Lactoferrin from Bovine Milk: A Protective Companion for Life
Abstract
1. Introduction
2. Bioavailability, Metabolism, Absorption and Delivery of Bovine Lactoferrin
3. Lactoferrin, Iron, Oxidative Stress and Anaemia
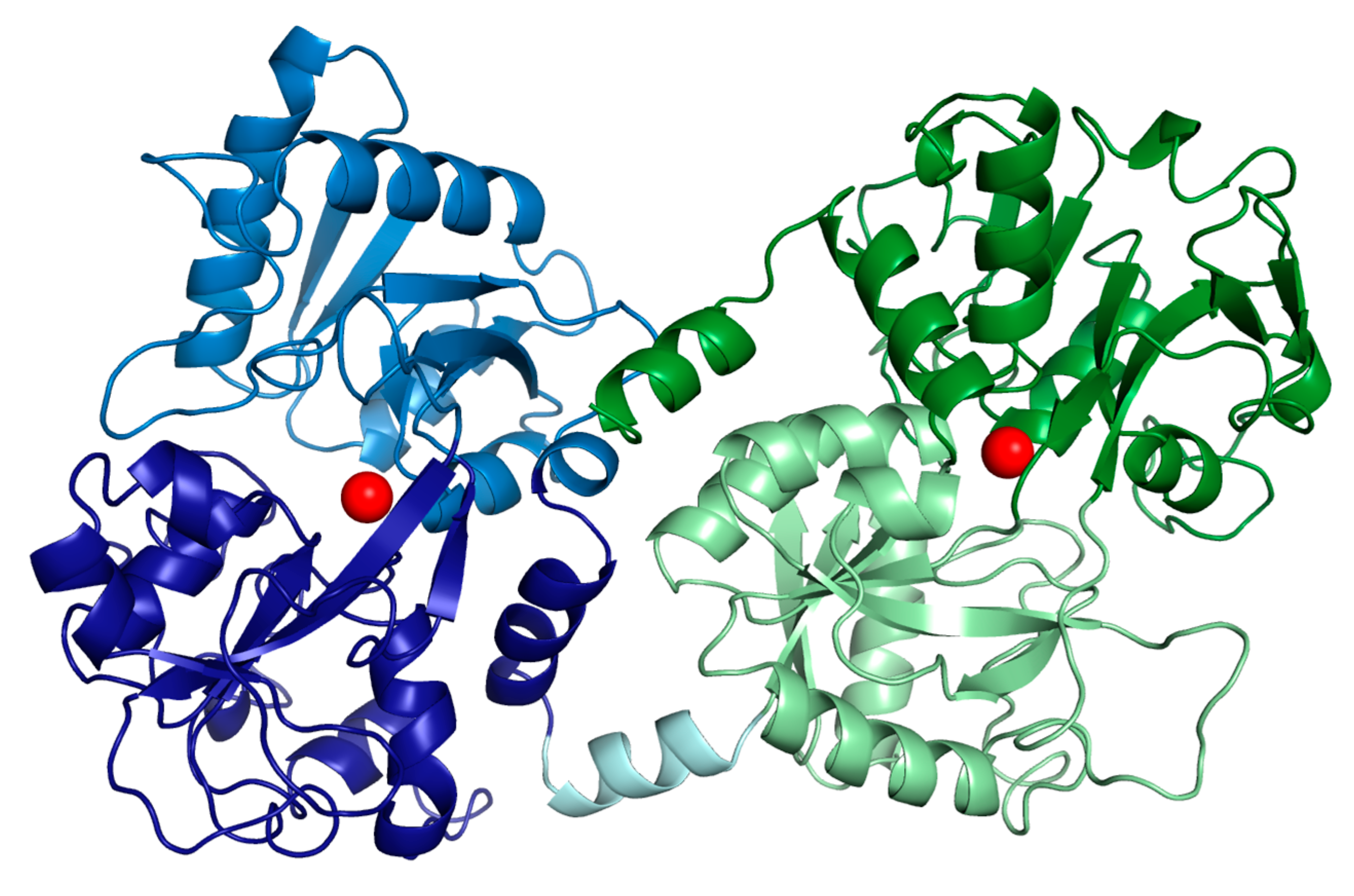
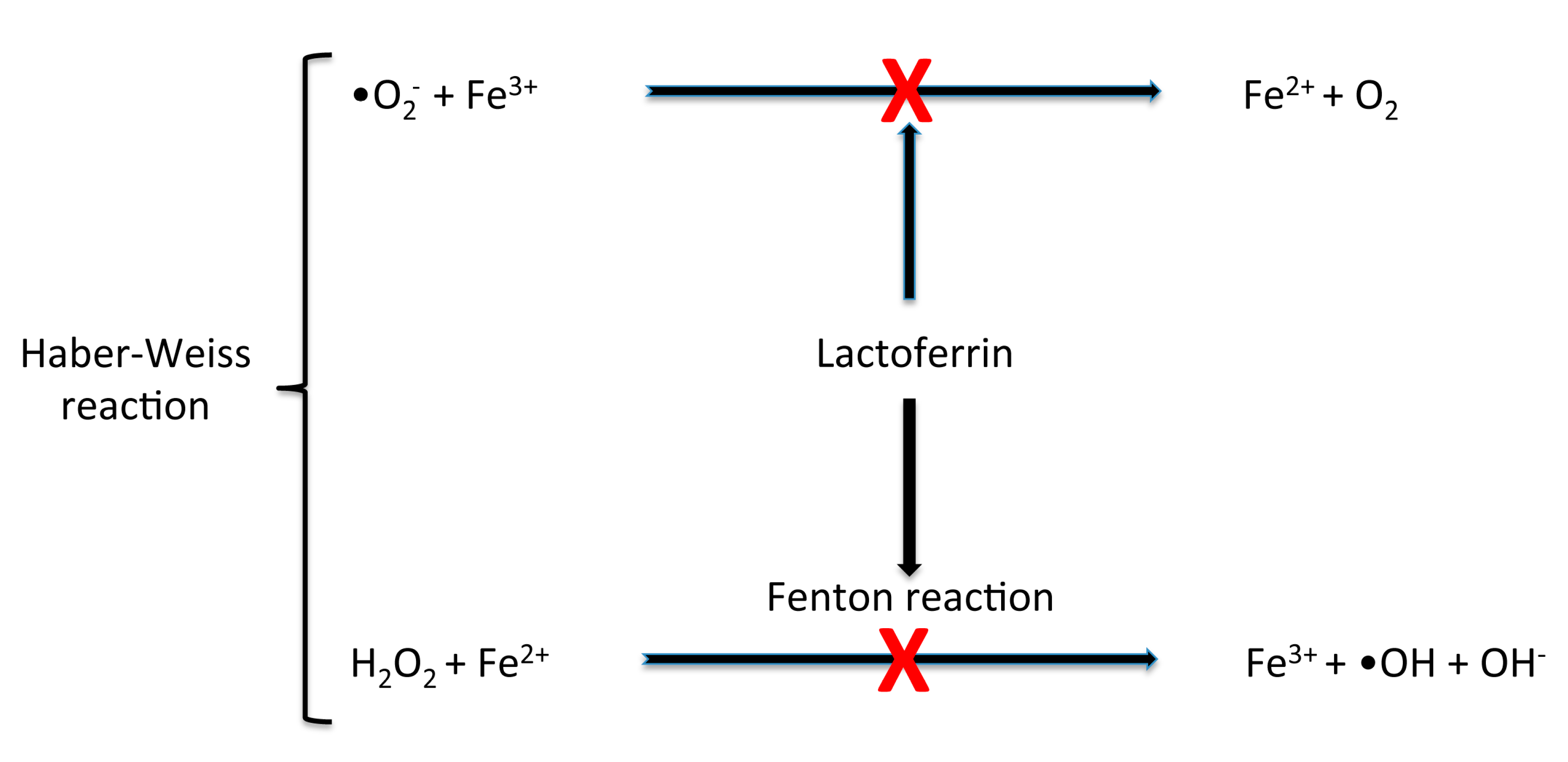
3.1. bLf Protection against Iron Deregulation and Oxidative Stress
3.2. bLf in the Prevention and Treatment of Iron Deficiency Anaemia
4. Lactoferrin in the Defences of the Babies: Decreased Risk of Sepsis and Necrotizing Enterocolitis in Preterm Infants
5. Antimicrobial Activity of bLf
6. Anti-Inflammatory Activity of bLf
6.1. Lactoferrin and Dermatitis
6.2. Lactoferrin and Inflammatory Bowel Diseases
6.3. Lactoferrin and Pulmonary Inflammation Disorders
6.4. Lactoferrin and Hepatitis
7. Anticancer Activity of bLf
8. Other Therapeutic Properties of Lactoferrin
8.1. Lactoferrin and Obesity
8.2. Lactoferrin and Bone Metabolism
8.3. Lactoferrin and Dry Eye Disease
9. bLf on the Market
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorensen, M.; Sorensen, S.P.L. The proteins in whey. Comptes-rendus des Trav. du Lab. Carlsberg Ser. Chim. 1939, 23, 55–99. [Google Scholar]
- Groves, M.L. The isolation of a red protein from milk. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar] [PubMed]
- Iyer, S.; Lönnerdal, B. Lactoferrin, lactoferrin receptors and iron metabolism. Eur. J. Clin. Nutr. 1993, 47, 232–241. [Google Scholar] [PubMed]
- Johanson, B. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a pleiotropic protein in health and disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Sekine, K.; Fujita, K.; Iigo, M. Cancer prevention by bovine lactoferrin and underlying mechanisms—A review of experimental and clinical studies. Biochem. Cell Biol. 2002, 80, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Iyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional roles of lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Vorland, L.H. Lactoferrin: A multifunctional glycoprotein. APMIS 1999, 107, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer effects of lactoferrin: Underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a context of inflammation-induced pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Jiang, R.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and holo- lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells. J. Cell Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.Y.; Wen, J. Oral Delivery of Bovine Lactoferrin Using Pectin-and Chitosan-Modified Liposomes and Solid Lipid Particles: Improvement of Stability of Lactoferrin. Chem. Biol. Drug Des. 2015, 86, 466–475. [Google Scholar] [CrossRef]
- Buccigrossi, V.; de Marco, G.; Bruzzese, E.; Ombrato, L.; Bracale, I.; Polito, G.; Guarino, A. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr. Res. 2007, 61, 410–414. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive Proteins in Human Milk: Health, Nutrition, and Implications for Infant Formulas. J. Pediatr. 2016, 173 (Suppl. S4–S9). [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M.J. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. Appl. Bacteriol. 1992, 73, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, M.I.; Nazmi, K.; Teeken, A.; Groenink, J.; van’t Hof, W.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol. Chem. 2005, 386, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Vorland, L.H.; Ulvatne, H.; Andersen, J.; Haukland, H.H.; Rekdal, Ø.; Svendsen, J.S.; Gutteberg, T.J. Lactoferricin of bovine origin is more active than lactoferricins of human, murine and caprine origin. Scand. J. Infect. Dis. 1998, 30, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Flores-Villaseñor, H.; Canizalez-Román, A.; Reyes-Lopez, M.; Nazmi, K.; de la Garza, M.; Zazueta-Beltrán, J.; León-Sicairos, N.; Bolscher, J.G. Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 2010, 23, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Bellamy, W.; Takase, M.; Tomita, M. Inactivation of Listeria monocytogenes by lactoferricin, a potent antimicrobial peptide derived from cow’s milk. J. Food Prot. 1992, 55, 238–240. [Google Scholar]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, L.T.; Berge, G.; Sveinbjørnsson, B.; Svendsen, J.S.; Vorland, L.H.; Rekdal, Ø. Evidence for a direct antitumor mechanism of action of bovine lactoferricin. Anticancer Res. 2002, 22, 2703–2710. [Google Scholar] [PubMed]
- Chen, H.Y.; Mollstedt, O.; Tsai, M.H.; Kreider, R.B. Potential clinical applications of multi-functional milk proteins and peptides in cancer management. Curr. Med. Chem. 2014, 21, 2424–2437. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell Biol. 2017, 95, 99–109. [Google Scholar] [CrossRef]
- Yan, D.; Chen, D.; Shen, J.; Xiao, G.; van Wijnen, A.J.; Im, H.J. Bovine lactoferricin is anti-inflammatory and anti-catabolic in human articular cartilage and synovium. J. Cell. Physiol. 2013, 228, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides and their health promoting effects: A potential role in atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and peptide-derivatives: Antimicrobial agents with potential use in nonspecific immunity modulation. Curr. Pharm. Des. 2018, 24, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.Y.; Wen, J. Oral Delivery of Lactoferrin: A Review. Int. J. Pept. Res. Ther. 2013, 19, 125–134. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Mild thermal treatment and in-vitro digestion of three forms of bovine lactoferrin: Effects on functional properties. Int. Dairy J. 2017, 64, 22–30. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Liu, W.; Liu, C.; Singh, H. Stability during in vitro digestion of lactoferrin-loaded liposomes prepared from milk fat globule membrane-derived phospholipids. J. Dairy Sci. 2013, 96, 2061–2070. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef]
- Fleming, R.E.; Bacon, B.R. Orchestration of Iron Homeostasis. N. Engl. J. Med. 2005, 352, 1741–1744. [Google Scholar] [CrossRef]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta. Mol. Cell. Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef]
- Zimecki, M.; Mazurier, J.; Spik, G.; Kapp, J.A. Human lactoferrin induces phenotypic and functional changes in murine splenic B cells. Immunology 1995, 86, 122–127. [Google Scholar] [PubMed]
- Zimecki, M.; Kocieba, M.; Kruzel, M. Immunoregulatory activities of lactoferrin in the delayed type hypersensitivity in mice are mediated by a receptor with affinity to mannose. Immunobiology 2002, 205, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Zimecki, M.; Wise, J.; Ploszaj, P.; Mirza, S.; Kruzel, M.; Hwang, S.A.; Ba, X.; Boldogh, I. Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression. J. Biotechnol. 2013, 168, 666–675. [Google Scholar] [CrossRef]
- Okazaki, Y.; Kono, I.; Kuriki, T.; Funahashi, S.; Fushimi, S.; Iqbal, M.; Okada, S.; Toyokuni, S. Bovine lactoferrin ameliorates ferric nitrilotriacetate-induced renal oxidative damage in rats. J. Clin. Biochem. Nutr. 2012, 51, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Gozzellino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Waalen, J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood 2006, 107, 1747–1750. [Google Scholar] [CrossRef]
- Scholl, T.O. Maternal iron status: Relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr. Rev. 2011, 69, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr. Bull. 2007, 28, S621–S627. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef] [PubMed]
- Fransson, G.B.; Keen, C.L.; Lönnerdal, B. Supplementation of milk with iron bound to lactoferrin using weanling mice: L. Effects on hematology and tissue iron. J. Pediatr. Gastroenterol. Nutr. 1983, 2, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell. Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Pantanella, F.; Pacifici, E.; Goolsbee, W.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. Biometals 2010, 23, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Nappi, C.; Tommaselli, G.A.; Morra, I.; Massaro, M.; Formisano, C.; Di Carlo, C. Efficacy and tolerability of oral bovine lactoferrin compared to ferrous sulfate in pregnant women with iron deficiency anemia: A prospective controlled randomized study. Acta Obstet. Gynecol. Scand. 2009, 88, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Abu Hashim, H.; Foda, O.; Ghayaty, E. Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: A meta-analysis of randomized trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 219, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Koikawa, N.; Nagaoka, I.; Yamaguchi, M.; Hamano, H.; Yamauchi, K.; Sawaki, K. Preventive effect of lactoferrin intake on anemia in female long distance runners. Biosci. Biotechnol. Biochem. 2008, 72, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Lan, Z.; Hua, L.; Ying, Z.; Humina, X.; Jia, S.; Weizheng, T.; Ping, Y.; Lingying, C.; Meng, M. Iron metabolism in infants: Influence of bovine lactoferrin from iron-fortified formula. Nutrition 2015, 31, 304–309. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Pezo, A.; Cruz, K.; Chea-Woo, E.; Cleary, T.G. Clinical studies of lactoferrin in children. Biochem. Cell. Biol. 2012, 90, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C. Iron Nutriture of the Fetus, Neonate, Infant, and Child. Ann. Nutr. Metab. 2017, 71 (Suppl. 3), 8–14. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, Italian Society of Neonatology. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef]
- Manzoni, P.; Meyer, M.; Stolfi, I.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Decembrino, L.; Laforgia, N.; et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial. Early Hum. Dev. 2014, 90 (Suppl. 1), S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Akin, I.M.; Atasay, B.; Dogu, F.; Okulu, E.; Arsan, S.; Karatas, H.D.; Ikinciogullari, A.; Turmen, T. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am. J. Perinatol. 2014, 31, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Gathwala, G. Efficacy of Bovine Lactoferrin Supplementation in Preventing Late-onset Sepsis in low Birth Weight Neonates: A Randomized Placebo-Controlled Clinical Trial. J. Trop. Pediatr. 2015, 61, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Zegarra, J.; Cam, L.; Llanos, R.; Pezo, A.; Cruz, K.; Zea-Vera, A.; Carcamo, C.; Campos, M.; Bellomo, S. Randomized controlled trial of lactoferrin for prevention of sepsis in peruvian neonates less than 2500 g. Pediat. Infect. Dis. J. 2015, 34, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.P.; Alexander, T.J. Reduction in necrotizing enterocolitis and improved outcomes in preterm infants following routine supplementation with Lactobacillus GG in combination with bovine lactoferrin. Neonatal Perinatal Med. 2017, 10, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; García Sánchez, R.; Meyer, M.; Stolfi, I.; Pugni, L.; Messner, H.; Cattani, S.; Betta, P.M.; Memo, L.; Decembrino, L.; et al. Italian Task Force for the Study, and Prevention of Neonatal Fungal Infections and the Italian Society of Neonatology. Exposure to gastric acid inhibitors increases the risk of infection in preterm very low birth weight infants but concomitant administration of lactoferrin counteracts this effect. J. Pediatr. 2018, 193, 62–67.e1. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Higgins, R.D.; Fanaroff, A.A.; Duara, S.; Goldberg, R.; Laptook, A.; Walsh, M.; Oh, W.; Hale, E.; et al. Very low birth weight preterm infants with early onset neonatal sepsis: The predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr. Infect. Dis. J. 2005, 24, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Shastri, S.; Sharma, P. Role of lactoferrin in neonatal care: A systematic review. J. Matern. Fetal Neonatal Med. 2017, 30, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Superti, F.; Berlutti, F.; Paesano, R.; Valenti, P. Structure and activity of lactoferrin-a multi functional protective agent for human health. In Iron Metabolism and Disease; Fuchs, H., Ed.; Publisher Research Signpost: Trivandrum, India, 2008; Volume 8, pp. 1–32. [Google Scholar]
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. Biometals 2004, 17, 189–196. [Google Scholar] [CrossRef]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Superti, F.; De Seta, F. Warding Off Recurrent Yeast and Bacterial Vaginal Infections: Lactoferrin and Lactobacilli. Microorganisms 2020, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Aiba, Y.; Shimizu, K.; Takagi, A.; Miwa, T.; Koga, Y. The therapeutic effect of bovine lactoferrin in the host infected with Helicobacter pylori. Scand. J. Gastroenterol. 1999, 34, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Teraguchi, S.; Wakabayashi, H.; Kuwata, H.; Yamauchi, K.; Tamura, Y. Protection against infections by oral lactoferrin: Evaluation in animal models. Biometals 2004, 17, 231–234. [Google Scholar] [CrossRef]
- Okuda, M.; Nakazawa, T.; Yamauchi, K.; Miyashiro, E.; Koizumi, R.; Booka, M.; Teraguchi, S.; Tamura, Y.; Yoshikawa, N.; Adachi, Y.; et al. Bovine lactoferrin is effective to suppress Helicobacter pylori colonization in the human stomach: A randomized, double-blind, placebo-controlled study. J. Infect. Chemother. 2005, 11, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Superti, F. Recent developments in antiviral research. In Antiviral Activity of Lactoferrin; Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, India, 2001; pp. 193–203. [Google Scholar]
- Seganti, L.; Di Biase, A.M.; Marchetti, M.; Pietrantoni, A.; Tinari, A.; Superti, F. Antiviral activity of lactoferrin towards naked viruses. Biometals 2004, 17, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Trybala, E.; Superti, F.; Johansson, M.; Bergström, T. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology 2004, 318, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Pietrantoni, A.; Fortuna, C.; Remoli, M.E.; Ciufolini, M.G.; Superti, F. Bovine lactoferrin inhibits Toscana virus infection by binding to heparan sulphate. Viruses 2015, 7, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Pietrantoni, A.; Di Biase, A.M.; Tinari, A.; Marchetti, M.; Valenti, P.; Seganti, L.; Superti, F. Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob. Agents Chemother. 2003, 47, 2688–2691. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Tamura, Y.; Kurokawa, M.; Shiraki, K. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J. Med. Microbiol. 2005, 54, 717–723. [Google Scholar] [CrossRef]
- Tanaka, K.; Ikeda, M.; Nozaki, A.; Kato, N.; Tsuda, H.; Saito, S.; Sekihara, H. Lactoferrin inhibits hepatitis C virus viremia in patients with chronic hepatitis C: A pilot study. Jpn. J. Cancer Res. 1999, 90, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Sato, T.; Yamamoto, S.; Tanaka, K.; Ohkawa, S.; Takagi, H.; Yokosuka, O.; Furuse, J.; Saito, H.; Sawaki, A.; et al. Randomized, double-blind, placebo-controlled trial of bovine lactoferrin in patients with chronic hepatitis C. Cancer Sci. 2006, 97, 1105–1110. [Google Scholar]
- Egashira, M.; Takayanagi, T.; Moriuchi, M.; Moriuchi, H. Does daily intake of bovine lactoferrin-containing products ameliorate rotaviral gastroenteritis? Acta Paediatr. 2007, 96. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Chea-Woo, E.; Baiocchi, N.; Pecho, I.; Campos, M.; Prada, A.; Valdiviezo, R.N.; Lluque, A.; Lai, D.; Cleary, T.G. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J. Pediatr. 2013, 162, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Coulson, S.; Beck, S.L.; Gramotnev, H.; Du, S.; Lewis, S. The clinical efficacy of bovine lactoferrin/whey protein Ig-rich fraction (Lf/IgF) for the common cold: A double blind randomized study. Complement Ther. Med. 2013, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Yamano, E.; Miyauchi, M.; Furusyo, H.; Kawazoe, A.; Ishikado, A.; Makino, T.; Tanne, K.; Tanaka, E.; Takata, T. Inhibitory effects of orally administrated liposomal bovine lactoferrin on the LPS-induced osteoclastogenesis. Lab. Investig. 2010, 90, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Wyde, P.R.; Six, H.R.; Wilson, S.Z.; Gilbert, B.E.; Knight, V. Activity against rhinoviruses, toxicity, and delivery in aerosol of enviroxime in liposomes. Antimicrob. Agents Chemother. 1988, 32, 890–895. [Google Scholar] [CrossRef]
- Taylor, K.M.G.; Fan, S.J. Liposomes for Drug Delivery to the Respiratory Tract. Drug Dev. Ind. Pharm. 1993, 19, 123–142. [Google Scholar]
- Kirkpatrick, C.H.; Green, I.; Rich, R.R.; Schade, A.L. Inhibition of growth of Candida albicans by iron un saturated lactoferrin: Relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 1971, 124, 539–544. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kakeya, H.; Miyazaki, T.; Izumikawa, K.; Yanagihara, K.; Ohno, H.; Yamamoto, Y.; Tashiro, T.; Kohno, S. Synergistic antifungal effect of lactoferrin with azole antifungals against Candida albicans and a proposal for a new treatment method for invasive candidiasis. Jpn. J. Infect. Dis. 2011, 64, 292–296. [Google Scholar] [PubMed]
- Lai, Y.W.; Campbell, L.T.; Wilkins, M.R.; Pang, C.N.I.; Chen, S.; Carter, D.A. Synergy and antagonism between iron chelators and antifungal drugs in Cryptococcus. Int. J. Antimicrob. Agents 2016, 48, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Uchida, K.; Yamauchi, K.; Teraguchi, S.; Hayasawa, H.; Yamaguchi, H. Lactoferrin given in food facilitates dermatophytosis cure in guinea pig models. J. Antimicrob. Chemother. 2000, 46, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Takakura, N.; Wakabayashi, H.; Ishibashi, H.; Teraguchi, S.; Tamura, Y.; Yamaguchi, H.; Abe, S. Oral lactoferrin treatment of experimental oral candidiasis in mice. Antimicrob. Agents Chemother. 2003, 47, 2619–2623. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Sontag, M.K.; Accurso, F.J. Relationship between antimicrobial proteins and airway inflammation and infection in cystic fibrosis. Pediatr. Pulmonol. 2009, 44, 402–409. [Google Scholar] [CrossRef]
- Pfefferkorn, M.D.; Boone, J.H.; Nguyen, J.T.; Juliar, B.E.; Davis, M.A.; Parker, K.K. Utility of fecal lactoferrin in identifying Crohn disease activity in children. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 425–428. [Google Scholar] [CrossRef]
- Ashida, K.; Sasaki, H.; Suzuki, Y.A.; Lönnerdal, B. Cellular internalization of lactoferrin in intestinal epithelial cells. Biometals 2004, 17, 311–315. [Google Scholar] [CrossRef]
- Bertuccini, L.; Costanzo, M.; Iosi, F.; Tinari, A.; Terruzzi, F.; Stronati, L.; Aloi, M.; Cucchiara, S.; Superti, F. Lactoferrin prevents invasion and inflammatory response following E. coli strain LF82 infection in experimental model of Crohn’s disease. Dig. Liver Dis. 2014, 46, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Elass-Rochard, E.; Legrand, D.; Salmon, V.; Roseanu, A.; Trif, M.; Tobias, P.D.; Mazurier, J.; Genevieve Spik, G. Lactoferrin Inhibits the Endotoxin Interaction with CD14 by Competition with the Lipopolysaccharide-Binding Protein. Infect. Immun. 1998, 66, 486–491. [Google Scholar] [PubMed]
- Zimecki, M.; Spiegel, K.; Właszczyk, A.; Kübler, A.; Kruzel, M.L. Lactoferrin increases the output of neutrophil precursors and attenuates the spontaneous production of TNF-alpha and IL-6 by peripheral blood cells. Arch. Immunol. Ther. Exp. (Warsz) 1999, 47, 113–118. [Google Scholar] [PubMed]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef]
- Legrand, D.; Mazurier, J. A critical review of the roles of host lactoferrin in immunity. Biometals 2010, 23, 365–376. [Google Scholar] [CrossRef]
- Hwang, S.A.; Kruzel, M.L.; Actor, J.K. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int. Immunopharmacol. 2005, 5, 591–599. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Draelos, Z.D. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr. Med. Res. Opin. 2008, 24, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Katoh, N. Future perspectives in the treatment of atopic dermatitis. J. Dermatol. 2009, 36, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Artym, J.; Kocieba, M.; Kruzel, M.L. Effects of lactoferrin on elicitation of the antigen-specific cellular and humoral cutaneous response in mice. Postepy Hig. Med. Dosw. 2012, 66, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Togawa, J.; Nagase, H.; Tanaka, K.; Inamori, M.; Umezawa, T.; Nakajima, A.; Naito, M.; Sato, S.; Saito, T.; Sekihara, H. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G187–G195. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Fuss, I.; Pasparakis, M.; Alexopoulou, L.; Haralambous, S.; Meyer zum Buschenfelde, K.H.; Strober, W.; Kollias, G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur. J. Immunol. 1997, 27, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Togawa, J.; Nagase, H.; Tanaka, K.; Inamori, M.; Nakajima, A.; Ueno, N.; Saito, T.; Sekihara, H. Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. J. Gastroenterol. Hepatol. 2002, 17, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, F.; Yun, Z.; An, Y.; Wang, C.; Yan, X. Determination of the effects of lactoferrin in a preclinical mouse model of experimental colitis. Mol. Med. Rep. 2013, 8, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Jiang, P.; Stensballe, A.; Bendixen, E.; Sangild, P.T.; Chatterton, D.E.W. Bovine lactoferrin regulates cell survival, apoptosis and inflammation in intestinal epithelial cells and preterm pig intestine. J. Proteomics 2016, 139, 95–102. [Google Scholar] [CrossRef]
- Holgate, S.T. The epidemic of allergy and asthma. Nature 1999, 402, B2–B4. [Google Scholar] [CrossRef]
- Boldogh, I.; Bacsi, A.; Choudhury, B.K.; Dharajiya, N.; Alam, R.; Hazra, T.K.; Mitra, S.; Goldblum, R.M.; Sur, S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Investig. 2005, 115, 2169–2179. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Bacsi, A.; Choudhury, B.; Sur, S.; Boldogh, I. Lactoferrin decreases pollenantigen-induced allergic airway inflammation in a murine model of asthma. Immunology 2006, 119, 159–166. [Google Scholar] [CrossRef]
- Zimecki, M.; Artym, J.; Kocieba, M.; Kaleta-Kuratewicz, K.; Kruzel, M.L. Lactoferrin restrains allergen-induced pleurisy in mice. Inflamm. Res. 2012, 61, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.J.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas aeruginosa Chronic Lung Infection. Int. J. Mol. Sci. 2019, 20, 2128. [Google Scholar] [CrossRef]
- Tsubota, A.; Yoshikawa, T.; Nariai, K.; Mitsunaga, M.; Yumoto, Y.; Fukushima, K.; Hoshina, S.; Fujise, K. Bovine lactoferrin potently inhibits liver mitochondrial 8-OHdG levels and retrieves hepatic OGG1 activities in Long-Evans Cinnamon rats. J. Hepatol. 2008, 48, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Tanaka, A.; Yamauchi, K.; Iwatsuki, K. Bovine lactoferrin ingestion protects against inflammation via IL-11 induction in the small intestine of mice with hepatitis. Br. J. Nutr. 2014, 111, 1801–1810. [Google Scholar] [CrossRef]
- Konishi, M.; Iwasa, M.; Yamauchi, K.; Sugimoto, R.; Fujita, N.; Kobayashi, Y.; Watanabe, S.; Teraguchi, S.; Adachi, Y.; Kaito, M. Lactoferrin inhibits lipid peroxidation in patients with chronic hepatitis C. Hepatol. Res. 2006, 36, 27–32. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; Technical Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sekine, K.; Watanabe, E.; Nakamura, J.; Takasuka, N.; Kim, D.J.; Asamoto, M.; Krutovskikh, V.; Baba-Toriyama, H.; Ota, T.; Moore, M.A.; et al. Inhibition of azoxymethane-initiated colon tumor by bovine lactoferrin administration in F344 rats. Jpn. J. Cancer Res. 1997, 88, 523–526. [Google Scholar] [CrossRef]
- Ushida, Y.; Sekine, K.; Kuhara, T.; Takasuka, N.; Iigo, M.; Maeda, M.H.; Tsuda, H. Possible chemopreventive effects of bovine lactoferrin on esophagus and lung carcinogenesis in the rat. Jpn. J. Cancer Res. 1999, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kawabata, K.; Kohno, H.; Honjo, S.; Murakami, M.; Ota, T.; Tsuda, H. Chemopreventive effect of bovine lactoferrin on 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male F344 rats. Jpn. J. Cancer. Res. 2000, 91, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K.; Mattsby-Baltzer, I.; Innocenti, M.; Tuneberg, S. Orally administered bovine lactoferrin systemically inhibits VEGF(165)-mediated angiogenesis in the rat. Int. J. Cancer 2001, 91, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Chandra Mohan, K.V.; Kumaraguruparan, R.; Prathiba, D.; Nagini, S. Modulation of xenobiotic-metabolizing enzymes and redox status during chemoprevention of hamster buccal carcinogenesis by bovine lactoferrin. Nutrition 2006, 22, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Yamamoto, Y.; Ashino, H.; Oikawa, T.; Hazato, T.; Tsuda, H.; Iigo, M. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer 2004, 111, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Iigo, M.; Itoh, T.; Ushida, Y.; Sekine, K.; Terada, N.; Okamura, H.; Tsuda, H. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr. Cancer 2000, 38, 192–199. [Google Scholar] [CrossRef]
- Iigo, M.; Shimamura, M.; Matsuda, E.; Fujita, K.I.; Nomoto, H.; Satoh, J.; Kojima, S.; Alexander, B.D.; Moore, M.A.; Tsuda, H. Orally administered bovine lactoferrin induces caspase-1 and interleukin-18 in the mouse intestinal mucosa: A possible explanation for inhibition of carcinogenesis and metastasis. Cytokine 2004, 25, 36–44. [Google Scholar] [CrossRef]
- Fischer, R.; Debbabi, H.; Dubarry, M.; Boyaka, P.; Tomé, D. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem. Cell. Biol. 2006, 84, 303–311. [Google Scholar] [CrossRef]
- Iigo, M.; Alexander, D.B.; Long, N.; Xu, J.; Fukamachi, K.; Fukamachi, M.; Takase, M.; Tsuda, H. Anticarcinogenesis pathways activated by bovine lactoferrin in the murine small intestine. Biochimie 2009, 91, 86–101. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Watanabe, S.; Watanabe, R.; Hata, K.; Shimazaki, K.; Azuma, I. Bovine lactoferrin and Lactoferricin inhibit tumor metastasis in mice. Adv. Exp. Med. Biol. 1998, 443, 285–291. [Google Scholar] [CrossRef]
- Iigo, M.; Kuhara, T.; Ushida, Y.; Sekine, K.; Moore, M.A.; Tsuda, H. Inhibitory effects of bovine lactoferrin on colon carcinoma 26 lung metastasis in mice. Clin. Exp. Metastasis 1999, 17, 35–40. [Google Scholar] [CrossRef]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Okamoto, K.; Haing, S.; Thao Nguyen, P.; Imanaka, H.; Takata, T. Bovine lactoferrin reverses programming of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2018, 507, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.M.; Ramadan, G.; Zoheiry, M.K.; El-Beih, N.M. Antihepatocarcinogenic activity of whey protein concentrate and lactoferrin in diethylnitrosamine-treated male albino mice. Environ. Toxicol. 2019, 34, 1025–1033. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s anti-cancer properties: Safety, selectivity, and wide range of action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Roseanu, A.; Florian, P.E.; Moisei, M.; Sima, L.E.; Evans, R.W.; Trif, M. Liposomalization of lactoferrin enhanced its anti-tumoral effects on melanoma cells. Biometals 2010, 23, 485–492. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Y.; Zhang, Q.; Jiang, W.; Tang, L.; Liu, J.; He, Q. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur. J. Pharm. Sci. 2011, 44, 164–173. [Google Scholar] [CrossRef]
- Chen, H.; Tang, L.; Qin, Y.; Yin, Y.; Tang, J.; Tang, W.; Sun, X.; Zhang, Z.; Liu, J.; He, Q. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur. J. Pharm. Sci. 2010, 40, 94–102. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Palmano, K.P.; Sun, X.; Kanvar, R.K.; Gupta, R.; Haggarty, N.; Rowan, A.; Ram, S.; Krissansen, G.W. ’Iron-saturated’ lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy. Immunol. Cell. Biol. 2008, 86, 277–288. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Mahidhara, G.; Kanwar, R.K. Novel alginate-enclosed chitosan-calcium phosphate-loaded iron-saturated bovine lactoferrin nanocarriers for oral delivery in colon cancer therapy. Nanomedicine (Lond) 2012, 7, 1521–1550. [Google Scholar] [CrossRef]
- Sabra, S.; Agwa, M.M. Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Pilvi, T.K.; Harala, S.; Korpela, R.; Mervaala, E.M. Effects of high-calcium diets with different whey proteins on weight loss and weight regain in high-fat-fed C57BL/6J mice. Br. J. Nutr. 2009, 102, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Ono, T.; Fujisaki, C.; Ishihara, Y.; Murakoshi, M.; Kato, H.; Hosokawa, M.; Miyashita, K.; Sugiyama, K.; Nishino, H. Bovine lactoferrin reduces visceral fat and liver triglycerides in ICR mice. J. Oleo Sci. 2013, 62, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Sun, J.; Ren, F.; Xiong, L.; Zhao, L.; Guo, H. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods 2016, 22, 189–200. [Google Scholar] [CrossRef]
- Shi, J.; Finckenberg, P.; Martonen, E.; Ahiroos-Lehmus, A.; Pilvi, T.; Korpela, R. Metabolic effects of lactoferrin during energy restriction and weight regain in diet-induced obese mice. J. Funct. Foods 2012, 4, 66–78. [Google Scholar] [CrossRef]
- Xiong, L.; Ren, F.; Lv, J.; Zhang, H.; Guo, H. Lactoferrin attenuates high-fat diet-induced hepatic steatosis and lipid metabolic dysfunctions by suppressing hepatic lipogenesis and down-regulating inflammation in C57BL/6J mice. Food Funct. 2018, 9, 4328–4339. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.Q.; Qin, L.Q.; Sun, Z.Z.; Zuo, W.T.; Zhao, L.; Xu, J.Y. Effects of metformin combined with lactoferrin on lipid accumulation and metabolism in mice fed with high-fat diet. Nutrients 2018, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Murakoshi, M.; Suzuki, N.; Iida, N.; Ohdera, M.; Iigo, M.; Yoshida, T.; Sugiyama, K.; Nishino, H. Potent antiobesity effect of enteric-coated lactoferrin: Decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br. J. Nutr. 2010, 104, 1688–1695. [Google Scholar] [CrossRef]
- Nikonorov, A.A.; Skalnaya, M.G.; Tinkov, A.A.; Skalny, A.V. Mutual interaction between iron homeostasis and obesity pathogenesis. J. Trace Elem. Med. Biol. 2015, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lorget, F.; Clough, J.; Oliveira, M.; Daury, M.C.; Sabokbar, A.; Offord, E. Lactoferrin reduces in vitro osteoclast differentiation and resorbing activity. Biochem. Biophys. Res. Commun. 2002, 296, 261–266. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U.; Watson, M.; Lin, J.M.; Tong, P.C.; Chen, Q.; et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef]
- Cornish, J.; Naot, D. Lactoferrin as an effector molecule in the skeleton. Biometals 2010, 23, 425–430. [Google Scholar] [CrossRef]
- Inubushi, T.; Kawazoe, A.; Miyauchi, M.; Yanagisawa, S.; Subarnbhesaj, A.; Chanbora, C.; Ayuningtyas, N.F.; Ishikado, A.; Tanaka, E.; Takata, T.; et al. Lactoferrin inhibits infection-related osteoclastogenesis without interrupting compressive force-related osteoclastogenesis. Arch. Oral Biol. 2014, 59, 226–232. [Google Scholar] [CrossRef]
- Cornish, J. Lactoferrin promotes bone growth. Biometals 2004, 17, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Jiang, L.; Ibrahim, S.A.; Zhang, L.; Zhang, H.; Zhang, M.; Ren, F.Z. Orally administered lactoferrin preserves bone mass and microarchitecture in ovariectomized rats. J. Nutr. 2009, 139, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, J.; Ji, P.; Zhu, S.; Zhu, Y. Oral administration of bovine lactoferrin accelerates the healing of fracture in ovariectomized rats. J. Bone Miner. Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, A.; Inubushi, T.; Miyauchi, M.; Ishikado, A.; Tanaka, E.; Tanne, K.; Takata, T. Orally administrated liposomal lactoferrin inhibits inflammation-related bone breakdown without interrupting orthodontic tooth movement. J. Periodontol. 2013, 84, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Ishikado, A.; Uesaki, S.; Suido, H.; Nomura, Y.; Sumikawa, K.; Maeda, M.; Miyauchi, M.; Takata, T.; Makino, T. Human trial of liposomal lactoferrin supplementation for periodontal disease. Biol. Pharm. Bull. 2010, 33, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, T.; Kosai, A.; Yanagisawa, S.; Chanbora, C.; Miyauchi, M.; Yamasaki, S.; Sugiyama, E.; Ishikado, A.; Makino, T.; Takata, T. Bovine lactoferrin enhances osteogenesis through Smad2/3 and p38 MAPK activation. J. Oral Biosci. 2020, 62, 147–154. [Google Scholar] [CrossRef]
- Danjo, Y.; Lee, M.; Horimoto, K.; Hamano, T. Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol. (Copenh.) 1994, 72, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Kawakita, T.; Inaba, T.; Okada, N.; Ito, M.; Shimmura, S.; Watanabe, M.; Shinmura, K.; Tsubota, K. Dietary lactoferrin alleviates age-related lacrimal gland dysfunction in mice. PLoS ONE 2012, 7, e33148. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Matsumoto, Y.; Yamamoto, Y.; Goto, E.; Saiki, M.; Shimazaki, J.; Takebayashi, T.; Tsubota, K. Lactoferrin in Sjögren’s syndrome. Ophthalmology 2007, 114, 2366–2367. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Medina, I.; Tombo, I.; Satué-Gracia, M.T.; German, J.B.; Frankel, E.N. Effects of natural phenolic compounds on the antioxidant activity of lactoferrin in liposomes and oil-in-water emulsions. J. Agric. Food Chem. 2002, 50, 2392–2399. [Google Scholar] [CrossRef][Green Version]
- Duran, A.; Kahve, H.I. The use of lactoferrin in food industry. Acad. J. Sci. 2017, 7, 89–94. [Google Scholar]
| Infants | Study Design | Intervention | Main Results | References |
|---|---|---|---|---|
| VLBW neonates (<1000 g at birth) | Multicentre, double-blind, placebo-controlled, randomized trial | bLf (100 mg/d) or bLf + LGG 6.109 CFU/d) or placebo. | bLf alone or in combination with LGG reduced the incidence of a first episode of late-onset sepsis in VLBW neonates. | [58] |
| VLBW neonates (<1500 g) | Multicentre, double-blind, placebo-controlled, randomized trial | bLf (100 mg/d), or bLf + LGG 6.109 CFU/d) or placebo. | NEC or death incidence was significantly lower in groups bLf and bLf + LGG. No adverse effects or intolerances to treatment occurred. | [59] |
| VLBW (<1500 g) or <32 weeks neonates | Single-centre, double-blind, placebo-controlled, randomized trial | bLf (200 mg/d) or placebo, during hospitalization period | bLf prophylaxis decreased nosocomial sepsis episodes and increased Treg levels. Treg level increasing is suggested to be responsible for decreased sepsis. | [60] |
| VLBW (neonates <2000 g) | Placebo-controlled, double-blind, randomized trial | bLf 80–140 mg/kg/d or placebo for 4 weeks | bLf supplementation decreased the incidence of first episode of LOS. | [61] |
| Neonates, (<2500 g at birth) | Placebo-controlled, double-blind, randomized trial | bLf 200 mg/kg/d or placebo for 4 weeks | bLf supplementation decreased nosocomial sepsis episodes, especially in VLBW neonates. | [62] |
| Neonates <32 weeks or <1500 g | Placebo-controlled, double-blind, randomized trial | bLf (100 mg/d) or bLf + LGG 1.106 CFU/d) or placebo. | bLf supplementation decreased the incidence of severe NEC. | [63] |
| VLBW preterm neonates | Multicentre, double-blind, placebo-controlled, randomized trial | bLf (100 mg/d) or bLf + LGG 1.106 CFU/d) or placebo. | bLf supplementation, alone or in combination with LGG, reduced the risk for infections related to inhibitors of gastric acidity. bLf decreased the incidence of LOS and NEC. | [64] |
| Animal Species (Male) | Dose | Administration | Treatment Length | Outcomes | References |
|---|---|---|---|---|---|
| ICR mice | 100 mg/kg | Gastric intubation | 4 weeks | Reduced mesenteric fat tissue and hepatic lipid accumulation | [147] |
| High-fat diet induced obese C57BL/6J mice | (190 mL/kg) 100 mg/kg | Oral | 7 weeks | Reduced body weight, obesity, adipose tissue (visceral, abdominal) and plasma glucose | [146] |
| High-fat diet induced obese C57BL/6J mice | 100 mg/kg | Oral | 12 weeks | Reduced body weight, fat accumulation, inflammation and relieve liver steatosis | [149] |
| High-fat diet induced obese C57BL/6J mice | 100 mg/kg | Oral | 15 weeks | Reduced weight gain, visceral adiposity, serum glucose, inflammation and improved hepatic steatosis | [151] |
| High-fat diet induced obese C57BL/6J mice | 100 mg/kg | Oral | 50 days | Ameliorated fatty liver formation, exerted beneficial effects on glucose tolerance and adipocyte tissue inflammation without interfering energy intake. | [150] |
| C57BL/6 mice | 2 g/100 mL alone or in combination with metformin | Oral | 12 weeks | Improved high-fat diet-induced obesity and lipid metabolism. | [152] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. https://doi.org/10.3390/nu12092562
Superti F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients. 2020; 12(9):2562. https://doi.org/10.3390/nu12092562
Chicago/Turabian StyleSuperti, Fabiana. 2020. "Lactoferrin from Bovine Milk: A Protective Companion for Life" Nutrients 12, no. 9: 2562. https://doi.org/10.3390/nu12092562
APA StyleSuperti, F. (2020). Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients, 12(9), 2562. https://doi.org/10.3390/nu12092562





