |
| Glucosamine | 2000 mg |
| Sucralose | 24 mg |
| Other components | |
| Malic acid | 1200 mg |
| Zinc sulfate | 80 mg |
| Manganese sulfate | 4 mg |
| Sodium benzoate | 100 mg |
| Potassium sorbate | 100 mg |
| Maracuya Aroma | 50 mg |
| Study | Findings | Conclusions | Hospitals | Patients | Drugs for Combinations | Reference |
|---|---|---|---|---|---|---|
| OOS given to patients with end-stage hepatocellular carcinoma (HCC) as monotherapy. | Increased survival of patients with end-stage hepatocellular carcinoma due to intake of OOS. | Terminally ill and end stage HCC patients may be managed by food supplements such as OOS and OOS capsules. | Department of Hepatology, Bangabandhu Sheikh Mujib Medical university, Shahbagh, Dhaka, Bangladesh and Department of Medical Sciences, Toshiba General Hospital, Tokio, Japan. | 29 | Monotherapy with OOS and OOS capsules. | NCT 01392131 [79] |
| Patients with terminal stage of hepatocellular carcinoma were studied from the micronutritional point of view after OOS administration. | In patients with terminal stage HCC, OOS improved appetite, quality of life and well-being. OOS also improved overall survival in this group of patients. | The use of micronutrients and aminoacids in cancer patients undergoing chemotherapy is essential to maintain patients quality of life. | Multidisciplinary Oncology Institute, Murcia, Spain. | Not indicated. | Monotherapy with OOS. | [78] |
| Proof of concept study of OOS plus chemo and radiotherapy in patients with Stage IIB-III of cutaneous melanoma. | OOS showed a good safety profile in patients with stage IIB-III cutaneous melanoma. Patients kept a stable quality of life at the end of study and a high progression-free survival rate. | OOS increased progression-free survival rate in cutaneous melanoma. | Department of Dermatology and Infectious Diseases, Manuel Fajardo University Hospital, La Habana, Cuba. Department of Oncology, National Institute of Oncology (INOR), La Habana, Cuba | 20 | Surgery, Interferon, Paclitaxel and Temozolomide. | NCT 03541148 [80] |
| OOS administered with chemo and radiotherapy in gastric cancer IIB-IIIC and non-small cell lung cancer IIB-IIIA was studied from the point of view of quality of life and toxicities induced by cancer therapies. | Toxicity and side effects due to chemotherapy were diminished. | OOS helps to maintain appetite, body mass and quality of life in patients with advanced cancer treated with chemotherapy. | Kazakh Research Institute of Oncology and Radiology, Almaty, Republic of Kazakhstan. Department of Pharmacology and Clinical Pharmacology, Khanty-Mansiysk State Medical Academy, Khanty-Mansiysk, Russia. Department of Chemotherapy, Moscow Clinical Scientific Center n. a. A.S. Loginov, Moscow. | 133 | Xelox or paclitaxel plus carboplatin. | NCT 03550482 [75] |
| Phase II randomised double-blind study of patients with head and neck cancer which undergo cancer protocol therapies associated with OOS. | OOS during radiotherapy or concomitant with chemotherapy in patients with head and neck cancer, improved the quality of life and decreased the number and level of toxicities from these treatments without interfering with their mechanism of action. | OOS had a positive effect on quality of life in patients treated with radio/chemotherapy. | Department of Otolaryngology, Radiotherapy and Chemotherapy, National Institute of Oncology and Radiobiology (INOR), La Habana, Cuba. | 60 | Radiotherapy and Cisplatin. | NCT 03541772 [74] |
| OOS in the Management of chemotherapy – and or radiotherapy-associated oral mucositis. | OOS rapidly improved oral mucositis, as measured by the WHO Oral Toxicity Scale, maintained body mass and decreased the toxicity of anticancer therapy. | OOS helped to maintain normal eating habits and decreased side effects of radio/chemotherapy. | Medical Scientific Centre of Professor Shumsky, Samara, Russia. Khanty-Mansiysk Regional Hospital, Khanty-Mansiysk, Russia. | 15 | Monotherapy with OOS. | NCT 03577535 [76] |
| Findings of the 3 months supportive treatment with OOS beside the standard modalities of patients with different neoplastic diseases. | OOS along with protocol anticancer therapy improved the quality of life of patients with head and neck, breast and cervix cancer. OOS decreased episodes of depression and increased optimism. OOS reduced toxicities due to chemo and radiotherapies and increased survival rates. | The administration of OOS along with conventional chemo and radiotherapy lead to relevant anti-tumor synergy as well as to the inhibition of conventional therapy’s toxicity. | Department of Radiotherapy, Rajshahi Medical College, Rajshahi, Bangladesh. | 90 | Conventional radiotherapy and chemotherapy for head and neck, breast and cervix cancer. | [81] |
| Efficacy of OOS-VIUSID on the reduction of adverse reactions to chemotherapy and radiotherapy in patients diagnosed with cervical cancer and endometrial adenocarcinoma. | OOS-VIUSID significantly reduced the number of patients who suffered adverse events to onco-specific treatment. OOS-VIUSID stopped the fall in haemoglobin levels, and platelet and leukocyte counts, compared to patients receiving traditional treatment. | The administration of OOS along with chemo and radiotherapy lead to inhibition of conventional therapy’s toxicity and consequently less interruption of the cancer treatments. | Hospital Ramón González Coro, La Habana, Cuba. National Institute of Oncology and Radiobiology (INOR), La Habana, Cuba. | 63 | Radiotherapy and Cisplatin | NCT 03540407 [77] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandiella-Alonso, A.; Díaz-Rodríguez, E.; Sanz, E. Antitumoral Properties of the Nutritional Supplement Ocoxin Oral Solution: A Comprehensive Review. Nutrients 2020, 12, 2661. https://doi.org/10.3390/nu12092661
Pandiella-Alonso A, Díaz-Rodríguez E, Sanz E. Antitumoral Properties of the Nutritional Supplement Ocoxin Oral Solution: A Comprehensive Review. Nutrients. 2020; 12(9):2661. https://doi.org/10.3390/nu12092661
Chicago/Turabian StylePandiella-Alonso, Atanasio, Elena Díaz-Rodríguez, and Eduardo Sanz. 2020. "Antitumoral Properties of the Nutritional Supplement Ocoxin Oral Solution: A Comprehensive Review" Nutrients 12, no. 9: 2661. https://doi.org/10.3390/nu12092661
APA StylePandiella-Alonso, A., Díaz-Rodríguez, E., & Sanz, E. (2020). Antitumoral Properties of the Nutritional Supplement Ocoxin Oral Solution: A Comprehensive Review. Nutrients, 12(9), 2661. https://doi.org/10.3390/nu12092661




