Abstract
Chlorella is a green unicellular alga that is commercially produced and distributed worldwide as a dietary supplement. Chlorella products contain numerous nutrients and vitamins, including D and B12, that are absent in plant-derived food sources. Chlorella contains larger amounts of folate and iron than other plant-derived foods. Chlorella supplementation to mammals, including humans, has been reported to exhibit various pharmacological activities, including immunomodulatory, antioxidant, antidiabetic, antihypertensive, and antihyperlipidemic activities. Meta-analysis on the effects of Chlorella supplementation on cardiovascular risk factors have suggested that it improves total cholesterol levels, low-density lipoprotein cholesterol levels, systolic blood pressure, diastolic blood pressure, and fasting blood glucose levels but not triglycerides and high-density lipoprotein cholesterol levels. These beneficial effects of Chlorella might be due to synergism between multiple nutrient and antioxidant compounds. However, information regarding the bioactive compounds in Chlorella is limited.
Keywords:
antioxidants; Chlorella; dietary fibers; dietary supplements; folate; lutein; vitamin B12; vitamin D2 1. Introduction
Microalgae are primarily found in aquatic ecosystems, living in both seawater and freshwater, and are photosynthetic eukaryotic organisms that contain chloroplasts and nuclei, similar to plants. Microalgae more efficiently yield biomass than land-based plants owing to their higher performance in utilizing sunlight and CO2, leading to their extremely high growth rates [1]. Therefore, microalgae have been used in the food, pharmaceutical, and cosmetic industries, and their pigments, nutrients, bioactive compounds and whole biomass are already in use worldwide. Recently, various bioactive compounds and nutrients have been detected in both seawater and freshwater microalgae, including cyanobacteria. These compounds and nutrients have been reported to promote human health [1,2]. However, there is limited information regarding the bioactive compounds of freshwater-living Chlorella species, which are classified as green algae.
Chlorella species can be mass-cultured, and their dietary supplement products are commercially available worldwide. However, the commercial cultivation of their biomass has started only several years ago. Chlorella vulgaris was discovered and reported in 1890 by Dr. Martinus Willem Beijerinck, a famous microbiologist and botanist [3]. Another Chlorella species, distinguished by the presence pyrenoids in chloroplasts, was identified and accordingly named C. pyrenoidosa in 1903 [4]. Since then, more than 20 Chlorella species have been characterized, with over 100 strains described [5]. At present, Chlorella species are divided into three varieties: C. vulgaris, C. lobophora, and C. sorokiniana [6]. C. sorokiniana is a sub-species first isolated in 1953 by Sorokin and originally thought to be a thermotolerant mutant of C. pyrenoidosa [7,8]. C. pyrenoidosa, the subject of many scientific studies, is now called C. sorokiniana.
Investigations of the dietary value of Chlorella in human health began in the early 1950s, when the use of Chlorella as a food source was initiated in the midst of a global food crisis [9]. Chlorella was first produced and consumed in Asia, mainly in Japan, and then used as a dietary supplement worldwide [10]. Chlorella is produced commercially for use in foods and as a source of its intrinsic compounds. Using large-scale cultivation technology, C. vulgaris and C. pyrenoidosa are prepared as commercial sources for dietary supplements [11]. Studies have shown that Chlorella cells contain a variety of nutrients and bioactive compounds that promote human health and prevent certain diseases [10,12], suggesting that Chlorella-derived natural compounds might provide substitutes for synthetic compounds or drugs. The content of natural compounds in Chlorella differs greatly between culture conditions and Chlorella species [13,14].
Here, we present updated information on the Chlorella content of nutrients and bioactive compounds that promote human health. However, at present, there is limited information available regarding the bioactive compounds responsible for its pharmacological activities, which might be due to the synergistic effects of various nutrients and antioxidant compounds in Chlorella.
2. Nutrients in Commercial Chlorella Products
2.1. Macronutrients
The macronutrient content of 13 commercially available Chlorella products, based on information provided on the packaging label, are summarized in Table 1. Humans cannot digest Chlorella cells in their natural state because their cell walls are made of cellulose. Therefore, Chlorella cell walls are mechanically broken down in most dietary supplements. An animal study has shown that more than 80% of Chlorella proteins are digestible [15].

Table 1.
Nutrient content of 13 commercially available Chlorella products.
These Chlorella products contain a large amount of proteins (approximately 59% based on dry weight), coinciding with the analytical data of the protein contents of C. pyrenoidosa (57%) [16] and C. vulgaris (51–58%) [17]. This protein content is higher than that of soybeans (approximately 33%, dry weight). The amino acid composition of Chlorella products C and M are shown in Table 2. These amino acid profiles indicate that all essential amino acids for humans (isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, and histidine) are present in substantial concentrations in these products. According to the essential amino acid index (EAAI) used to evaluate protein quality for human nutrition, the quality of C. pyrenoidosa (EAAI, 1.35) [18] and a commercially available Chlorella product (EAAI, 0.92) [19] are higher than that of soybean protein (EAAI, 0.66) [18]. These results indicate that proteins in Chlorella products are of high or good quality. Notably, Chlorella products contain a considerable amount of arginine (approximately 3200 mg/100 g dry weight), which serves as a substrate for the production of NO, a potent intracellular signaling molecule that influences every mammalian system [20]. Arginine also serves as a potent modulator of immune functions [21].

Table 2.
Amino acid content of commercially available Chlorella products C and M.
Approximately 17% (dry weight) of carbohydrates are found in the commercially available Chlorella products. Similar results have been reported for C. vulgaris [17]. As shown in Table 1, more than 65% of the carbohydrate is dietary fiber, which appears to be derived from the Chlorella cell wall. Various polysaccharides have been extracted and characterized [22,23,24,25]. Chlorella polysaccharides exhibited a variety of biologically active compounds, including antioxidants [24] and stimulators of plant growth [25]. Tabarsa et al. [26] characterized an immune-enhancing water-soluble α-glucan prepared from C. vulgaris.
Commercially available Chlorella products contain a small amount of fats (approximately 11%, dry weight) (Table 1), which coincides with the analytical data of the fat content of C. vulgaris (14–22%) [17]. Chlorella products contain α-linolenic acid (approximately 10–16% of total fatty acids) and linoleic acid (approximately 18% of total fatty acids) but not eicosapentaenoic acid, docosahexenoic acid, or arachidonic acid [19,27]. Approximately 65–70% of the total fatty acids found in commercially available Chlorella products are derived from polyunsaturated fatty acids [19,27].
Different growth conditions, such as temperature, nutrient composition, and light availability, can readily alter the levels of biomass, macro- and micronutrients, and other valuable bioactive compounds, including antioxidants, in Chlorella cells [28,29,30].
2.2. Micronutrients
2.2.1. Vitamins
As shown in Table 3, commercially available Chlorella products contain all the vitamins required by humans, i.e., B1, B2, B6, B12, niacin, folate, biotin, pantothenic acid, C, D2, E, and K, and α- and β-carotenes. Chlorella products contain substantial amounts of vitamins D2 and B12, both of which are well known to be absent in plants. Commercially available Chlorella (C. vulgaris) products contain higher amounts of folate (approximately 2.5 mg/100 g dry weight) than spinach [31]. Vitamin B12 and folate deficiencies induce the accumulation of serum homocysteine, which is involved in cardiovascular diseases. In this section, we discuss vitamin D2, vitamin B12, and folate.

Table 3.
Content of vitamins and related compounds in 13 commercially available Chlorella products.
Vitamin D, a major regulator of calcium absorption, reduces the risk of osteomalacia in adults and rickets in children [32]. The two main dietary forms of vitamin D are vitamin D2 and D3, which are found in fungi such as mushrooms [33,34] and animal-derived foods such as fish and fish products [35], respectively. Mushrooms have the ability to synthesize ergosterol (known as provitamin D2), which is converted into ergocalciferol as vitamin D2 upon ultraviolet irradiation [34,36]. Thus, ultraviolet-irradiated mushrooms are suitable for use as vitamin D2 sources in strict vegetarians [36]. Cell walls of mushrooms contain high concentrations of ergosterol, which plays a physiological role in modulating cell membrane strength and fluidity similar to cholesterol in animals [37]. Sun-dried, commercially available mushrooms reportedly contain approximately 17 µg of vitamin D2 per g dry weight [38]. The bioavailability of vitamin D2 from mushrooms has been studied in humans [39,40].
Ergosterol was first reported as the main sterol compound in C. pyrenoidosa in the early 1950S [41]. C. vulgaris also contains a substantial amount of ergosterol [42,43]. Our unpublished data show that one commercially available Chlorella product contains both ergosterol (1.68 mg/g dry weight) and vitamin D2 (15.2 µg/g dry weight), similar in amounts to those in sun-dried mushrooms. The vitamin D2 in this Chlorella product is synthesized from ergosterol upon exposure to sunlight during cultivation (Figure 1). Although it has been reported that vitamin D3 is more effective than vitamin D2 at increasing the concentration of circulating 25-hydroxyvitamin D [44], Chlorella products and sun-dried mushrooms could become sources of vitamin D for vegetarians.
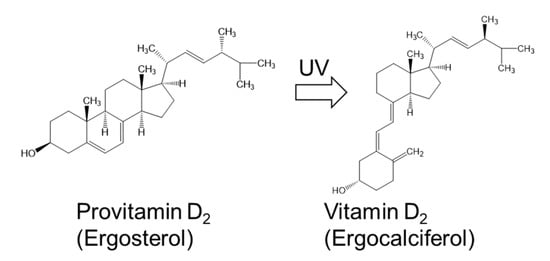
Figure 1.
Structures of provitamin D2 and vitamin D2 found in commercially available Chlorella products.
Serum homocysteine (Hcy) is an established biomarker of cardiovascular disease in humans [45,46]. Hcy is a non-protein forming amino acid (Figure 2) produced as an intermediate compound of methionine metabolism and is further metabolized to cystathionine via cystathionine β-synthetase, a vitamin B6-dependent enzyme [46]. Alternatively, Hcy can be remethylated back to methionine by methionine synthase, a vitamin B12-dependent enzyme. Folate is also required for the remethylation of Hcy by providing 5-methyltetrahydrofolate. Deficiencies in vitamin B12 [47], vitamin B6 [48], and folate [49] cause hyper-homocysteinemia. Several clinical studies report a correlation between atherosclerosis and deficiencies in vitamin B12 and folate [50,51]. Folate deficiency in women before and during pregnancy is associated with neural tube defects in newborns [52]. Plants can synthesize folate compounds de novo, but animals cannot [53]. Thus, plant-derived foods are sources of dietary folates for humans. High concentrations of folate (approximately 1.69–2.45 mg/100 g dry weight) are reported in commercially available Chlorella (C. vulgaris) products [31], with concentrations similar to those of the products shown in Table 3 (0.3–3.6 mg/100 g dry weight). The main folate compounds identified in Chlorella products are 5-CHO-H4 folate (60–62%) and 5-CH3-H4 folate (24–26%) and the minor folate compounds are 10-CHO-folate (5–7%), H4 folate (4%), and fully oxidized folate (3–6%) [31]. The chemical structures of the Chlorella folate compounds are shown in Figure 3. The main dietary sources of folates are vegetables (25%), bread and cereal products (22%), dairy products (10%), fruit (10%), and oils and fats (5%) [31]. Spinach has high folate content (165 µg/100 g fresh weight; 1.7 mg/100 g dry weight) [31,54], which is similar to that of Chlorella products. Thus, Chlorella products are an excellent source of folate for humans.
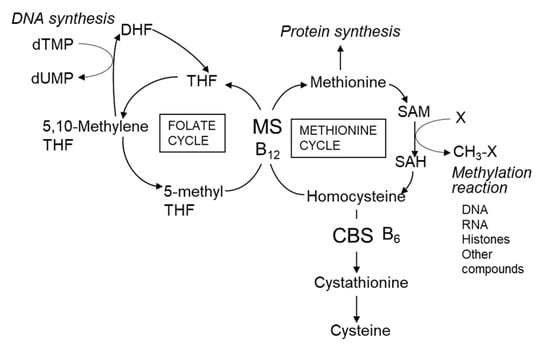
Figure 2.
Homocysteine metabolic pathway in mammals. Abbreviations: B6, vitamin B6; B12, vitamin B12; CBS, cystathionine β-synthetase; DHF, dihydrofolate; MS, cobalamin-dependent methionine synthase; SAM, S-adenosyl methionine; SAH, S-adenosyl homocysteine; THF, tetrahydrofolate.
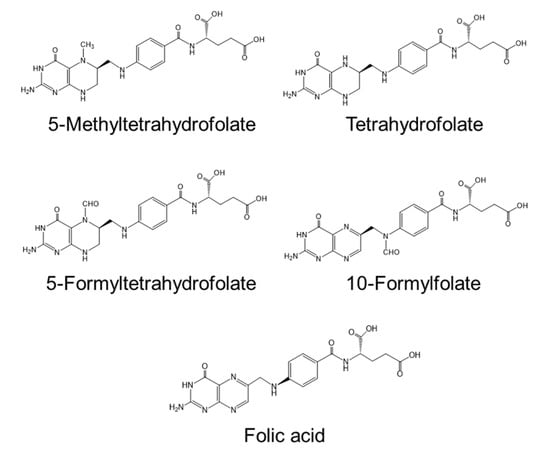
Figure 3.
Chemical structures of folate compounds found in commercially available Chlorella products.
Vitamin B12 (B12) is synthesized by certain bacteria and archaea but not by plants [55]. Animal-derived foods, such as meats, milk, fish, and shellfish, are the major dietary sources of B12 for humans [56]. B12 content is high in beef, pork, and chicken livers (approximately 25–53 µg/100 g fresh weight) [56] and in edible bivalves such as clams (approximately 60 µg/100 g fresh weight) [57]. The reported B12 content of Chlorella products varies from <0.1 to 400 µg per 100 g of dry weight [58,59], consistent with that of the products shown in Table 3 (6–500 µg/100 g dry weight). Among Chlorella species, the B12 content is much higher in C. pyrenoidosa than in C. vulgaris when grown under open culture conditions [59]. B12 is not essential for the growth of these Chlorella species [59,60], suggesting that Chlorella cells absorb and accumulate large amounts of exogenous B12. Some of the high B12-containing Chlorella products contain inactive corrinoid compounds such as 5-methoxybenzimidazolylcobamide and cobalt-free corrinoid (Figure 4). Thus, if Chlorella products with high B12 are consumed as a sole B12 source, accurate content estimation requires the identification of B12 compounds using liquid chromatography–tandem mass spectrometry [59].
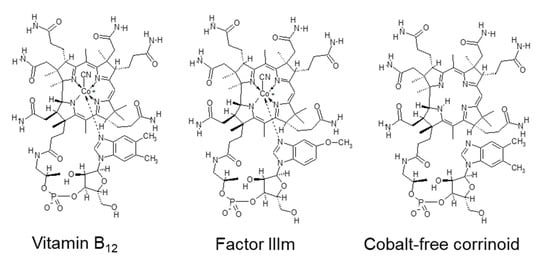
Figure 4.
Chemical structures of vitamin B12 and related compounds found in commercially available Chlorella products. Abbreviations: Factor IIIm, 5-methoxybenzimidazolylcobamide.
Rauma et al. [61] demonstrated that substantial consumption of Chlorella products can supply adequate amounts of B12. Another study of strict vegetarians (vegans) with an elevated baseline of serum methylmalonic acid (as an index of B12 deficiency) showed that ingestion of 9 g of C. pyrenoidosa daily for 60 days resulted in significant decreases in serum methylmalonic acid in 88% of the subjects [62]; serum Hcy decreased and serum B12 tended to increase, although the mean corpuscular volume, hemoglobin, and hematocrit levels were unchanged. These results suggest that Chlorella products with high B12 and without inactive corrinoid compounds are suitable for use as B12 sources in humans, particularly vegans.
2.2.2. Minerals
As shown in Table 4, commercially available Chlorella products contain a variety of minerals that are required in humans. In particular, Chlorella products contain substantial amounts of iron (104 mg/100 g dry weight) and potassium (986 mg/100 g dry weight), of which adequate intake prevents anemia [63] and hypertension [64], respectively. Iron plays physiological roles in respiration, energy production, DNA synthesis, and cell proliferation [65]. The phytates in grains potently inhibit the intestinal absorption of iron because they chelate iron to form an insoluble complex [66]. Thus, people on vegan and vegetarian diets may be at risk for iron-deficiency anemia [63]. Studies in rats and humans have investigated whether Chlorella supplementation can prevent iron-deficiency anemia [67,68]. In a cohort of 32 women in the second and third trimester of pregnancy, oral Chlorella supplementation (6 g/day) for 12–18 weeks decreased markers of anemia as compared to the control group [68], suggesting that Chlorella supplementation significantly reduces the risk of pregnancy-associated anemia.

Table 4.
Mineral content of 13 commercially available Chlorella products.
Selenium (Se) is an essential trace mineral that serves as a fundamental nutrient to human health. It is a component of selenoproteins, such as thioredoxin reductase and glutathione peroxidases, and protects against intercellular oxidative damage [69,70,71]. Therefore, low levels of Se show various pharmaceutical activities, including antitumor and antiaging effects; however, high levels of Se induce the generation of reactive oxygen species. Generally, the organic forms of Se are more bioavailable and less toxic than the inorganic forms of Se. Selenite (SeO32−) and selenite (SeO42−) are the predominant forms of Se in freshwater. Microalgae act as a major transporter of Se from water to filter-feeders and other organisms. Although most plant species accumulate less than 25 µg Se/g dry weight [72], some microalgae species can accumulate Se at high concentrations (100 µg Se/g dry weight) [73]. Se is essential for many algae and functions to protects them from oxidative damage. Sun et al. [74] indicated that C. vulgaris can accumulate Se at high concentrations (857 µg/g dry weight) when grown under Se concentrations of 0–200 mg/L in a growth medium and that relatively low Se concentrations (75 mg selenite/L medium) positively promotes C. vulgaris growth and acts as an antioxidant by inhibiting lipid peroxidation and intracellular reactive oxygen species. The maximum accumulation of organic Se was found at 316 µg/g dry weight under relatively low Se (75 mg selenite/L medium) conditions [75], indicating that C. vulgaris is an efficient Se accumulator and that Se-enriched Chlorella cells might be useful for human supplementation.
2.3. Pigments
Carotenoids are secondary metabolites in the most abundant naturally occurring pigments that participate in various biological processes in plants, including photosynthesis, photomorphogenesis, photoprotection, and development [76]. They also serve as colorants and critical components of the human diet, such as antioxidants and provitamin A [76]. More than 400 carotenoids have been identified in living organisms [77], and β-carotene, astaxanthin, lutein, zeaxanthin, and lycopene are widely known as the major carotenoids among them. The green microalgae Dunaliella salina produces high concentrations of β-carotene of up to 14% of algal dry weight [78]. Haematococcus pluvialis increases astaxanthin concentration up to 4–5% of algal dry weight [79] under stressful conditions. Chlorella products contain lower contents of total carotenoids (approximately 1.3%) [80], compared with the above-mentioned green algae. C. vulgaris reportedly produces lutein as the primary carotenoid [81,82]. However, C. zofingiensis reportedly accumulates significant amounts of astaxanthin, and it might be a suitable organism for the mass production of astaxanthin [83].
3. Pharmacological Activities of Chlorella Products
Because Chlorella cells contain various nutrients and biologically active compounds, the effects of Chlorella supplementation on preventing the development of various diseases has been studied in rats and mice, including disease-specific model animals. These animal studies have been useful for elucidating the specific health effects of Chlorella supplementation. Moreover, the effects of Chlorella supplementation on mitigating a variety of diseases in humans have been investigated. These studies have used either C. vulgaris or C. pyrenoidosa because these species are commercially available as Chlorella products.
3.1. Antihypertensive Effects
Hypertension increases the risk of cardiovascular disease [84]. Antihypertensive compounds in foods have been identified using a stroke-prone spontaneously hypertensive (SHRSP) rat model, which is genetically predisposed to hypertension and cerebral stroke [85]. Sansawa et al. [86] investigated the effects of dried Chlorella powder (C. regularis) on blood pressure, cerebral stroke lesions, and the life span of SHRSP rats. In 12-week-old SHRSP rats fed Chlorella (5%, 10%, and 20%) for 13 weeks, elevated blood pressure significantly decreased in the 10% and 20% Chlorella groups compared with the untreated controls. Serum total cholesterol levels were significantly lower in all Chlorella groups, and their average life span was more than that of the controls. To characterize the antihypertensive compounds in Chlorella, Chlorella powder was fractionated into hot-water-soluble, lipid-soluble, and residual fractions. Blood pressure was significantly lower in rats fed the lipid or residual fraction but not in those fed the hot-water-soluble fraction. The lipid fraction contained substantial amounts of carotenoids, which are potent antioxidants, and phospholipids, which mediate aorta collagen and elastin metabolism. The residual fraction contained a high level of arginine, which increases the production of endothelium-derived relaxing factor. These beneficial effects of Chlorella powder on SHRSP rats might result from synergism between its numerous bioactive compounds.
To evaluate whether daily Chlorella supplementation can reduce blood pressure in subjects with mild to moderate hypertension, a pilot study was conducted in 24 participants administered C. pyrenoidosa (10 g of Chlorella tablets and 100 mL Chlorella extract) [87]. After two months of Chlorella supplementation, the average heart rate and sitting systolic and diastolic blood pressures only slightly changed. On the other hand, for some subjects with mild to moderate hypertension, Chlorella supplementation reduced or maintained their sitting diastolic blood pressure.
Arterial stiffness is a well-established risk factor of cardiovascular disease [88]. Previous studies have reported that antioxidants [89], potassium [90], and n-3 unsaturated fatty acids [91] decrease arterial stiffness. Nitric oxide (NO), derived from arginine in the vascular endothelium, is an important modulator of arterial stiffness [92]. Chlorella products contain antioxidants, vitamins, potassium, arginine, and n-3 unsaturated fatty acids. To evaluate the effects of Chlorella supplementation on arterial stiffness, a single-blinded, placebo-controlled crossover study was conducted in 14 young participants who were administered C. pyrenoidosa (6 g/day) or placebo for four weeks, with a 12-week washout period between trials, in a randomized order [93]. No differences were observed in blood pressure or heart rate before and after supplementation in both the placebo and Chlorella groups. Brachial-ankle pulse wave velocity, a measure of arterial stiffness, decreased in the Chlorella group but not in the placebo group [93]. A similar trial in 32 middle-aged and older subjects reports that the brachial-ankle pulse wave velocity decreased after Chlorella supplementation but not after placebo supplementation [94]. These changes in brachial-ankle pulse wave velocity with Chlorella supplementation correlated with the plasma NOx level. These results suggest that Chlorella supplementation decreases arterial stiffness in both younger and older subjects.
The efficacy of Chlorella supplementation in reducing cardiovascular risk factors was assessed in a meta-analysis of 19 randomized controlled trials including 797 subjects [95]. This study concluded that Chlorella supplementation improves total cholesterol levels, low-density lipoprotein cholesterol levels, systolic blood pressure, diastolic blood pressure, and fasting blood glucose levels but not triglyceride levels, high-density lipoprotein cholesterol levels, and body mass index.
3.2. Antihypercholesterolemic and Antihyperlipemic Effects
Elevated total cholesterol and triglycerides and abnormal metabolism of lipoproteins and apolipoproteins are responsible for an increased risk of cardiovascular disease [96,97,98]. The indigestible components of foods, such as dietary fiber, decrease serum cholesterol by inhibiting the intestinal absorption of neutral steroids [99]. Chlorella supplementation reportedly decreases serum cholesterol levels in model animals [100]. To identify the bioactive compounds responsible for this effect, the indigestible fraction of C. regularis powder was isolated and characterized, revealing a content of 43% crude protein, 37.3% dietary fiber, 6.9% carbohydrate, 5.4% moisture, 4.3% crude fat, and 2.7% ash [101]. Rats fed a diet with 5.3% of this indigestible fraction demonstrated lower serum and liver cholesterol levels and higher fecal neutral steroid levels as compared with those fed a diet of 12.7% Chlorella powder. Both Chlorella powder and the indigestible fraction exhibited a high bile-acid binding capacity in vitro. Furthermore, the indigestible fraction increased the hepatic mRNA levels of cholesterol 7α-hydroxylase, which is the rate limiting enzyme for cholesterol catabolism and bile-acid synthesis [102]. These results indicate that the indigestible fraction of Chlorella possesses hypercholesteromic activity, which improves cholesterol catabolism via the upregulation of hepatic cholesterol 7α-hydroxylase expression.
Chlorella supplementation is also reported to decrease serum cholesterol levels in hyperlipemia and mild hypercholesterolemic patients in a small, open-label trial [103]. To evaluate the preventive role of Chlorella in maintaining serum cholesterol levels against excess dietary cholesterol intake, a double-blind, randomized, placebo-control study was conducted in 63 mildly hypercholesterolemic subjects treated with either C. vulgaris (5 g/day) or placebo for four weeks [104]. A similar trial investigated cholesterol levels in 34 participants administered 510 mg of dietary cholesterol from three eggs concomitantly with either Chlorella (C. vulgaris) (5 g/day) or a matched placebo for 4 weeks [105]. Participants on the three-egg diet alone exhibited significant elevation in serum total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels. The administration of Chlorella in addition to the three-egg diet significantly suppressed these increases in total cholesterol and low-density lipoprotein cholesterol levels and significantly increased serum lutein and α-carotene levels [105]. In mildly hypercholesterolemic subjects, Chlorella administration resulted in marked changes in total cholesterol, triglycerides, lutein/zeaxanthin, and α-carotene levels as well as a significant decrease in very low-density lipoprotein cholesterol, apolipoprotein B, non-high-density lipoprotein, and high-density lipoprotein/triglyceride levels [104]. These results suggest that Chlorella might inhibit the intestinal absorption of dietary and endogenous lipids. In addition, the observed changes in serum lipids may be associated with changes in serum carotenoids. These results suggest that daily consumption of Chlorella provides potential health benefits by reducing the levels of serum lipid risk factors, such as triglycerides and total cholesterol, in mild hypercholesterolemic subjects.
3.3. Antidiabetic Effect
Type 2 diabetes, accounting for 90–95% of all diabetes cases, is a severe health problem affecting over 380 million people worldwide [106]. Elevated blood glucose levels, insulin resistance, and low insulin sensitivity are the main characteristics of patients with type 2 diabetes [107], resulting in serious conditions, including arteriosclerosis, renal damage, and retinopathy [108]. In a streptozotocin-induced animal model of diabetes, several studies have been conducted to elucidate the mechanisms underlying the antidiabetic activity of Chlorella supplementation [109,110,111]. Shibata et al. [109] evaluated the effects of Chlorella supplementation on antioxidant status and cataracts by feeding a diet containing 7.3% (w/w) Chlorella powder (C. regularis) to 11-week old rats with streptozotocin-induced diabetes. After 11 weeks of supplementation, serum lipid peroxide levels (an index of oxidative status) and blood glycated hemoglobin were lower in Chlorella-supplemented rats than in control rats; however, the serum glucose level did not differ between groups. Chlorella supplementation delayed the development of lens opacities. These results indicate that Chlorella supplementation might be beneficial for preventing diabetes complications such as cataracts, possibly due to the activity of its antioxidant compounds.
Cherng and Shih reported potential hypoglycemic effects of Chlorella supplementation in streptozotocin-induced diabetic mice [110]. Oral administration of Chlorella 60 min before glucose administration (0.5 g/kg body weight) resulted in a transient hypoglycemic effect at 90 min after glucose administration without an increase in insulin secretion. Chlorella supplementation increased 2-deoxyglucose uptake in the liver and soleus muscles of streptozotocin-treated mice and was likely the cause of the observed hypoglycemic effects [111].
The prophylactic effect of Chlorella (C. vulgaris) supplementation on diabetes was studied by Vecina et al. [112], who investigated body weight, lipid profile, blood glucose, and insulin signaling in liver, skeletal muscle, and adipose tissue in high-fat diet-induced obese mice. Chlorella supplementation improves glycemic control in obesity and diabetes because it decreases insulin resistance caused by increased expression of glucose transporter 4 via the activation of protein kinase B phosphorylation in skeletal muscle. Chlorella supplementation combined with aerobic exercise training showed more pronounced effects on the improvement of glycemic control via increased activation of muscle phosphorylation signaling in type 2-diabetic rats [113].
A randomized, double-blind, placebo-controlled human study was conducted in 28 borderline-diabetic participants treated with either Chlorella (8 g/day) or placebo for 12 weeks [114]. The expression levels of 252 genes, including six associated with type 2 diabetes, differed between the two groups. Notably, the mRNA expression level of resistin, an insulin resistance inducer, was significantly lower in the Chlorella group than in the placebo group and correlated with the expression levels of hemoglobin A1c, tumor necrosis factor-a, and interleukin-6 [114], all of which are involved in glucose metabolism and/or inflammation.
3.4. Hepatoprotective Effect
Li et al. [115] demonstrated that C. vulgaris extract has a potent hepatoprotective effect on carbon tetrachloride-induced acute hepatic injury in mice. Chlorella extract of 50, 100, or 200 mg/kg of diet, was administered to mice every other day for four weeks, and carbon tetrachloride was administrated intraperitoneally 3 h after the final Chlorella supplement. Carbon tetrachloride treatment increased serum alanine and aspartate aminotransferases levels, lipid peroxidation, and cytochrome P450 expression and decrease in reduced glutathione and cellular antioxidant defense enzyme levels; all of these changes were significantly lower in the Chlorella (100 and 200 mg/kg diet) groups. Although hepatocyte necrosis was mildly diminished in the 50 mg/kg Chlorella-treated group, it was absent in the 100 and 200 mg/kg Chlorella-treated groups. These results indicate that Chlorella extract has a protective effect on carbon tetrachloride-induced acute hepatic injury in mice, presumably due to the inhibition of carbon tetrachloride-induced cytochrome P450 activation and the activation of antioxidant enzymes and free radical scavengers.
Non-alcoholic fatty liver disease (NAFLD) is a group of metabolic disorders that involving abnormal fat accumulation of more than 5–10% in hepatocytes [116]. It affects 10–35% of the world population [117]. NAFLD includes steatosis, non-alcoholic steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma [118]. Most NAFLD patients have at least one characteristic metabolic syndrome, including insulin resistance, hypertension, dyslipidemia, diabetes, and obesity [119]. Seventy NAFLD patients were randomly administered C. vulgaris (1.2 g/day) or placebo for eight weeks [120]. The mean body weight and serum concentrations of liver enzymes were significantly lower in the Chlorella group than in the placebo group, and the serum insulin concentration was significantly higher in the Chlorella group than in the placebo group. Therefore, Chlorella supplementation may have beneficial effects on reducing weight and serum glucose levels and improving inflammatory biomarkers as well as liver function in NAFLD patients [120,121].
To evaluate the safety and efficacy of Chlorella (C. pyrenoidosa) in patients chronically infected with hepatitis C virus genotype 1, patients received daily oral supplement of Chlorella (both of Chlorella extract and tablets) for 12 weeks [122]. The majority (approximately 85%) of the patients exhibited a significant decrease in alanine aminotransferase levels from Week 0 to Week 12. Patients with decreased alanine aminotransferase level showed a tendency toward decreased hepatitis C virus load.
3.5. Detoxification Effect
Dioxins are a group of polychlorinated dibenzo-p-dioxin and dibenzofuran-related compounds that are industrial contaminants and ubiquitous environmental pollutants [123]. These compounds are easily absorbed in the mammalian gastrointestinal tract [124] and then stored in the liver, adipose tissue, and breast milk due to their lipophilic properties [125]. An incident involving the consumption of cooking oil contaminated with dioxins had tragic effects [126]. To investigate the effects of Chlorella supplementation on fecal excretion of dioxins, rats were administered dioxin-contaminated rice oil [127]. The rats were fed 4 g of a 10% (w/w) Chlorella (C. vulgaris) diet or a control diet (without Chlorella) once during the five-day experimental period, and the amounts of fecal dioxins were measured. The fecal dioxin levels were significantly greater in the Chlorella group than in the control group. In addition, Chlorella supplementation significantly inhibited the gastrointestinal absorption of dioxins (approximately 2–53% decrease). These results indicate that Chlorella supplementation might be useful in promoting dioxin excretion.
Heterocyclic amines have been established as carcinogenic chemicals that form when amino acids, sugars, and creatine in muscle meats (beef, pork, fish, and poultry) react with one another during cooking at high temperatures [128]. To evaluate the effect of Chlorella supplementation on the detoxification of carcinogenic heterocyclic amines, a randomized, double-blind, placebo-controlled crossover study with Chlorella supplementation (100 mg/day) for two weeks was conducted [129]. Chlorella supplementation decreased urinary excretion of the predominant metabolite of carcinogenic heterocyclic amines [129], suggesting that Chlorella either inhibits the intestinal absorption of heterocyclic amines or inactivates carcinogenic compounds.
Methylmercury is a neurotoxic metal compound that is converted from inorganic mercury by microorganisms in aquatic environments and is then accumulated in fish and shellfish through marine food chains [130]. Therefore, the major route of human exposure to methylmercury is the consumption of seafood [130]. In many countries, pregnant women are cautioned against consuming large fish, such as tuna, to prevent fetal exposure [131]. As Chlorella consumption is reported to increase the excretion of methylmercury and lower tissue mercury levels in methylmercury-treated mice [132], an open-label clinical trial was performed to estimate the effects of Parachlorella beijerinckii supplementation (9 g/day) for three months on mercury concentrations in the hair and blood of healthy subjects [133]. Chlorella supplementation reduced mercury levels in both the hair and blood [133]. Fecal excretion is the major route of methylmercury elimination (90%) in humans [134]. Most of the methylmercury in the liver is secreted as a glutathione complex via the bile duct, with a small portion excreted in the feces [135]. The dietary fiber in Chlorella cells increases the amount of feces excreted by humans [136]. Dietary fiber has been shown to absorb some methylmercury in vitro [132]. These observations suggest that the observed lowering of hair and blood mercury levels in Chlorella-treated participants may result from the promotion of fecal methylmercury excretion via accelerated bile secretion, the binding of methylmercury to dietary fiber in the intestinal tract, and increased feces production.
3.6. Immunomodulatory Effects
Allergic disease is a prevalent aberrant immune responsive against innocuous environmental proteins (antigens) [137]. Allergen-specific CD4+ T cells involved in the initiation of allergic reactivity can develop into either type 1 or type 2 helper T cells [138]. CD4+ T cells stimulated in the presence of interleukin-12 and γ-interferon can develop into type 1 helper T cells [138], while interleukin-4 promotes the development of type 2 helper T cells and inhibits the generation of type 1 helper T cells [139]. Since type 1 and 2 helper T cells regulate each other, interleukin-12 functions not only to induce the type 1 helper T-cell response but also to regulate the type 2 helper T-cell response [140]. Interleukin-12 strongly suppresses the production of IgE by preventing type 2 helper T-cell development [141]. Allergen-specific IgE induces the pathogenesis of allergic disorder [142].
Hasegawa et al. [143] described the effects of a Chlorella (C. vulgaris) hot-water extract on antigen specific response in mice. A 2% (w/w) Chlorella hot-water extract diet or control diet (without Chlorella extract) was given to mice for two weeks before intraperitoneal administration of casein/complete Freund’s adjuvant (an immunostimulant). Mice that received the hot-water extract exhibited suppressed IgE production and mRNA expression of interleukin-6 involved in the type 2 helper T-cell response. They also exhibited increased levels of interleukin-12 and g-interferon mRNA, increasing the type 1 helper T-cell response and suppressing the type 2 helper T-cell response. These results suggest that Chlorella hot-water extract supplementation might be useful for suppressing allergic responses with a predominant type 2 helper T-cell response. To clarify the mechanisms underlying the immunomodulatory activity of Chlorella hot-water extract, soluble polysaccharides were isolated from C. pyrenoidosa hot-water extract and characterized [144]. GC-MS analysis indicated that the major monosaccharide components of the soluble polysaccharides are rhamnose (31.8%), glucose (20.4%), galactose (10.3%), mannose (5.2%), and xylose (1.3%). These soluble polysaccharides were intraperitoneally administrated (100 mg/kg of body weight) to 6–8-week-old mice. After 24 h, lipopolysaccharide as an antigen was administrated to mice, and their serum was collected 1.5 h later [144]. The soluble polysaccharides induced interleukin-1b secretion in macrophages via the toll-like receptor protein kinase signaling pathway. Interleukin-1β is one of the most important mediators of inflammation and host responses to infection [145]. These results suggest that Chlorella hot-water-soluble polysaccharides could be used as an agent source to stimulate anti-microorganism activity.
Halperin et al. [146] evaluated the effect of C. pyrenoidosa supplementation (200 or 400 mg) on the immune response to influenza vaccination. After 28 days of Chlorella supplementation, the antibody response to the influenza vaccine was not elevated in the overall study population but was increased in participants aged 50–55 years.
Salivary secretory immunoglobulin A (SIgA) plays a crucial role in mucosal immune function and is the first line of defense against pathogenic microbial invasion in human [147]. To evaluate whether Chlorella supplementation increases salivary SIgA secretion in humans, a blind, randomized, crossover study was conducted in participants administered Chlorella (C. pyrenoidosa) (6 g/day) or placebo for four weeks [148]. Although no difference was observed in salivary SIgA levels before and after placebo ingestion, salivary SIgA levels were significantly elevated after Chlorella ingestion than at baseline. The SIgA secretion rate increased significantly after Chlorella supplementation. These results suggest that four-week Chlorella supplementation increases salivary SIgA secretion and improves mucosal immune function in humans.
Natural killer cells are the predominant innate lymphocyte subsets that mediate antitumor and antiviral responses [149]. To evaluate the effect of Chlorella supplementation on natural killer cell activity and early inflammatory response in humans, a randomized, double-blinded, placebo-controlled trial was conducted in healthy adults ingested with Chlorella (C. vulgaris) (5 g/day) or placebo [150]. After eight weeks of supplementation, serum interferon-γ and interleukin-1β levels were significantly elevated and that of interleukin-12 tended to increase in the Chlorella group. Natural killer cell activities were significantly elevated in the Chlorella group. These results suggest a beneficial immunostimulatory effect of short-term Chlorella supplementation that increases natural killer cell activity and produces interferon-γ, interleukin-12, and interleukin-1β.
3.7. Antioxidant Effects
C. vulgaris hot-water extract [151] and acetone extract [152] are reported to have antitumor activity. A Chlorella aqueous extract containing substantial amounts of antioxidants also exhibit antiproliferative activity in human hepatoma cells [153]. Lipophilic pigments, including carotenoids antheraxanthin, zeaxanthin, and lutein, extracted from Chlorella cells were observed to significantly inhibit the growth of human colon cancer cells [154]. These results suggest that the antitumor activity of Chlorella might be the synergistic effect of multiple bioactive compounds. Romos et al. [155] reported that Chlorella supplementation can modulate immunomyelopoietic activity and disengage tumor-induced suppression of various cytokines and related cell activities in tumor-bearing mice. Interestingly, a 63.1-kD antitumor glycoprotein was isolated from the culture supernatant of C. vulgaris strain CK22 [156,157], and its chemical and antitumor properties were characterized [158], suggesting possible contribution of this glycoprotein toward the observed antitumor activity.
Alzheimer’s disease is a severe neurodegenerative condition affecting humans [159]. The erythrocytes of Alzheimer’s disease patients are known to be in an excessively oxidized state [160]. α-Tocopherol and carotenoids such as lutein are important lipophilic antioxidants in human erythrocytes [161]. Erythrocyte lutein levels were found to be significantly lower in Alzheimer’s disease patients than in normal subjects [162]. Oral intake of lutein capsules increases lutein levels and prevents phospholipid hydroperoxide accumulation in human erythrocytes [163], suggesting that dietary lutein has the potential to act as an important antioxidant in erythrocytes and thus may have beneficial effects in Alzheimer’s disease patients. According to the labels on Chlorella products D and M, the products contain substantial amounts of lutein (approximately 200 mg/100 g dry weigh). A randomized, double-blind, placebo-controlled human study was conducted to evaluate the effects of Chlorella supplementation (8 g Chlorella/day/person; equivalent to 22.9 mg lutein/day/person) on phospholipid hydroperoxide and lutein levels in erythrocytes [164]. After two months of Chlorella supplementation, erythrocyte lutein levels increased 4.6-fold, but tocopherol levels did not change [164], suggesting that daily Chlorella intake may be effective for improving and maintaining erythrocyte antioxidant status and lutein levels in humans. These results suggest that Chlorella supplementation contributes to maintaining the normal function of erythrocytes and has beneficial effects on Alzheimer’s disease-related dementia in humans.
Major depressive disorder is a widespread mental disorder that greatly impairs the quality of life of humans. Approximately 12% of people experience at least one episode of depression during their lifetime [165]. Although various antidepressant drugs are available for treating depression, a considerable proportion of patients are not responsive to these drugs and some experience side effects [166,167]. Therefore, alternative antidepressant drugs with adequate efficacy and safety are needed. The therapeutic effect of dried C. vulgaris extract administration (1.8 g/day) for six weeks was evaluated in patients with major depressive disorder [168]. After treatment, the participants exhibited improvements in physical and cognitive symptoms of depression [168]. As oxidative stress is an important pathophysiological mechanism underlying major depressive disorder, major depressive disorder has been effectively reversed via antioxidant therapy [169,170]. These observations suggest that the therapeutic effectiveness of Chlorella supplementation may result from the action of its antioxidant nutrients and compounds [171].
3.8. Other Effects
Stress is well known to disturb homeostasis, impairing immunological functions. Chlorella supplementation reportedly stimulates the pool of hematopoietic stem cells and activates leukocytes [172]. To further understand the influence of Chlorella (C. vulagaris) supplementation on hematopoiesis, hematopoietic cell populations in the bone marrow of mice subjected to a single or repeated stressor were measured [173]. Reduced numbers of hematopoietic progenitors in the bone marrow were observed after treatment with either stressor. Both stressors induced a decrease in mature myeloid and lymphoid populations but did not affect pluripotent hematopoietic progenitors. Both stressors reduced the levels of interleukin-1α and interleukin-6. Chlorella supplementation prevented the changes produced by both stressors in all of the parameters tested, suggesting that Chlorella supplementation is an effective tool for the prophylaxis of myelosuppression caused by single or repeated stressors.
Stressors are processed in the brain through the activation of several types of neurons. Immediate early genes such as c-fos are extensively used to map brain areas involved in stress responses [174]. Using c-fos expression, Oueiroz et al. [175] evaluated the effect of acute pretreatment with Chlorella (C. vulgaris) on the peripheral and central responses to forced swimming stress in rats. Chlorella supplementation produced a significant reduction in stress-related hypothalamic–pituitary–adrenal axis activation due to decreased corticotrophin releasing factor gene expression in the hypothalamic paraventricular nucleus and a lower adrenocorticotropic hormone response. Hyperglycemia induced by the stressor was similarly reduced. These results suggest that Chlorella supplementation might reduce the impact of stressors.
A hot-water extract of Chlorella (C. pyrenoidosa) increased the lifespan of superoxide dismutase-1 mutant adults of Drosophila melanogaster in a dose-dependent manner (200–800 µg/mL) [176]. An active compound was purified and identified as phenethylamine, an aromatic amine, which exhibited no superoxide dismutase-like activity. Treatment with this compound extended the lifespan of the mutant flies at very low concentration (60 ng/g diet) [176]. Similarly, supplementation of C. sorokiniana (4 mg/mL) reportedly increased the lifespan of D. melanogaster by 10% increase as compared to a control diet, likely due to the increased mRNA expression of antioxidative enzymes (Cu/Zn-superoxide dismutase and catalase) [177].
However, for the beneficial effects described above, no human study has been conducted.
4. Conclusions
Commercially available Chlorella products contain a variety of nutrients essential for humans, as well as a large amount of good quality protein, dietary fibers, and polyunsaturated fatty acids, including α-linolenic and linoleic acids. In particular, Chlorella products contain vitamins D2 and B12, which are absent from plant-derived food sources, and larger amounts of folate and iron than other plant-derived foods. Mounting scientific evidence of the health benefits of daily Chlorella consumption has been presented in animal and human studies. The pharmacological activities reported in Chlorella studies include immunomodulation, antioxidative activity, and effects against diabetes, hypertension, and hyperlipidemia. The beneficial effects of Chlorella might involve synergism between multiple nutrient and antioxidant compounds. Overall, the information regarding bioactive compounds in Chlorella is limited. Thus, new bioactive compounds responsible for its pharmacological activities may be identified in future studies.
Author Contributions
T.B., E.O., M.F., and F.W. conceived of and designed the study. T.B. and F.W. wrote the original draft. E.O. and M.F. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The potential for microalgae as bioreactors to produce pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Beijerinck, M.W. Culturversuche mit Zoochlorellen, Lichenengonidien und anderen niederen Algen. Botanische Zeitung 1890, 47, 725–739. [Google Scholar]
- Chick, H. A study of a unicellular green alga, occurring in polluted water, with especial reference to its nitrogenous metabolism. Proc. Royal Soc. Biol. Sci. Ser. B 1903, 71, 458–476. [Google Scholar]
- Wu, H.L.; Hseu, R.S.; Lin, L.P. Identification of Chlorella spp. isolates using ribosomal DNA sequences. Bot. Bull. Acad. Sin. 2001, 42, 115–121. [Google Scholar]
- Krienitz, L.; Hegewald, E.H.; Hepperle, D.; Huss, V.A.R.; Rohr, T.; Wolf, M. Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chloropyta, Trebouxiophyceae). Phycologia 2004, 43, 529–542. [Google Scholar] [CrossRef]
- Sorokin, C.; Myers, J. A high-temperature strain of Chlorella. Science 1953, 117, 330–331. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. [Google Scholar] [CrossRef]
- Montoya, E.Y.O.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Converti, A.; Carvalho, M. Production of Chlorella vulgaris as a source of essential fatty acids in a tubular photobioreactor continuously fed with air enriched with CO2 at different concentrations. Biotechnol. Prog. 2014, 30, 916–922. [Google Scholar] [CrossRef]
- Rani, K.; Sandal, N.; Sahoo, P.K. A comprehensive review on chlorella-its composition, health benefits, market and regulation scenario. Pharma Innov. J. 2018, 7, 583–589. [Google Scholar]
- De Ortega, A.R.; Roux, J.C. Production of Chlorella biomass in different types of Flat bioreactors in temperate zones. Biomass 1986, 10, 141–156. [Google Scholar] [CrossRef]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. App. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Shukla, S.P.; Kvíderová, J.; Tríska, J.; Elster, J. Chlorella mirabilis as a potential species for biomass production in low-temperature environment. Front. Microbiol. 2013, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Ward, V.C.A.; Rehmann, L. Rast media optimization for mixotrophic cultivation of Chlorella vulgaris. Sci. Rep. 2019, 9, 19262. [Google Scholar] [CrossRef]
- Komaki, H.; Yamashita, M.; Niwa, Y.; Tanabe, Y.; Kamiya, N.; Ando, Y.; Furuse, M. The effect of processing of Chlorella vulgaris: K-5 on in vitro and in vivo digestibility in rats. Anim. Feed Sci. Technol. 1998, 70, 363–366. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a sourse of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pynenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Recent advances in arginine metabolism: Roles and regulation of the arginases. Br. J. Pharm. 2009, 157, 922–930. [Google Scholar] [CrossRef]
- Bansal, V.; Ochoa, J.B. Arginine availability, arginase, and the immune response. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, S.A.; Northcote, D.H. Polysaccharides of Chlorella pyrenoidosa. Biochem. J. 1962, 82, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Gizaw, Y.; BeMiller, J.N. Extraction of polysaccharides from a species of Chlorella. Carbohydr. Polym. 2012, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, M.; Gui, J.; Huang, S.; Liu, Y.; Shentu, H.; He, J.; Fang, Z.; Wang, W.; Zhag, Y. Preparation of Chlorella vulgaris polysaccharides and their antioxidant activity in vitro and in vivo. Int. J. Biol. Macromol. 2019, 137, 139–150. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharaides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef]
- Tabarsa, M.; Shin, I.S.; Lee, J.H.; Surayot, U.; Park, W.J.; You, S.G. An immune-enhancing water-soluble a-glucan from Chlorella vulgaris and structural characteristics. Food Sci. Biotechnol. 2015, 24, 1933–1941. [Google Scholar] [CrossRef]
- Ötles, S.; Pire, R. Fatty acid composition of Chlorella and Spirulina microalgae species. J. AOAC Int. 2001, 84, 1708–1714. [Google Scholar] [CrossRef]
- Panahi, Y.; Khosroshahi, A.Y.; Sahebkar, A.; Heidari, H.R. Impact of cultivation condition and media content on Chlorella vulgaris composition. Adv. Pharm. Bull. 2019, 9, 182–194. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, T.Y.; Chang, Y.B.; Kuo, C.M.; Lin, C.S. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef]
- Mao, X.; Wu, T.; Sun, D.; Zhang, Z.; Chen, F. Differential responses of the green microalga Chlorella zofingiensis to the starvation of various nutrients for oil and astaxanthin production. Bioresour. Technol. 2018, 249, 791–798. [Google Scholar] [CrossRef]
- Woortman, D.V.; Fuchs, T.; Striegel, L.; Fuchs, M.; Weber, N.; Brück, T.B.; Rychlik, M. Microalae a superior source of folates: Quantification of folates in halophile microalgae by stable isotope dilution assay. Front. Bioeng. Biotechnol. 2020, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Fernandes, A.; Barreiro, M.F.; Ferreira, I.C.F.R. UV-irradiated mushrooms as a source of vitamin D2: A review. Trends Food Sci. Technol. 2017, 70, 82–94. [Google Scholar] [CrossRef]
- Holman, E.H.; Martin, B.R.; Lackcik, P.L.; Godeon, D.T.; Fleet, J.C.; Weaver, C.M. Bioavailability and efficacy of vitamin D2 from UV-irradiated yeast in growing, vitamin D-deficient rats. J. Agric. Food Chem. 2011, 59, 2341–2346. [Google Scholar]
- Ložnjak, P.; Jakobsen, J. Stability of vitamin D3 and vitamin D2 in oil, fish and mushrooms after household cooking. Food Chem. 2018, 254, 144–149. [Google Scholar] [CrossRef]
- Cardwell, G.C.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef]
- Weete, J.D.; Abril, M.; Blackwell, M. Phylogenetic distribution of fungal sterols. PLoS ONE 2010, 5, e10899. [Google Scholar] [CrossRef]
- Huang, G.; Cai, W.; Xu, B. Vitamin D2, ergosterol, and vitamin B2 content in commercially dried mushrooms. Int. J. Vitam. Nutr. Res. 2016, 1, 1–10. [Google Scholar]
- Urbain, P.; Singler, F.; Ihorst, G.; Biesalski, H.K.; Bertz, H. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: A randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 965–971. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kiely, M.; Seamans, K.M.; Urbain, P. Effect of Ultraviolet light-exposed mushrooms on vitamin D status: Liquid chromatography-tandem mass spectrometry reanalysis of biobanked sera from a randomized controlled trial and a systematic review plus meta-analysis. J. Nutr. 2016, 146, 565–575. [Google Scholar] [CrossRef]
- Klosty, M.; Bergmann, W. Sterols of algae. III. The occurrence of ergosterol in Chlorella pyranoidosa. J. Am. Chem. Soc. 1952, 74, 1601. [Google Scholar] [CrossRef]
- Patterson, G.W. Sterols of Chlorella. II. The occurrence of an unusual sterol mixture in Chlorella vulgaris. Plant. Physiol. 1967, 42, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, C.; Lu, Y. Preparative separation of phytosterol analogues from green alga Chlorella vulgaris using recycling counter-current chromatography. J. Separ. Sci. 2017, 40, 2326–2334. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 106, 481–490. [Google Scholar] [CrossRef]
- Selhub, J.; Jacques, P.F.; Bostom, A.G.; D’Agostino, R.B.; Wilson, P.W.F.; Belanger, A.J.; O’Leary, D.H.; Wolf, P.A.; Schaffer, E.J.; Rosenberg, I.H. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N. Engl. J. Med. 1995, 332, 286–291. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the develop of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjorke-Mercado, A.L.; Brto, A.; Gueant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Prim. 2017, 3, 17040. [Google Scholar] [CrossRef]
- Miller, J.W.; Ribaya-Meercado, J.D.; Russell, R.M.; Shepard, D.C.; Morrow, F.D.; Cochary, E.F.; Sadowski, J.A.; Gershoff, S.N.; Selhub, J. Effect of vitamin B6-deficiency on fasting plasma homocysteine concentrations. Am. J. Clin. Nutr. 1992, 55, 1154–1160. [Google Scholar] [CrossRef]
- Forges, T.; Monnier-Barbarino, P.; Alberto, J.M.; Gueant-Rodriguez, R.M.; Daval, J.L.; Gueant, J.L. Impact of folate and homocysteine metabolism on human reproductive health. Hum. Reprod. Update 2007, 13, 225–238. [Google Scholar] [CrossRef]
- Celik, S.F.; Celik, E. Subclinical atherosclerosis and impaired cardiac autonomic control in pediatric patients with vitamin B12 deficiency. Niger. J. Clin. Pract. 2018, 21, 1012–1016. [Google Scholar]
- Bunout, D.; Petermann, M.; Hirsch, S. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition 2000, 16, 434–438. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bránhidy, F. The prevention of neural-tube defects and congenital heart defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef] [PubMed]
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Van der Straeten, D. Fotes in plants: Research advances and progress in crop biofortification. Front. Chem. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Delchier, N.; Herbig, A.L.; Rychlik, M.; Renard, M.G.C. Folates in fruits and vegetables: Contents, processing and stability. Comp. Rev. Food Sci. Food Saf. 2016, 15, 506–528. [Google Scholar] [CrossRef]
- Watanabe, F.; Bito, T. Corrinoids in food and biological samples. In Frontiers in Natural Product Chemistry; Atta-ur-Rahman, Ed.; Bentham Science Publishers: Dubai, UAE, 2016; Volume 2, pp. 229–244. [Google Scholar]
- Watanabe, F.; Bito, T. Vitamin B12 sources and microbial interaction. Exp. Biol. Med. 2018, 243, 148–158. [Google Scholar] [CrossRef]
- Bito, T.; Tanioka, Y.; Watanabe, F. Characterization of vitamin B12 compounds from marine foods. Fish. Sci. 2018, 84, 747–755. [Google Scholar] [CrossRef]
- Kittaka-Katsura, H.; Fujita, T.; Watanabe, F.; Nakano, Y. Purification and characterization of a corrinoid compound from Chlorella Tablets as an algal health food. J. Agric. Food Chem. 2002, 50, 4994–4997. [Google Scholar] [CrossRef]
- Bito, T.; Bito, M.; Asai, Y.; Takenaka, S.; Yabuta, Y.; Tago, K.; Ohnishi, M.; Mizoguchi, T.; Watanabe, F. Characterization and quantitation of vitamin B12 compounds in various Chlorella supplements. J. Agric. Food Chem. 2016, 64, 8516–8524. [Google Scholar] [CrossRef]
- Watanabe, F.; Abe, K.; Takenaka, S.; Tamura, Y.; Maruyama, I.; Nakano, Y. Occurrence of cobalamin coenzymes in the photosynthetic green alga, Chlorella vulgaris. Biosci. Biotechnol. Biochem. 1997, 61, 896–897. [Google Scholar] [CrossRef]
- Rauma, A.L.; Törrönen, R.; Hänninen, O.; Mykkänen, H. Vitamin B12 status of long-term adherents of strict uncooked vegan diet (“living food diet”) is comprised. J. Nutr. 1995, 125, 2511–2515. [Google Scholar]
- Merchant, R.E.; Phillips, T.W.; Udani, J. Nutritional supplementation with Chlorella pyrenoidosa lowers serum methylmalonic acid in vegans and vegetarians with a suspected vitamin B12 deficiency. J. Med. Food 2015, 18, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Adrogué, H.J.; Madias, N.E. Sodium and potassium in pathogenesis of hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Raboy, V.; King, J.C. Implication of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr. Rev. 2018, 76, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Guo, W.; Zeng, M.; Feng, Y.; Feng, G. Effect of microalgae as iron supplements on ion-deficiency anemia in rats. Food Funct. 2019, 10, 723–732. [Google Scholar] [CrossRef]
- Nakano, S.; Takekoshi, H.; Nakano, M. Chlorella pyrenoidosa supplementation reduces the risk of anemia, proteinuria and edema in pregnant women. Plant Food Hum. Nutr. 2010, 65, 25–30. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Allen, C.B.; Lacourciere, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium. Ann. Rev. Nutr. 1999, 19, 1–16. [Google Scholar] [CrossRef]
- Sunde, R.A. Handbook of nutritionally essential mineral elements. In Handbook of Nutritionally Essential Mineral Elements; O’Dell, B.L., Sunde, R.A., Eds.; Marcel Dekker Inc: New York, NY, USA, 1997; pp. 493–556. [Google Scholar]
- White, P.J.; Brown, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Trueman, L.J.; Smith, B.M.; Thomas, B.; et al. Interactions between selenium and Sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K.; Kotrbáček, V.; Zachleder, V. Production of Chlorella biomass enriched by selenium and its use in animal nutrition: A review. Appl. Microbiol. Biotechnol. 2009, 83, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhong, Y.; Huang, Z.; Yang, Y. Selenium accumulation in unicellular green alga Chlorella vulgaris and its effects on antioxidant enzymes and content of photosynthetic pigments. PLoS ONE 2014, 9, e112270. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.F.; Zheng, W.J.; Wong, Y.S.; Yang, F. Selenium-induced changes in activities of antioxidant enzymes and content of photosynthetic pigments in Spirulina platensis. J. Integr. Plant. Biol. 2008, 50, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant. 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef]
- Boussiba, S.; Bing, W.; Yuan, J.P.; Zarka, A.; Chen, F. Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 20, 601–604. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Connor, T.P.; O’Brien, N.M. The content and bioaccessibility of carotenoids from selected commercially available health supplements. Proc. Nutr. Soc. 2011, 70, E62. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibάañez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Lui, J.; Sun, Z.; Gerken, H.; Liu, S.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar]
- McFarlane, S.I.; Jean-Lours, G.; Zizi, F.; Whaley-Connel, A.T.; Ogedegbe, O.; Nakaryus, A.N.; Maraj, I. Hypertension in the high-cardiovascular-risk populations. Int. J. Hypertens. 2012, 2011, 746369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamori, Y. Experimental evidence for dietary prevention of cardiovascular disease. Clin. Exp. Pharm. Physiol. 1989, 16, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Sansawa, H.; Takahashi, M.; Tsuchikura, S.; Endo, H. Effect of Chlorella and its fractions on blood pressure, cerebral stroke lesions, and life-span in stroke-prone spontaneously hypertensive rats. J. Nutr. Sci. Vitam. 2006, 52, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.E.; Andre, C.A.; Sica, D.A. Nutritional supplementation with Chlorella pyrenoidosa for mild to moderate hypertension. J. Med. Food 2002, 5, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Terentes-Printzios, D.; Ioakeimidis, N.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 2012, 60, 556–562. [Google Scholar] [CrossRef]
- Plantinga, Y.; Ghiadoni, L.; Magagna, A.; Giannarelli, C.; Franzoni, F.; Salvetti, A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am. J. Hypertens. 2007, 20, 392–397. [Google Scholar] [CrossRef]
- Nestel, P.J.; Pomeroy, S.E.; Sasahara, T.; Yamashita, T.; Ling, Y.L.; Dart, A.M.; Jennings, G.L.; Abbey, M.; Cameron, J.D. Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. Arter. Thromb. Vasc. Biol. 1997, 17, 1163–1170. [Google Scholar] [CrossRef]
- He, F.J.; Marciniak, M.; Carney, C.; Markandu, N.D.; Anand, V.; Fraser, W.D.; Dalton, R.N.; Kaski, J.C.; MacGregor, G.A. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension 2010, 55, 681–688. [Google Scholar] [CrossRef]
- Kinlay, S.; Creager, M.A.; Fukumoto, M.; Hikita, H.; Fang, J.C.; Selwyn, A.P.; Ganz, P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension 2001, 38, 1049–1053. [Google Scholar] [CrossRef]
- Osuki, T.; Shimizu, K.; Iemitsu, M.; Kono, I. Multicomponent supplement containing Chlorella decreases arterial stiffness in healthy young men. J. Clin. Biochem. Nutr. 2013, 53, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Otuski, T.; Shimizu, K.; Maeda, S. Changes in arterial stiffness and nitric oxide production with Chlorella-derived multicomponent supplementation in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Sarmast, E.D.; Dehkordi, S.H.; Engardeh, J.; Mahmoodnia, L.; Khaledifar, A.; Jafari, T. Effect of Chlorella supplementation on cardiovascular risk factors: A meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Otvos, J.D.; Rifai, N.; Rosenson, R.S.; Buring, J.E.; Ridker, P.M. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009, 119, 931–939. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. J. Am. Med Assoc. 2007, 298, 299–308. [Google Scholar] [CrossRef]
- Sacks, F.M.; Alaupovic, P.; Moye, L.A.; Cole, T.G.; Sussex, B.; Stampfer, M.J.; Pfeffer, M.A.; Braunwald, E. VLDL, apolipoproteins B, CIII and E, and risk of recurrent coronary events in the cholesterol and recurrent events (CARE) trial. Circulation 2000, 102, 1886–1892. [Google Scholar] [CrossRef]
- Adnerson, J.W.; Deakins, D.A.; Bridges, S.R. Soluble fiber: Hypocholesteromic effects and proposed mechanisms. In Dietary Fiber: Chemistry, Physiology and Health Effects; Kritchevsky, D., Bonfield, C., Anderson, J.W., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 339–363. [Google Scholar]
- Cherng, J.Y.; Shih, M.F. Preventing dyslipidemia by Chlorella pyrenoidosa in rats and hamsters after chronic high fat diet treatment. Life Sci. 2005, 76, 3001–3013. [Google Scholar] [CrossRef]
- Shibata, S.; Oda, K.; Onodera-Masuoka, N.; Matsubara, S.; Kikuchi-Hayakawa, H.; Ishikawa, F.; Iwabuchi, A.; Sansawa, H. Hypocholesteromic effect of indigestible fraction of Chlorella regularis in cholesterol-fed rats. J. Nutr. Sci. Vitam. 2001, 47, 373–377. [Google Scholar] [CrossRef]
- Shibata, S.; Hayakawa, K.; Egashira, Y.; Sanada, H. Hypocholesteromic mechanism of Chlorella: Chlorella and its indigestible fraction enhance hepatic cholesterol catabolism through up-regulation of cholesterol 7α-hydroxylase in rats. Biosci. Biotechnol. Biochem. 2007, 71, 916–925. [Google Scholar] [CrossRef]
- Sansawa, H.; Inoue, K.; Shirai, T. Effect of Chlorella tablet ingestion on mild hypercholesterolemic patients. J. Jpn. Soc. Food Sci. Technol. 2002, 49, 167–173. [Google Scholar] [CrossRef][Green Version]
- Ryu, N.H.; Lim, Y.; Park, J.E.; Kim, J.; Kim, J.Y.; Kwon, S.W.; Kwon, O. Impact of daily Chlorella consumption on serum lipid and carotenoid profiles in mild hypercholesterolemic adults: A double-blinded, randomized, placebo-controlled study. Nutr. J. 2014, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Lim, Y.; Kim, Y.J.; Kim, J.Y.; Kwon, O. A dietary cholesterol challenge study to assess Chlorella supplementation in maintaining healthy lipid levels in adults: A double-blinded, randomized, placebo-controlled study. Nutr. J. 2016, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Magon, A.; Baroni, I.; Dellafiore, F.; Arrigoni, C.; Pittella, F.; Ausili, D. Health literacy in type 2 diabetes patients: A systematic review of systematic reviews. Acta Diabetol. 2018, 55, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. Impact of different dietary approaches on glycemic control and cardiovascular risk factors in patients with type 2 diabetes: A protocol for a systematic review and network meta-analysis. Sys. Rev. 2017, 6, 57. [Google Scholar] [CrossRef]
- De Groot, M.; Anderson, R.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. Association for depression and diabetes complications: A meta-analysis. Psychosom. Med. 2001, 63, 619–630. [Google Scholar] [CrossRef]
- Shibata, S.; Natori, Y.; Nishihara, T.; Tomisaka, K.; Matsumoto, K.; Sansawa, H.; Nguyen, V.C. Antioxidant and anti-cataract effects of Chlorella on rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitam. 2003, 49, 334–339. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Shih, M.F. Potential hypoglycemic effects of Chlorella in streptozotocin-induced diabetic mice. Life Sci. 2005, 77, 980–990. [Google Scholar]
- Cherng, J.Y.; Shih, M.F. Improving glycogenesis in streptozotocin (STZ) diabetic mice after administration of green algae Chlorella. Life Sci. 2006, 78, 1181–1186. [Google Scholar] [CrossRef]
- Vecina, J.F.; Oliveira, A.G.; Araujo, T.G.; Baggio, S.R.; Torello, C.O.; Saad, M.J.A.; Queiroz, M.L.S. Chlorella modulates insulin signaling pathway and prevents high-fat diet-induced insulin resistance in mice. Life Sci. 2014, 95, 45–52. [Google Scholar] [CrossRef]
- Horii, N.; Hasegawa, N.; Fujii, S.; Uchida, M.; Iemitsu, K.; Inoue, K.; Iemitsu, M. Effect of combination of chlorella intake and aerobic exercise training on glycemic control in type 2 diabetic rats. Nutrition 2019, 63–64, 45–50. [Google Scholar] [CrossRef]
- Itakura, H.; Kobayashi, M.; Nakamura, S. Chlorella ingestion suppresses resistin gene expression in peripheral blood cells of borderline diabetics. Clin. Nutr. ESPEN 2015, 10, e95–e101. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, Y.; Lee, Y.W. Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp. Toxicol. Pathol. 2013, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Paradis, V.; Bedossa, P. Definition and natural history of metabolic steatosis: Histology and cellular aspects. Diabetes Metab. 2008, 34, 638–642. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Ben, M.D.; Polimeni, L.; Baratta, F.; Pastori, D.; Loffredo, L.; Angelico, F. Modern approach to the clinical management of non-alcoholic fatty liver disease. World J. Gastroentrol. 2014, 20, 8341–8350. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Farhangi, M.A.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2017, 36, 1001–1006. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Aliashrafi, S.; Javadzadeh, Y.; AsghariJafarabadi, M. The effect of Chlorella vulgaris supplementation on liver enzymes, serum glucose and lipid profile in patients with non-alcoholic fatty liver disease. Health Promot. Perspect. 2014, 4, 107–115. [Google Scholar]
- Azocar, J.; Diaz, A. Efficacy and safety of Chlorella supplementation in adults with chronic hepatitis C virus infection. World J. Gastroenterol. 2013, 19, 1085–1090. [Google Scholar] [CrossRef]
- Schecter, A.; Startin, J.; Wright, C.; Kelly, M.; Papke, O.; Lis, A.; Ball, M.; Olson, J.R. Dioxins in, U.S. food and estimated daily intake. Chemosphere 1994, 29, 2261–2265. [Google Scholar] [CrossRef]
- Ven den Berg, M.; Jongh, J.; Poiger, H.; Olson, J.R. The toxicokinetics and metabolism of polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) and their relevance for toxicity. Crit. Rev. Toxicol. 1994, 24, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Papke, O.; Lis, A.; Ball, M.; Ryan, J.J.; Olson, J.R.; Li, L.; Kessler, H. Decrease in milk and blood dioxin levels over two years in a mother nursing twins: Estimates of decreased maternal and increased infant dioxin body burden from nursing. Chemosphere 1996, 32, 543–549. [Google Scholar] [CrossRef]
- Masuda, Y.; Kuroki, H.; Haraguchi, H.; Nagayama, J. PCB and PCDF congeners in the blood and tissues of Yusho and Yu-cheng patients. Envion. Health Perspect. 1985, 59, 53–58. [Google Scholar]
- Morita, K.; Matsueda, T.; Iida, T.; Hasegawa, T. Chlorella accelerates dioxin excretion in rats. J. Nutr. 1999, 129, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Kinze, M.G.; Dolbere, F.A.; Carroll, K.L.; Moore, D.H., II; Felton, J.S. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food Chem. Toxicol. 1994, 32, 595–603. [Google Scholar]
- Lee, I.; Tran, M.; Evans-Nguyen, T.; Stickle, D.; Kim, S.; Han, J.; Park, J.Y.; Yang, M. Detoxification of chlorella supplement on heterocyclic amines in Korean young adults. Environ. Toxicol. Pharm. 2015, 39, 441–446. [Google Scholar] [CrossRef]
- Harding, G.; Dalziel, J.; Vass, P. Bioaccumulation of methylmercury within the marine food web of the outer bay of Fundy, gulf of maine. PLoS ONE 2018, 13, e0197220. [Google Scholar] [CrossRef]
- His, H.C.; Hsu, Y.W.; Chang, T.C.; Chien, L.C. Methylmercury concentration in fish and risk-benefit assessment of fish intake among pregnant versus infertile women in Taiwan. PLoS ONE 2016, 11, e0155704. [Google Scholar]
- Uchikawa, T.; Yasutake, A.; Kumamoto, Y.; Maruyama, I.; Kumamoto, S.; Ando, Y. The influence of Parachlorella beijerinckii CK-5 on the absorption and excretion of methylmercury (MeHg) in mice. J. Toxicol. Sci. 2010, 35, 101–105. [Google Scholar] [CrossRef]
- Maruyama, I.; Uchikawa, T.; Kanno, T.; Ando, Y.; Kitsuki, H.; Yasutake, A. Chlorella supplementation decreases methylmercury concentrations of hair and blood in healthy volunteers. Fundam. Toxicol. Sci. 2018, 5, 117–122. [Google Scholar] [CrossRef]
- Miettinen, J.K.; Rahola, T.; Hattula, T.; Rissanen, K.; Tillander, M. Elimination of 203Hg-methylmercury in man. Ann. Clin. Res. 1971, 3, 116–122. [Google Scholar] [PubMed]
- Ballatori, N.; Clarkson, T.W. Biliary transport of glutathione and methylmercury. Am. J. Physiol 1983, 244, G435–G441. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Sinpo, K.; Imae, Y.; Nonomura, M.; Hirakawa, K. Effect of Chlorella vulgaris strain CK-5 on the frequency of bowel movement in humans. Ipn. J. Nutr. 1998, 56, 253–263. [Google Scholar]
- Nauta, A.; Engels, F.; Knippples, L.M.; Garssen, J.; Nijkamp, F.; Redegeld, F.A. Mechanisms of allergy and asthma. Eur. J. Pharmcol. 2008, 585, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Bancroft, G.J.; Schreiber, R.D.; Unanue, E.R. Natural immunity: A T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol. Rev. 1991, 124, 5–24. [Google Scholar] [CrossRef]
- Wynn, T.A.; Jankovic, D.; Hieny, S.; Cheever, A.W.; Sher, A. IL-12 enhances vaccine induced immunity to Schistosomiasia mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J. Immumol. 1995, 154, 4701–4709. [Google Scholar]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef]
- Hasegawa, T.; Ito, K.; Ueno, S.; Kumamoto, S.; Ando, Y.; Yamada, A.; Nomoto, K.; Yasunobu, Y. Oral administration of hot water extracts of Chlorella vulgaris reduces IgE production against milk casein in mice. Int. J. Immunopharmacol. 1999, 21, 311–323. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Jeyashoke, N.; Yeh, C.H.; Song, Y.J.; Hua, K.F.; Chao, L.K. Immunostimulatory bioactivity of algal polysaccharides from Chlorella pyrenoidosa activates macrophages via Toll-like receptor 4. J. Agric. Food Chem. 2010, 58, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Eder, C. Mechanisms of interkeukin-1β release. Immnobiology 2009, 214, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Smith, B.; Nolan, C.; Shay, J.; Kralovec, J. Safety and immunoenhancing effect of a Chlorella-derived dietary supplement in healthy adults undergoing influenza vaccination: Randomized, double-bind, placebo-controlled trial. Can. Med Assoc. J. 2003, 169, 111–117. [Google Scholar]
- Lamm, M.E.; Nedrud, J.G.; Kaetzel, C.S.; Mazanec, M.B. IgA and mucosal defense. Acta Pathol. Microbiol. Immunol. Scand. 1995, 103, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Shimizu, K.; Iemitsu, M.; Kono, I. Salivary secretory immunoglobulin a secretion increases after 4-weeks ingestion of chlorella-derived multicomponent supplement in humans: A randomized cross over study. Nutr. J. 2011, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural killer cells: Development, maturation, and clinical utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Kwak, J.H.; Baek, S.H.; Woo, Y.; Han, J.K.; Kim, B.G.; Kim, O.Y. Beneficial immunostimulatory effect of short-term Chlorella supplementation: Enhancement of Natural Killer cell activity and early inflammatory response (randomized, double-blinded, placebo-controlled trial). Nutr. J. 2012, 11, 53. [Google Scholar] [CrossRef]
- Konishi, F.; Tanaka, K.; Himeno, K.; Taniguchi, K.; Nomoto, K. Antitumor effect induced by a hot water extract of Chlorella vultaris (CE): Resistance to Meth-A tumor growth mediated by CE-induced polymorphonuclear leukocytes. Cancer Immunol. Immunother. 1985, 19, 73–78. [Google Scholar] [CrossRef]
- Tanaka, K.; Tomita, Y.; Tsuruta, M.; Konishi, F.; Okuda, M.; Himeno, K. Oral administration of Chlorella vulgaris augments concomitant antitumor immunity. Immunopharmacol. Immunotoxicol. 1990, 12, 277–291. [Google Scholar] [CrossRef]
- Wu, L.C.; Ho, J.A.; Shieh, M.C.; Lu, I.W. Antioxidant and antiproliferatives of Spirulina and Chlorella water extracts. J. Agric. Food Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef]
- Cha, K.H.; Koo, S.Y.; Lee, D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Romos, A.L.; Torello, C.O.; Queiroz, M.L.S. Chlorella vulgaris modulates immunomyelopoietic activity and enhances the resistance of tumor-bearing mice. Nutr. Cancer 2010, 62, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Tanaka, K. A water-soluble glycoprotein from Chlorella vulgaris. Planta Med. 1996, 62, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Ohno, N.; Tanaka, K. A new type of biological response modifier from Chlorella vulgaris which needs protein moiety to show antitumor activity. Phytother. Res. 1998, 12, 309–319. [Google Scholar] [CrossRef]
- Hasegawa, T.; Matsuguchi, T.; Noda, K.; Tanaka, K.; Kumamoto, S.; Shoyama, Y.; Yoshikai, Y. Toll-like receptor 2 is at least partly involved in the antitumor activity of glycoprotein from Chlorella vulgaris. Int. Immunopharmacol. 2002, 2, 579–589. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Bosman, G.J.; Barholomeus, I.G.; de Man, A.J.; van Kalmthout, P.J.; de Grip, W.J. Erythrocyte membrane characteristics indicate abnormal cellular aging in patients with Alzheimer’s disease. Neurobiol. Aging. 1991, 12, 13–18. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Hatade, K.; Asai, A.; Kumra, F.; Sookwong, P.; Tsuduki, T.; Arai, H.; Miyazawa, T. Development of a high-performance liquid chromatography-based assay for carotenoids in human red blood cells: Application to clinical studies. Anal. Biochem. 2008, 381, 129–134. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Suzuki, T.; Arai, H.; Miyazawa, T. Significance of lutein in red blood cells of Alzheimer’s disease patients. J. Alzheimers Dis. 2012, 28, 593–600. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Hatade, K.; Sookwong, P.; Arai, H.; Miyazawa, T. Antioxidant effect of lutein towards phospholipid hydroperoxidation in human erythrocytes. Br. J. Nutr. 2009, 102, 1280–1284. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Takekoshi, H.; Higuchi, O.; Koto, S.; Kondo, M.; Kimura, F.; Miyazawa, T. Ingestion of Chlorella reduced the oxidation of erythrocyte membrance lipids in senior Japanese subjects. J. Oleo Sci. 2013, 62, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.; Caraveo-Anduaga, J.J.; Berglung, P.; Bijl, R.V.; de Graaf, R.; Vollebergh, W.; Dragomirecka, E.; Kohn, R.; Keller, M.; Kessler, R.; et al. The epidemiology of major depressive episodes: Results from the international consortium of psychiatric epidemiology (ICPE) surveys. Int. J. Methods Psychiatry Res. 2003, 12, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B. Prevalence and management of treatment-resistant depression. J. Clin. Psychiatry 2007, 68, s17–s25. [Google Scholar]
- Khawam, E.; Laurence, G.; Malone, J.R.D. Side effects of antidepressants: An overview. Cleve Clin. J. Med. 2006, 73, 351–353. [Google Scholar] [CrossRef]
- Panahi, Y.; Badeli, R.; Karami, G.R.; Badeli, Z.; Sahebkar, A. A randomized controlled trial of 6-week Chlorella vulgaris supplementation in patients with major depressive disorder. Comp. Ther. Med. 2015, 23, 598–602. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M.P. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell Biochem. 2007, 303, 39–44. [Google Scholar] [CrossRef]
- Konishi, F.; Mitsuyama, M.; Okuda, M.; Tanaka, K.; Hasegawa, T.; Nomoto, K. Protective effect of an acidic glycoprotein obtained from culture of Chlorella vulgaris against myelosuppression by 5-fluorouraci. Cancer Immunol. Immunother. 1996, 42, 268–274. [Google Scholar] [CrossRef]
- De Souza Queiroz, J.; Barbosa, C.M.V.; da Rocha, M.; Bincoletto, C.; Paredes-Gamero, E.J.; de Souza Queiroz, M.L.; Neto, J.P. Chlorella vulgaris treatment ameliorates the suppressive effects of single and repeated stressors on hematopoiesis. Brain Behav. Immun. 2013, 29, 39–50. [Google Scholar] [CrossRef]
- Armario, A. The contribution of immediate early genes to the understanding of brain processing of stressors. In Immediate Early Genes in Sensory Processing Cognitive Performance and Neurological Disorders; Pinaud, R., Termere, L., Eds.; Springer: Berlin, Germany, 2006; pp. 199–221. [Google Scholar]
- Queiroz, J.S.; Blasco, I.M.; Gagliano, H.; Daviu, N.; Román, A.G.; Belda, X.; Carrasco, J.; Rocha, M.C.; Neto, J.P.; Armario, A. Chlorella vulgaris reduces the impact of stress on hypothalamic-pituitary-adrenal axis and brain c-fos expression. Psychoneuroendocrinology 2016, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Inoue, Y.H.; Kohno, N.; Fujishima, M.; Okumura, E.; Sato, K. Phenethylamine in hot water extract of Chlorella pyrenoidosa expands lifespan of SOD1 mutant adults of Drosophila melanogaster at very low dose. J. Food Bioact. 2020, 9, 52–57. [Google Scholar] [CrossRef]
- Qiu, S.; Shen, Y.; Zhang, L.; Ma, B.; Amadu, A.A.; Ge, S. Antioxidant assessment of wastewater-cultivated Chlorella sorokiniana in Drosophila melanogaster. Algal. Res. 2020, 46, 101795. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).