Plasma Lithium Levels in the General Population: A Cross-Sectional Analysis of Metabolic and Dietary Correlates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Clinical Evaluation and Definitions
2.3. Biochemical Measurements
2.4. Dietary Assessment
2.5. Statistical Analysis
2.6. Exploratory Dietary Pattern
3. Results
3.1. General Characteristics
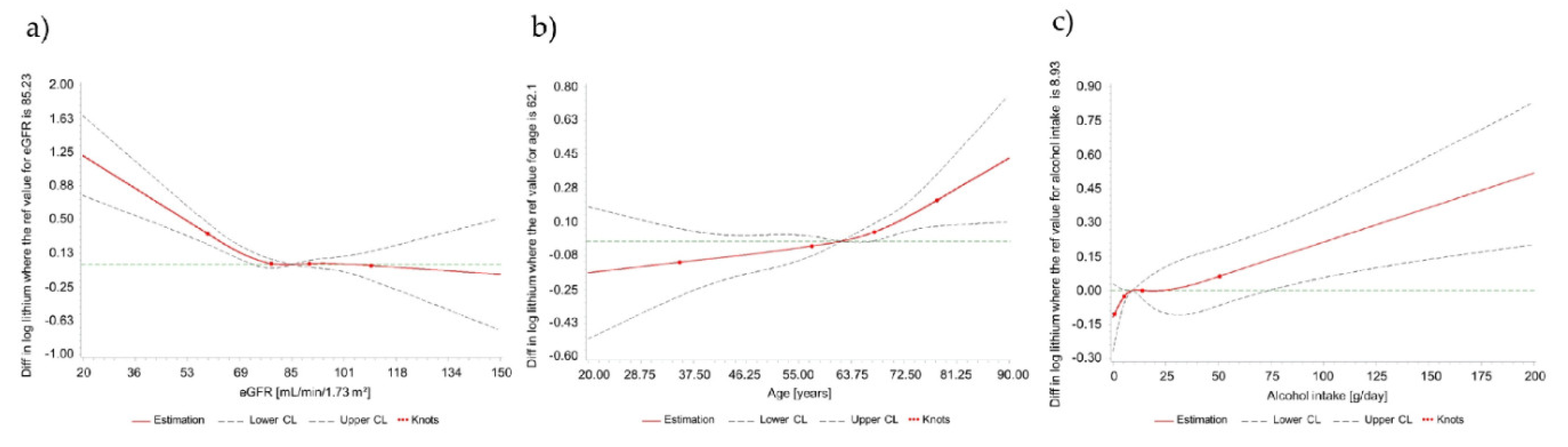
3.2. Correlates of Circulating Lithium Concentrations
3.3. Dietary Correlates of Circulating Lithium Concentrations
4. Discussion
4.1. Principal Observations
4.2. In the Context of the Published Literature
4.3. Association of Lithium Levels with Anthropometric, Metabolic and Lifestyle Characteristics
4.4. Dietary Correlates of Plasma Lithium Concentrations
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schrauzer, G.N. Lithium: Occurrence, dietary intakes, nutritional essentiality. J. Am. Coll. Nutr. 2002, 21, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Szklarska, D.; Rzymski, P. Is Lithium a Micronutrient? From Biological Activity and Epidemiological Observation to Food Fortification. Biol. Trace Elem. Res. 2019, 189, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Adams, D.; Berk, M. Is lithium in a class of its own? A brief profile of its clinical use. Aust. N. Z. J. Psychiatry 2009, 43, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Mughal, M.N.; Tanveer, M.; Gupta, D.; Abbas, G. Is lithium biologically an important or toxic element to living organisms? An overview. Environ. Sci. Pollut. Res. Int. 2017, 24, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, N.D.; Mossaheb, N.; Etzersdorfer, E.; Hlavin, G.; Thau, K.; Willeit, M.; Praschak-Rieder, N.; Sonneck, G.; Leithner-Dziubas, K. Lithium in drinking water and suicide mortality. Br. J. Psychiatry 2011, 198, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenbergh, R.; Hendrix, P.; Robberecht, H.; Deelstra, H. Daily dietary lithium intake in Belgium using duplicate portion sampling. Z. Lebensm. Forsch. A 1999, 208, 153–155. [Google Scholar] [CrossRef]
- Noël, L.; Chekri, R.; Millour, S.; Vastel, C.; Kadar, A.; Sirot, V.; Leblanc, J.C.; Guérin, T. Li, Cr, Mn, Co, Ni, Cu, Zn, Se and Mo levels in foodstuffs from the Second French TDS. Food Chem. 2012, 132, 1502–1513. [Google Scholar] [CrossRef]
- González-Weller, D.; Rubio, C.; Gutiérrez, Á.J.; González, G.L.; Mesa, J.M.C.; Gironés, C.R.; Ojeda, A.B.; Hardisson, A. Dietary intake of barium, bismuth, chromium, lithium, and strontium in a Spanish population (Canary Islands, Spain). Food Chem. Toxicol. 2013, 62, 856–868. [Google Scholar] [CrossRef]
- Seidel, U.; Baumhof, E.; Hägele, F.; Bosy-Westphal, A.; Birringer, M.; Rimbach, G. Lithium-Rich Mineral Water is a Highly Bioavailable Lithium Source for Human Consumption. Mol. Nutr. Food Res. 2019, 63, 1900039. [Google Scholar] [CrossRef]
- Pickett, E.E.; O’Dell, B.L. Evidence for dietary essentiality of lithium in the rat. Biol. Trace Elem. Res. 1992, 34, 299–319. [Google Scholar] [CrossRef]
- Anke, M.; Arnhold, W.; Schäfer, U.; Müller, R. Recent progress in exploring the essentiality of the ultratrace element lithium to the nutrition of animals and man. Biomed. Res. Trace Elem. 2005, 16, 169–176. [Google Scholar]
- Zarse, K.; Terao, T.; Tian, J.; Iwata, N.; Ishii, N.; Ristow, M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur. J. Nutr. 2011, 50, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Quan, J.I.; Li, L.; Kinghorn, K.J.; Ivanov, D.K.; Tain, L.S.; Slack, C.; Kerr, F.; Nespital, T.; Thornton, J.; Hardy, J.; et al. Lithium Promotes Longevity through GSK3/NRF2-Dependent Hormesis. Cell Rep. 2016, 15, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Kessing, L.V.; Gerds, T.A.; Knudsen, N.N.; Jorgensen, L.F.; Kristiansen, S.M.; Voutchkova, D.; Ernstsen, V.; Schullehner, J.; Hansen, B.; Andersen, P.K.; et al. Association of Lithium in Drinking Water With the Incidence of Dementia. JAMA Psychiatry 2017, 74, 1005–1010. [Google Scholar] [CrossRef]
- Fajardo, V.A.; Fajardo, V.A.; LeBlanc, P.J.; MacPherson, R.E.K. Examining the Relationship between Trace Lithium in Drinking Water and the Rising Rates of Age-Adjusted Alzheimer’s Disease Mortality in Texas. J. Alzheimer’s Dis. 2018, 61, 425–434. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Shrestha, K.P. Lithium in drinking water and the incidences of crimes, suicides, and arrests related to drug addictions. Biol. Trace Elem. Res. 1990, 25, 105–113. [Google Scholar] [CrossRef]
- Ohgami, H.; Terao, T.; Shiotsuki, I.; Ishii, N.; Iwata, N. Lithium levels in drinking water and risk of suicide. Br. J. Psychiatry 2009, 194, 464–465. [Google Scholar] [CrossRef]
- Giotakos, O.; Nisianakis, P.; Tsouvelas, G.; Giakalou, V.V. Lithium in the Public Water Supply and Suicide Mortality in Greece. Biol. Trace Elem. Res. 2013, 156, 376–379. [Google Scholar] [CrossRef]
- Fajardo, V.A.; LeBlanc, P.J.; Fajardo, V.A. Trace lithium in Texas tap water is negatively associated with all-cause mortality and premature death. Appl. Physiol. Nutr. Metab. 2017, 43, 412–414. [Google Scholar] [CrossRef]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 27. [Google Scholar] [CrossRef]
- Öhlund, L.; Ott, M.; Oja, S.; Bergqvist, M.; Lundqvist, R.; Sandlund, M.; Salander Renberg, E.; Werneke, U. Reasons for lithium discontinuation in men and women with bipolar disorder: A retrospective cohort study. BMC Psychiatry 2018, 18, 37. [Google Scholar] [CrossRef]
- Livingstone, C.; Rampes, H. Lithium: A review of its metabolic adverse effects. J. Psychopharmacol. 2006, 20, 347–355. [Google Scholar] [CrossRef]
- Shapiro, H.I.; Davis, K.A. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am. J. Psychiatry 2015, 172, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Albert, U.; De Cori, D.; Aguglia, A.; Barbaro, F.; Lanfranco, F.; Bogetto, F.; Maina, G. Lithium-associated hyperparathyroidism and hypercalcaemia: A case-control cross-sectional study. J. Affect. Disord. 2013, 151, 786–790. [Google Scholar] [CrossRef]
- Baird-Gunning, J.; Lea-Henry, T.; Hoegberg, L.C.G.; Gosselin, S.; Roberts, D.M. Lithium Poisoning. J. Intensive Care Med. 2017, 32, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, A.; Ardau, R.; Carta, P.; Ligas, F.; Sardu, C.; Pani, A.; Del Zompo, M. Duration of lithium treatment is a risk factor for reduced glomerular function: A cross-sectional study. BMC Med. 2013, 11, 33. [Google Scholar] [CrossRef]
- Rodrigo, C.; de Silva, N.L.; Gunaratne, R.; Rajapakse, S.; De Silva, V.A.; Hanwella, R. Lower estimated glomerular filtration rates in patients on long term lithium: A comparative study and a meta-analysis of literature. BMC Psychiatry 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, A.; Ardau, R.; Fanni, T.; Sardu, C.; Piras, D.; Pani, A.; Del Zompo, M. Renal function during long-term lithium treatment: A cross-sectional and longitudinal study. BMC Med. 2015, 13, 12. [Google Scholar] [CrossRef]
- Tondo, L.; Abramowicz, M.; Alda, M.; Bauer, M.; Bocchetta, A.; Bolzani, L.; Calkin, C.V.; Chillotti, C.; Hidalgo-Mazzei, D.; Manchia, M.; et al. Long-term lithium treatment in bipolar disorder: Effects on glomerular filtration rate and other metabolic parameters. Int. J. Bipolar Disord. 2017, 5, 27. [Google Scholar] [CrossRef]
- Paul, R.; Minay, J.; Cardwell, C.; Fogarty, D.; Kelly, C. Meta-analysis of the effects of lithium usage on serum creatinine levels. J. Psychopharmacol. 2010, 24, 1425–1431. [Google Scholar] [CrossRef]
- Bendz, H.; Schön, S.; Attman, P.O.; Aurell, M. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010, 77, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Lepkifker, E.; Sverdlik, A.; Iancu, I.; Ziv, R.; Segev, S.; Kotler, M. Renal insufficiency in long-term lithium treatment. J. Clin. Psychiatry 2004, 65, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Dastych, M.; Synek, O.; Gottwaldova, J. Impact of Long-Term Lithium Treatment on Renal Function in Patients With Bipolar Disorder Based on Novel Biomarkers. J. Clin. Psychopharmacol. 2019, 39, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Broberg, K.; Concha, G.; Engström, K.; Lindvall, M.; Grandér, M.; Vahter, M. Lithium in drinking water and thyroid function. Environ. Health Perspect. 2011, 119, 827–830. [Google Scholar] [CrossRef]
- Harari, F.; Akesson, A.; Casimiro, E.; Lu, Y.; Vahter, M. Exposure to lithium through drinking water and calcium homeostasis during pregnancy: A longitudinal study. Environ. Res. 2016, 147, 1–7. [Google Scholar] [CrossRef]
- Cetin, I.; Nalbantcilar, M.T.; Tosun, K.; Nazik, A. How Trace Element Levels of Public Drinking Water Affect Body Composition in Turkey. Biol. Trace Elem. Res. 2017, 175, 263–270. [Google Scholar] [CrossRef]
- Kraszewska, A.; Ziemnicka, K.; Jonczyk-Potoczna, K.; Sowinski, J.; Rybakowski, J.K. Thyroid structure and function in long-term lithium-treated and lithium-naive bipolar patients. Hum. Psychopharmacol. 2019, 34, e2708. [Google Scholar] [CrossRef]
- Aprahamian, I.; Santos, F.S.; dos Santos, B.; Talib, L.; Diniz, B.S.; Radanovic, M.; Gattaz, W.F.; Forlenza, O.V. Long-term, low-dose lithium treatment does not impair renal function in the elderly: A 2-year randomized, placebo-controlled trial followed by single-blind extension. J. Clin. Psychiatry 2014, 75, e672–e678. [Google Scholar] [CrossRef]
- Altinbas, K.; Guloksuz, S.; Caglar, I.M.; Caglar, F.N.; Kurt, E.; Oral, E.T. Electrocardiography changes in bipolar patients during long-term lithium monotherapy. Gen. Hosp. Psychiatry 2014, 36, 694–697. [Google Scholar] [CrossRef]
- Nunes, M.A.; Viel, T.A.; Buck, H.S. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 104–107. [Google Scholar] [CrossRef]
- Shine, B.; McKnight, R.F.; Leaver, L.; Geddes, J.R. Long-term effects of lithium on renal, thyroid, and parathyroid function: A retrospective analysis of laboratory data. Lancet 2015, 386, 461–468. [Google Scholar] [CrossRef]
- Prosser, J.M.; Fieve, R.R. Patients receiving lithium therapy have a reduced prevalence of neurological and cardiovascular disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Martinsson, L.; Westman, J.; Hallgren, J.; Osby, U.; Backlund, L. Lithium treatment and cancer incidence in bipolar disorder. Bipolar Disord. 2016, 18, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Meehan, A.D.; Udumyan, R.; Kardell, M.; Landen, M.; Jarhult, J.; Wallin, G. Lithium-Associated Hypercalcemia: Pathophysiology, Prevalence, Management. World J. Surg. 2018, 42, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, E.; Skinner, J.; Anderson, T.; Bazire, S.; Twigg, M.J.; Desborough, J.A. One lithium level >1.0 mmol/L causes an acute decline in eGFR: Findings from a retrospective analysis of a monitoring database. BMJ Open 2014, 4, e006020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kessing, L.V.; Gerds, T.A.; Feldt-Rasmussen, B.; Andersen, P.K.; Licht, R.W. Lithium and renal and upper urinary tract tumors results from a nationwide population-based study. Bipolar Disord. 2015, 17, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Hsieh, K.P.; Huang, W.W.; Yang, Y.H. Use of lithium and cancer risk in patients with bipolar disorder: Population-based cohort study. Br. J. Psychiatry 2016, 209, 393–399. [Google Scholar] [CrossRef]
- Aiff, H.; Attman, P.O.; Aurell, M.; Bendz, H.; Ramsauer, B.; Schon, S.; Svedlund, J. Effects of 10 to 30 years of lithium treatment on kidney function. J. Psychopharmacol. 2015, 29, 608–614. [Google Scholar] [CrossRef]
- Krawczak, M.; Nikolaus, S.; von Eberstein, H.; Croucher, P.J.; El Mokhtari, N.E.; Schreiber, S. PopGen: Population-based recruitment of patients and controls for the analysis of complex genotype-phenotype relationships. Community Genet. 2006, 9, 55–61. [Google Scholar] [CrossRef]
- Barbaresko, J.; Siegert, S.; Koch, M.; Aits, I.; Lieb, W.; Nikolaus, S.; Laudes, M.; Jacobs, G.; Nothlings, U. Comparison of two exploratory dietary patterns in association with the metabolic syndrome in a Northern German population. Br. J. Nutr. 2014, 112, 1364–1372. [Google Scholar] [CrossRef]
- Koch, M.; Borggrefe, J.; Barbaresko, J.; Groth, G.; Jacobs, G.; Siegert, S.; Lieb, W.; Müller, M.J.; Bosy-Westphal, A.; Heller, M.; et al. Dietary patterns associated with magnetic resonance imaging–determined liver fat content in a general population study. Am. J. Clin. Nutr. 2013, 99, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Moewes, D.; Koch, M.; Müller, H.P.; Jacobs, G.; Kassubek, J.; Lieb, W.; Nöthlings, U. MRI-determined total volumes of visceral and subcutaneous abdominal and trunk adipose tissue are differentially and sex-dependently associated with patterns of estimated usual nutrient intake in a northern German population. Am. J. Clin. Nutr. 2015, 101, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Seidel, U.; Jans, K.; Hommen, N.; Ipharraguerre, I.R.; Lüersen, K.; Birringer, M.; Rimbach, G. Lithium Content of 160 Beverages and Its Impact on Lithium Status in Drosophila melanogaster. Foods 2020, 9, 795. [Google Scholar] [CrossRef]
- Nothlings, U.; Hoffmann, K.; Bergmann, M.M.; Boeing, H. Fitting portion sizes in a self-administered food frequency questionnaire. J. Nutr. 2007, 137, 2781–2786. [Google Scholar] [CrossRef]
- Efron, B.; Hastie, T.; Johnstone, I.; Tibshirani, R. Least angle regression. Ann. Statist. 2004, 32, 407–499. [Google Scholar] [CrossRef]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef]
- Foley, R.N.; Wang, C.; Snyder, J.J.; Rule, A.D.; Collins, A.J. Kidney function and risk triage in adults: Threshold values and hierarchical importance. Kidney Int. 2011, 79, 99–111. [Google Scholar] [CrossRef]
- Thomas, L.; Xue, J.; Dominguez Rieg, J.A.; Rieg, T. Contribution of NHE3 and dietary phosphate to lithium pharmacokinetics. Eur. J. Pharm. Sci. 2019, 128, 1–7. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Cusin, C.; Serretti, A.; Mandelli, L.; Lucca, A.; Smeraldi, E. Seasonal variations of lithium plasma levels. Psychiatry Res. 2002, 111, 35–41. [Google Scholar] [CrossRef]
- Wilting, I.; Fase, S.; Martens, E.P.; Heerdink, E.R.; Nolen, W.A.; Egberts, A.C. The impact of environmental temperature on lithium serum levels. Bipolar Disord. 2007, 9, 603–608. [Google Scholar] [CrossRef]
- Van Westing, A.C.; Küpers, L.K.; Geleijnse, J.M. Diet and Kidney Function: A Literature Review. Curr. Hypertens. Rep. 2020, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Thongboonkerd, V. Protective Effects of Epigallocatechin-3-Gallate from Green Tea in Various Kidney Diseases. Adv. Nutr. 2019, 10, 112–121. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress-a review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, G.; Wu, Q. Recent scientific studies of a traditional chinese medicine, tea, on prevention of chronic diseases. J. Tradit. Complement. Med. 2014, 4, 17–23. [Google Scholar] [CrossRef]
- Cosola, C.; Sabatino, A.; di Bari, I.; Fiaccadori, E.; Gesualdo, L. Nutrients, Nutraceuticals, and Xenobiotics Affecting Renal Health. Nutrients 2018, 10, 808. [Google Scholar] [CrossRef]
- Cai, Q.; Dekker, L.; Bakker, S.; Borst, M.H.; Navis, G. Dietary Patterns Based on Estimated Glomerular Filtration Rate and Kidney Function Decline in the General Population: The Lifelines Cohort Study. Nutrients 2020, 12, 1099. [Google Scholar] [CrossRef]
| Overall | Men | Women | |
|---|---|---|---|
| n a | 928 | 532 (57.3) | 396 (42.7) |
| Plasma concentration of lithium (µg/L) b | 0.96 (0.70, 1.37) | 0.92 (0.69, 1.32) | 1.03 (0.72, 1.43) |
| Age (years) c | 61 ± 13 | 61 ± 12 | 60 ± 14 |
| Body mass index (kg/m2) c | 27.5 ± 4.9 | 27.7 ± 4.0 | 27.2 ± 5.9 |
| Waist circumference (cm) b | 96.1 (88.1, 105.0) | 99.9 (93.2, 108.2) | 89.8 (80.1, 99.3) |
| Waist-to-hip ratio b | 0.94 (0.88, 1.00) | 0.99 (0.94, 1.03) | 0.88 (0.83, 0.92) |
| Systolic blood pressure (mmHg) c | 140 ± 18 | 142 ± 18 | 137 ± 18 |
| Diastolic blood pressure (mmHg) c | 85 ± 9 | 86 ± 9 | 84 ± 9 |
| Prevalent hypertension a | 585 (63.0) | 355 (66.7) | 230 (58.1) |
| HbA1c (%) c | 5.73 ± 0.61 | 5.76 ± 0.67 | 5.70 ± 0.52 |
| Glucose (mg/dL) b | 98.0 (91.0, 105.0) | 99.0 (93.0, 107.0) | 95.0 (89.0, 103.0) |
| Prevalent diabetes a | 78 (8.4) | 54 (10.0) | 24 (6.1) |
| C-reactive protein (mg/L) b | 1.30 (0.45, 2.60) | 1.20 (0.45, 2.20) | 1.40 (0.45, 3.10) |
| Triglycerides (mg/dL) b | 106.0 (77.0, 140.5) | 111.0 (80.5, 148.0) | 99.5 (72.0, 131.5) |
| Total cholesterol (mg/dL) c | 222.8 ± 41.1 | 217.1 ± 40.3 | 230.6 ± 40.9 |
| High-density-lipoprotein cholesterol (mg/dL) c | 64.8 ± 18.1 | 58.0 ± 14.3 | 74.0 ± 18.5 |
| Low-density-lipoprotein cholesterol (mg/dL) c | 131.4 ± 33.5 | 131.2 ± 33.3 | 131.5 ± 33.8 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) c | 84.4 ± 15.4 | 84.2 ± 15.1 | 84.7 ± 15.6 |
| Creatinine (mg/dL) c | 0.88 ± 0.18 | 0.97 ± 0.17 | 0.76 ± 0.12 |
| Current smokers a | 129 (13.9) | 76 (14.3) | 53 (13.4) |
| Physical activity (MET-hours/week) b | 89.2 (58.2, 129.0) | 83.8 (55.0, 124.5) | 96.3 (65.3, 137.6) |
| High education (≥ 11 years) a | 299 (32.2) | 199 (37.4) | 100 (25.3) |
| Alcohol intake (g/day) b | 8.9 (3.2, 18.4) | 12.3 (5.5, 23.1) | 5.4 (1.9, 12.3) |
| Glutamate-Oxalacetate-Transaminase (U/L) b | 26.0 (22.0, 30.0) | 27.0 (23.0, 32.0) | 24.0 (21.0, 29.0) |
| Gamma-Glutamyl-Transferase (U/L) b | 25.0 (18.0, 37.0) | 28.0 (21.0, 42.0) | 19.0 (14.0, 29.5) |
| Glutamate-Pyruvate-Transaminase (U/L) b | 23.0 (17.0, 31.0) | 26.0 (20.0, 34.0) | 19.0 (15.0, 25.0) |
| Plasma Concentration of Lithium (µg/L) | T1 ≤ 0.78 µg/L | T2 0.79–1.21 µg/L | T3 ≥ 1.22 µg/L | p * | |
|---|---|---|---|---|---|
| n (% male) a | 309 (61.2) | 309 (57.9) | 310 (52.9) | 0.11 | |
| Plasma concentration of lithium (µg/L) b | 0.63 (0.52, 0.70) | 0.95 (0.87, 1.08) | 1.60 (1.37, 2.10) | <0.0001 | |
| Age (years) c | 57 ± 12 | 62 ± 12 | 64 ± 12 | <0.0001 | |
| Body mass index (kg/m2) c | 28.2 ± 5.4 | 26.9 ± 4.4 | 27.3 ± 4.8 | 0.004 | |
| Waist circumference (cm) b | male | 100.3 (93.3, 108.8) | 99.1 (92.7, 106.5) | 100.2 (94.1, 108.5) | 0.58 |
| female | 92.0 (80.4, 101.0) | 88.4 (79.0, 97.4) | 89.3 (82.5, 98.8) | 0.19 | |
| Waist-to-hip ratio b | male | 0.99 (0.95, 1.02) | 0.99 (0.94, 1.03) | 0.99 (0.94, 1.03) | 0.94 |
| female | 0.88 (0.83, 0.93) | 0.87 (0.81, 0.91) | 0.88 (0.83, 0.93) | 0.20 | |
| Systolic blood pressure (mmHg) c | 141 ± 18 | 139 ± 19 | 140 ± 19 | 0.58 | |
| Diastolic blood pressure (mmHg) c | 86 ± 9 | 84 ± 9 | 85 ± 9 | 0.07 | |
| Prevalent hypertension a | 198 (64.1) | 178 (57.6) | 209 (67.4) | 0.04 | |
| HbA1c (%) c | 5.65 ± 0.60 | 5.73 ± 0.54 | 5.82 ± 0.67 | 0.002 | |
| Glucose (mg/dL) b | 96.0 (90.0, 105.0) | 98.0 (91.0, 105.0) | 98.0 (92.0, 105.0) | 0.25 | |
| Prevalent diabetes a | 17 (5.5) | 23 (7.4) | 38 (12.3) | 0.01 | |
| C-reactive protein (mg/L) b | 1.40 (0.45, 2.90) | 1.10 (0.45, 2.50) | 1.30 (0.45, 2.40) | 0.16 | |
| Triglycerides (mg/dL) b | 103.0 (77.0, 137.0) | 106.0 (73.0, 140.0) | 107.0 (80.0, 145.0) | 0.47 | |
| Total cholesterol (mg/dL) c | 223.5 ± 41.4 | 223.8 ± 38.9 | 221.2 ± 43.0 | 0.71 | |
| High-density-lipoprotein cholesterol (mg/dL) c | 62.7 ± 16.8 | 66.6 ± 18.6 | 65.1 ± 18.6 | 0.03 | |
| Low-density-lipoprotein cholesterol (mg/dL) c | 134.8 ± 33.2 | 131.4 ± 31.7 | 127.9 ± 35.1 | 0.04 | |
| Estimated glomerular filtration rate (mL/min/1.73 m2) c | 89.0 ± 14.3 | 84.1 ± 14.1 | 80.2 ± 16.4 | <0.0001 | |
| Creatinine (mg/dL) c | 0.87 ± 0.17 | 0.88 ± 0.16 | 0.91 ± 0.21 | 0.02 | |
| Current smokers a | 45 (14.6) | 47 (15.2) | 37 (11.9) | 0.46 | |
| Physical activity (MET-hours/week) b | 86.5 (54.8, 123.0) | 94.5 (63.3, 132.6) | 85.4 (55.1, 134.3) | 0.07 | |
| High education (≥11 years) a | 96 (31.1) | 112 (36.3) | 91 (29.4) | 0.33 | |
| Alcohol intake (g/day) b | 8.6 (2.8, 15.8) | 9.3 (3.8, 18.8) | 8.8 (3.2, 20.7) | 0.17 | |
| Glutamate-Oxalacetate-Transaminase (U/L) b | 26.0 (22.0, 30.0) | 26.0 (22.0, 30.0) | 26.0 (23.0, 31.0) | 0.21 | |
| Gamma-Glutamyl-Transferase (U/L) b | 25.0 (17.0, 35.0) | 23.0 (17.0, 35.0) | 26.0 (18.0, 44.0) | 0.07 | |
| Glutamate-Pyruvate-Transaminase (U/L) b | 24.0 (18.0, 32.0) | 21.0 (17.0, 29.0) | 22.0 (17.0, 30.0) | 0.12 | |
| Least Angle Regression | |
|---|---|
| R2 | 0.0725 |
| Characteristic | Difference in plasma lithium concentration (µg/L) per 1-unit increment in the characteristic |
| Age (years) | 0.0028 |
| Diastolic blood pressure (mmHg) | −0.0016 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | −0.0069 |
| Smoking status (former vs. never smoking) | 0.0140 |
| Alcohol intake per drink (14 g/day) | 0.0184 |
| Plasma Concentration of Lithium [µg/L] | ||||
|---|---|---|---|---|
| Food Groups [g/day] | T1 ≤ 0.78 µg/L n = 307 | T2 0.79–1.21 µg/L n = 308 | T3 ≥ 1.22 µg/L n = 307 | p * |
| Potatoes | 83.2 (78.7, 87.8) | 84.4 (80.0, 88.9) | 81.0 (76.5, 85.5) | 0.56 |
| Leafy vegetables | 14.4 (12.7, 14.9) | 16.1 (16.6, 14.9) | 16.4 (14.7, 18.1) | 0.14 |
| Fruiting vegetables | 92.6 (86.2, 99.0) | 94.7 (88.4, 101.0) | 93.3 (87.0, 99.6) | 0.90 |
| Root vegetables | 22.1 (20.4, 23.7) | 23.4 (21.8, 25.0) | 25.4 (23.8, 27.1) | 0.02 |
| Cabbage | 25.7 (24.1, 27.4) | 27.4 (25.8, 29.1) | 26.0 (24.4, 27.6) | 0.29 |
| Other vegetables | 40.3 (38.1, 42.4) | 39.7 (37.6, 41.8) | 40.2 (38.1, 42.3) | 0.93 |
| Legumes | 3.0 (2.6, 3.4) | 3.1 (2.7, 3.4) | 3.1 (2.8, 3.5) | 0.89 |
| Fruits | 216.1 (198.4, 233.8) | 232.9 (215.6, 250.3) | 229.9 (212.4, 247.4) | 0.37 |
| Nuts and seeds | 3.8 (3.2, 4.4) | 4.3 (3.7, 4.9) | 3.9 (3.3, 4.5) | 0.51 |
| Milk | 110.6 (95.9, 125.3) | 108.9 (94.5, 123.3) | 104.5 (89.9, 119.0) | 0.84 |
| Dairy products | 105.1 (97.1, 113.0) | 110.6 (102.8, 118.3) | 105.0 (97.1, 112.8) | 0.52 |
| Cheese | 34.7 (32.6, 36.7) | 37.9 (35.9, 40.0) | 35.3 (33.2, 37.3) | 0.06 |
| Bread | 116.4 (107.0, 125.9) | 118.6 (109.3, 127.8) | 111.2 (101.8, 120.5) | 0.53 |
| Pasta and rice | 35.9 (33.0, 38.8) | 37.2 (34.3, 40.1) | 34.4 (31.5, 37.3) | 0.40 |
| Other cereals | 12.6 (11.6, 13.5) | 13.4 (12.5, 14.4) | 12.6 (11.6, 13.5) | 0.34 |
| Beef | 16.4 (14.5, 18.2) | 18.5 (16.7, 20.4) | 17.6 (15.7, 19.4) | 0.28 |
| Pork | 34.4 (30.4, 38.3) | 33.8 (30.0, 37.7) | 32.9 (28.9, 36.8) | 0.87 |
| Poultry | 14.6 (13.2, 15.9) | 13.6 (12.2, 14.9) | 14.4 (13.1, 15.8) | 0.54 |
| Processed meat | 50.5 (46.9, 54.0) | 49.3 (45.8, 52.8) | 49.7 (46.2, 53.2) | 0.89 |
| Other meat | 4.5 (4.0, 4.9) | 4.8 (4.3, 5.2) | 4.4 (4.0, 4.8) | 0.44 |
| Fish, fish products | 25.8 (23.4, 28.3) | 30.4 (28.0, 32.9) | 29.5 (27.0, 31.9) | 0.03 |
| Eggs | 18.3 (17.0, 19.7) | 16.0 (14.7, 17.4) | 18.0 (16.7, 19.4) | 0.04 |
| Butter | 12.3 (10.8, 13.8) | 12.4 (10.9, 13.9) | 10.4 (8.9, 11.9) | 0.11 |
| Margarine | 13.6 (12.0, 15.3) | 13.9 (12.3, 15.5) | 13.5 (11.9, 15.2) | 0.94 |
| Vegetable oils | 8.6 (7.9, 9.3) | 9.4 (8.7, 10.1) | 9.2 (8.5, 9.9) | 0.25 |
| Other fats | 2.2 (2.0, 2.3) | 2.0 (1.9, 2.1) | 2.0 (1.9, 2.1) | 0.20 |
| Sugar products (e.g., syrups, candy, ice cream, desserts) | 40.2 (37.6, 42.8) | 41.9 (39.3, 44.5) | 37.1 (34.5, 39.7) | 0.03 |
| Chocolate sweets | 12.5 (11.5, 13.5) | 12.0 (11.0, 13.0) | 11.1 (10.0, 12.1) | 0.14 |
| Cake, cookies | 61.3 (56.5, 66.1) | 61.6 (56.9, 66.3) | 57.6 (52.9, 62.3) | 0.43 |
| Non-alcoholic beverages | 1112.8 (1033.9, 1191.8) | 1114.4 (1037.0, 1191.8) | 1096.8 (1018.6, 1175.0) | 0.94 |
| Soft drinks | 208.3 (137.4, 279.2) | 190.1 (120.5, 259.7) | 191.1 (120.8, 261.3) | 0.92 |
| Coffee | 494.4 (450.4, 538.5) | 488.5 (445.3, 531.7) | 485.4 (441.8, 529.1) | 0.96 |
| Tea | 192.1 (150.9, 233.3) | 259.3 (218.9, 299.8) | 313.9 (273.1, 354.7) | 0.0003 |
| Beer | 116.2 (72.4, 160.0) | 132.7 (89.7, 175.7) | 212.7 (169.2, 256.1) | 0.01 |
| Wine | 62.8 (41.7, 83.9) | 90.3 (69.6, 111.0) | 101.8 (80.9, 122.6) | 0.03 |
| Alcoholic beverages | 17.1 (12.1, 22.0) | 15.8 (11.0, 21.0) | 11.4 (6.5, 16.3) | 0.24 |
| Sauces | 51.9 (49.0, 54.9) | 52.4 (49.4, 55.3) | 49.2 (46.3, 52.1) | 0.27 |
| Soups | 23.6 (21.4, 25.8) | 24.3 (22.1, 26.5) | 25.9 (23.8, 28.1) | 0.32 |
| Bouillon | 17.6 (16.3, 18.9) | 18.1 (16.8, 19.3) | 18.7 (17.5, 20.0) | 0.48 |
| Soya products | 2.1 (1.9, 2.4) | 2.2 (2.0, 2.5) | 2.4 (2.2, 2.7) | 0.23 |
| Dietetic products | 0.5 (0.3, 0.6) | 0.6 (0.4, 0.7) | 0.7 (0.6, 0.9) | 0.09 |
| Snacks | 1.0 (0.9, 1.1) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.23 |
| Reduced Rank Regression Factor Loading | |
|---|---|
| Overall | |
| Explained variation (%) | 8.63 |
| Food group (g/day) | |
| Potatoes | 0.2169 |
| Leafy vegetables | 0.1641 |
| Fruiting vegetables | - |
| Root vegetables | 0.2354 |
| Cabbage | - |
| Other vegetables | - |
| Legumes | - |
| Fruits | 0.1894 |
| Nuts and seeds | - |
| Milk | - |
| Dairy products | - |
| Cheese | - |
| Bread | - |
| Pasta and rice | −0.2083 |
| Other cereals | - |
| Beef | - |
| Pork | −0.1670 |
| Poultry | - |
| Processed meat | - |
| Other meat | - |
| Fish, fish products | - |
| Eggs | - |
| Butter | - |
| Margarine | - |
| Vegetable oils | - |
| Other fats | - |
| Sugar products (e.g., syrups, candy, ice cream, desserts) | - |
| Chocolate, sweets | −0.3668 |
| Cake, cookies | - |
| Non-alcoholic beverages | - |
| Soft drinks | −0.2014 |
| Coffee | - |
| Tea | 0.3196 |
| Beer | 0.1999 |
| Wine | 0.3024 |
| Other alcoholic beverages | −0.1544 |
| Sauces | −0.2222 |
| Soups | - |
| Bouillon | - |
| Soya products | - |
| Dietetic products | 0.1988 |
| Snacks | −0.3429 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enderle, J.; Klink, U.; di Giuseppe, R.; Koch, M.; Seidel, U.; Weber, K.; Birringer, M.; Ratjen, I.; Rimbach, G.; Lieb, W. Plasma Lithium Levels in the General Population: A Cross-Sectional Analysis of Metabolic and Dietary Correlates. Nutrients 2020, 12, 2489. https://doi.org/10.3390/nu12082489
Enderle J, Klink U, di Giuseppe R, Koch M, Seidel U, Weber K, Birringer M, Ratjen I, Rimbach G, Lieb W. Plasma Lithium Levels in the General Population: A Cross-Sectional Analysis of Metabolic and Dietary Correlates. Nutrients. 2020; 12(8):2489. https://doi.org/10.3390/nu12082489
Chicago/Turabian StyleEnderle, Janna, Urte Klink, Romina di Giuseppe, Manja Koch, Ulrike Seidel, Katharina Weber, Marc Birringer, Ilka Ratjen, Gerald Rimbach, and Wolfgang Lieb. 2020. "Plasma Lithium Levels in the General Population: A Cross-Sectional Analysis of Metabolic and Dietary Correlates" Nutrients 12, no. 8: 2489. https://doi.org/10.3390/nu12082489
APA StyleEnderle, J., Klink, U., di Giuseppe, R., Koch, M., Seidel, U., Weber, K., Birringer, M., Ratjen, I., Rimbach, G., & Lieb, W. (2020). Plasma Lithium Levels in the General Population: A Cross-Sectional Analysis of Metabolic and Dietary Correlates. Nutrients, 12(8), 2489. https://doi.org/10.3390/nu12082489






