The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Study Product
2.4. Cognitive and Mood Assessments
2.5. Vascular Assessments
2.6. Biochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Inclusion and Population Demographics
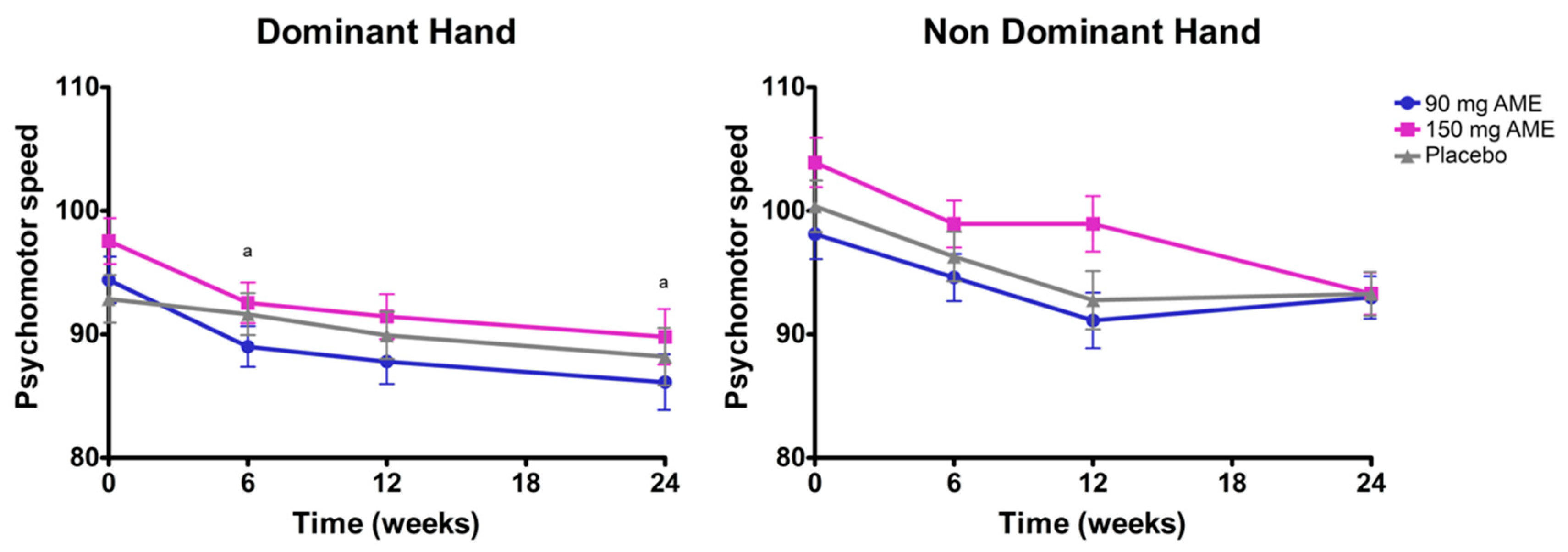
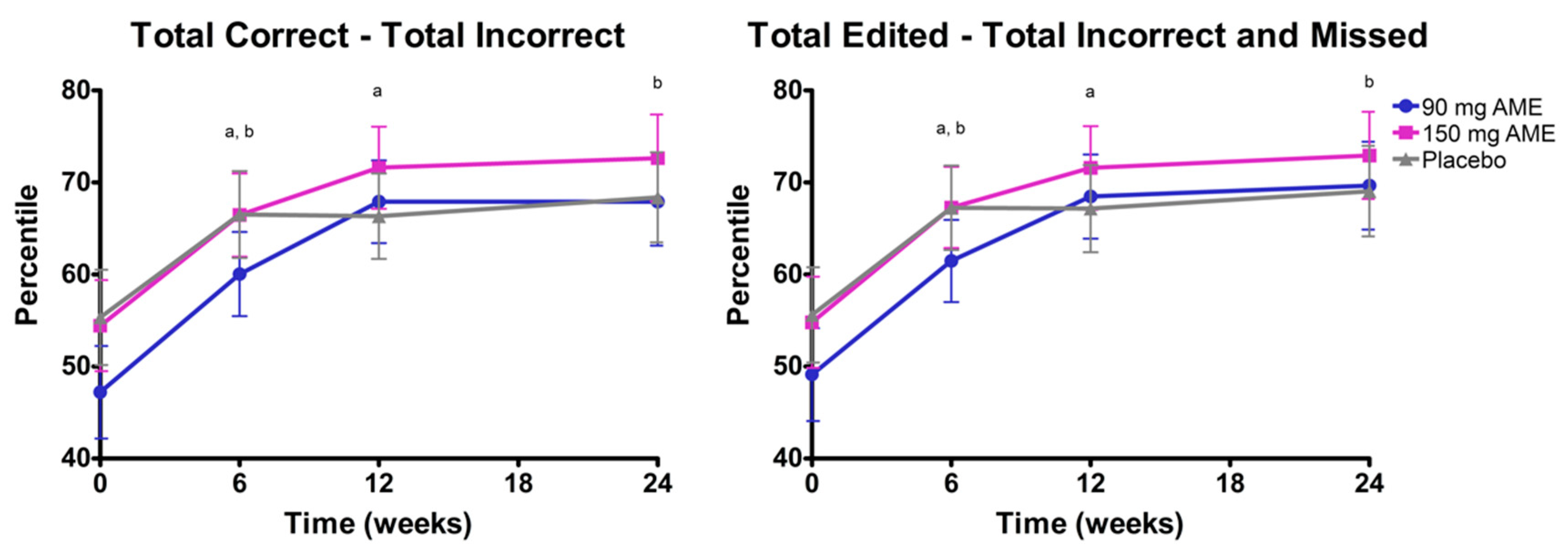
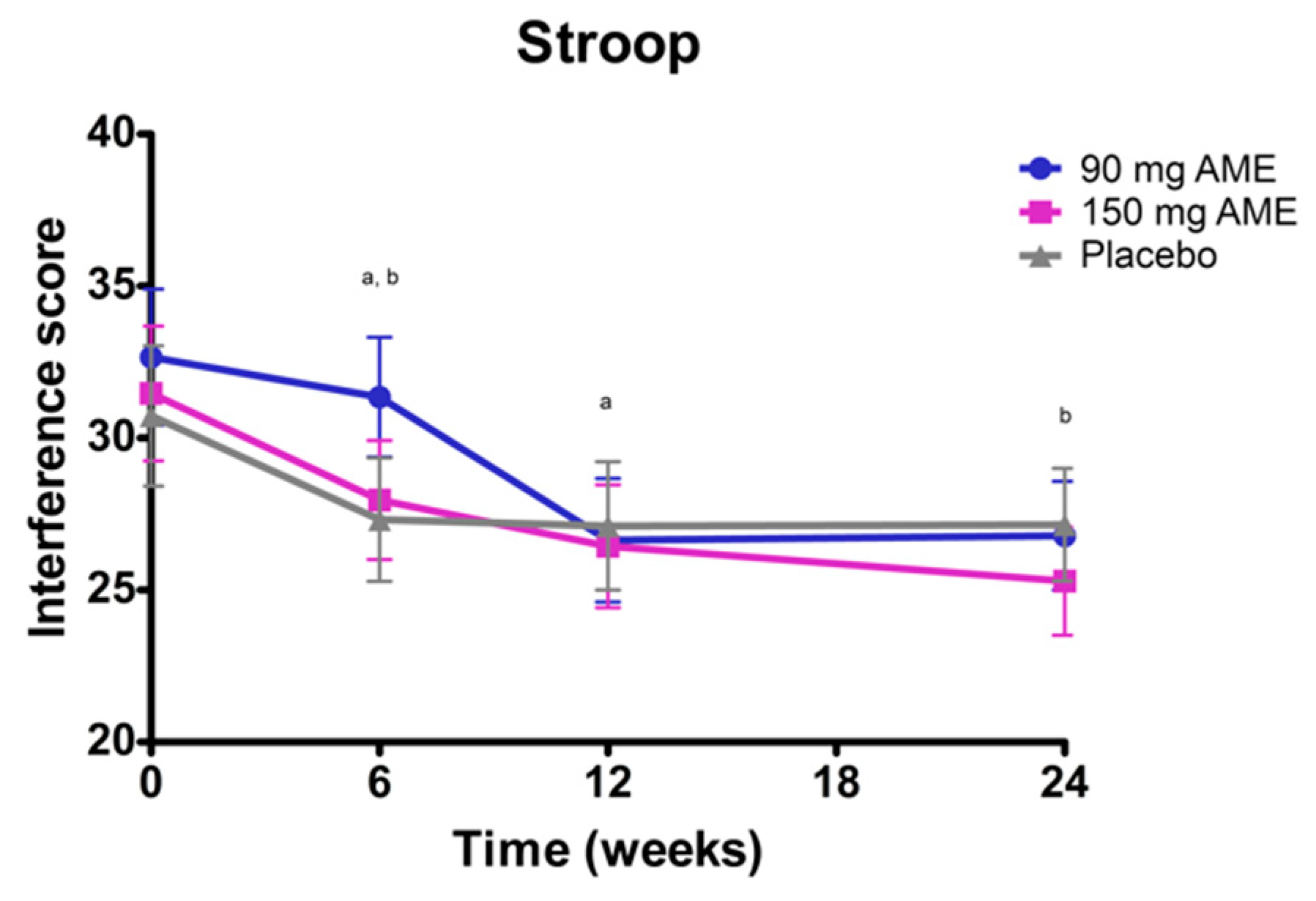
3.2. Aronia melanocarpa Extract Improves Psychomotor Speed, but Does Not Affect Attention, Cognitive Flexibility, Mood, and Serum Brain-Derived Neurotrophic Factor (BDNF) Levels
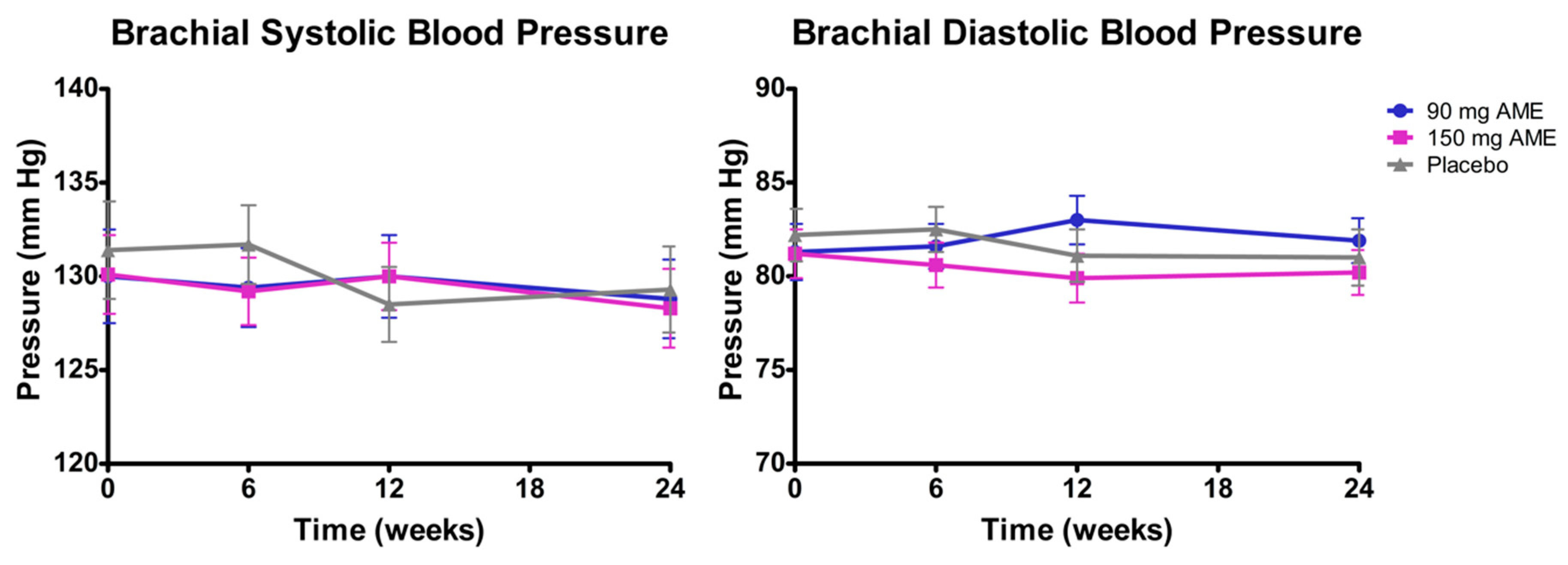
3.3. Aronia melanocarpa Extract Decreases Blood Pressure, but Does Not Affect Other Vascular Functions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakker, M.; Bánáti, D.; Calabrese, V.; Cederholm, T.; Cryan, J.; et al. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Daglia, M.; Nabavi, S.; Loizzo, M.; Sobarzo-Sánchez, E.; Nabavi, S. Flavonoids and dementia: An update. Curr. Med. Chem. 2015, 22, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Int. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Petersen, R.C.; Negash, S. Mild cognitive impairment: An overview. CNS Spectr. 2008, 13, 45–53. [Google Scholar] [CrossRef]
- Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2019. [Google Scholar]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2019, 11, 224–236. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharm. 2019, 858, 172500. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black chokeberry aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef]
- Rosi, A.; Paolella, G.; Biasini, B.; Scazzina, F. Dietary habits of adolescents living in North America, Europe or Oceania: A review on fruit, vegetable and legume consumption, sodium intake, and adherence to the mediterranean diet. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Guenther, P.M.; Oh, M.; Stuart, D.; Jensen, H.H. Assessing the consumption of berries and associated factors in the United States using the National Health and Nutrition Examination Survey (NHANES), 2007–2012. Food Funct. 2018, 9, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.E.; Netzel, M.; Fanning, K. Food-based anthocyanin intake and cognitive outcomes in human intervention trials: A systematic review. J. Hum. Nutr. Diet. 2017, 30, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Lee, S.; Park, H.; Suzuki, T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014, 6, 69. [Google Scholar] [CrossRef]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P.E. High-flavonoid intake induces cognitive improvements linked to changes in serum brain-derived neurotrophic factor: Two randomised, controlled trials. Nutr. Healthy Aging 2016, 4, 81–93. [Google Scholar] [CrossRef]
- Aprahamian, I.; Vanderlinde, F.; Biella, M.M. Chapter 19—Vascular function and cognitive decline. In Endothelium and Cardiovascular Diseases; Da Luz, P.L., Libby, P., Chagas, A.C.P., Laurindo, F.R.M., Eds.; Academic Press: New York, NY, USA, 2018; pp. 253–264. [Google Scholar]
- Crichton, G.E.; Elias, M.F.; Davey, A.; Alkerwi, A. Cardiovascular health and cognitive function: The maine-syracuse longitudinal study. PLoS ONE 2014, 9, e89317. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J.; Zhou, Z. Anthocyanins from Black chokeberry (Aroniamelanocarpa elliot) delayed aging-related degenerative changes of Brain. J. Agric. Food Chem. 2017, 65, 5973–5984. [Google Scholar] [CrossRef]
- Lee, H.Y.; Weon, J.B.; Jung, Y.S.; Kim, N.Y.; Kim, M.K.; Ma, C.J. Cognitive-enhancing effect of Aronia melanocarpa extract against memory impairment induced by Scopolamine in mice. Evid. Based Complement. Altern. Med. 2016, 2016, 6145926. [Google Scholar] [CrossRef]
- Daskalova, E.; Delchev, S.; Topolov, M.; Dimitrova, S.; Uzunova, Y.; Valcheva-Kuzmanova, S.; Kratchanova, M.; Vladimirova-Kitova, L.; Denev, P. Aronia melanocarpa (Michx.) Elliot fruit juice reveals neuroprotective effect and improves cognitive and locomotor functions of aged rats. Food Chem. Toxicol. 2019, 132, 110674. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Romaszko, E.; Piskula, M.K. Bioavailability of cyanidin glycosides from natural chokeberry (Aronia melanocarpa) juice with dietary-relevant dose of anthocyanins in humans. J. Agric. Food Chem. 2010, 58, 12130–12136. [Google Scholar] [CrossRef]
- Dennis, J.P.; Vander Wal, J.S. The cognitive flexibility inventory: Instrument development and estimates of reliability and validity. Cogn. Ther. Res. 2010, 34, 241–253. [Google Scholar] [CrossRef]
- Lohman, E.B.; Johnson, E.G.; Miguel, A.M.; Cordett, T.K.; Kang, D. Automated pegboard system: Reliability and validity of a new tool. J. Appl. Res. 2003, 3, 262–269. [Google Scholar]
- Dekker, R.; Mulder, J.; Dekker, P. De Ontwikkeling Van Vijf Nieuwe Nederlandstalige Tests; PITS: Leiden, The Netherlands, 2007. [Google Scholar]
- Nyenhuis, D.L.; Yamamoto, C.; Stern, R.A.; Luchetta, T.; Arruda, J.E. Standardization and validation of the visual analog mood scales. Clin. Neuropsychol. 1997, 11, 407–415. [Google Scholar] [CrossRef]
- Waal, T.; Zijta, F.; Stok, W.; Groot, E.; Kastelein, J.; Karemaker, J.M. 501 M-mode ultrasound imaging of arterial wall movement and arterial wall thickness of distal carotid arteries in atherosclerosis studies in healthy adults. Atheroscler. Suppl. 2004, 5, 115–116. [Google Scholar] [CrossRef]
- Łoboz-Rudnicka, M.; Jaroch, J.; Kruszyńska, E.; Bociąga, Z.; Rzyczkowska, B.; Dudek, K.; Szuba, A.; Łoboz-Grudzień, K. Gender-related differences in the progression of carotid stiffness with age and in the influence of risk factors on carotid stiffness. Clin. Interv. Aging 2018, 13, 1183–1191. [Google Scholar] [CrossRef]
- Kastelein, J.J.; Akdim, F.; Stroes, E.S.; Zwinderman, A.H.; Bots, M.L.; Stalenhoef, A.F.; Visseren, F.L.; Sijbrands, E.J.; Trip, M.D.; Stein, E.A.; et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 2008, 358, 1431–1443. [Google Scholar] [CrossRef]
- Kollias, A.; Ntineri, A.; Kyriakoulis, K.G.; Stambolliu, E.; Lagou, S.; Boubouchairopoulou, N.; Stergiou, G.S. Validation of the professional device for blood pressure measurement Microlife WatchBP Office in adults and children according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization standard. Blood Press. Monit. 2018, 23, 112–114. [Google Scholar]
- Cheng, H.M.; Sung, S.H.; Shih, Y.T.; Chuang, S.Y.; Yu, W.C.; Chen, C.H. Measurement accuracy of a stand-alone oscillometric central blood pressure monitor: A validation report for Microlife WatchBP Office Central. Am. J. Hypertens. 2013, 26, 42–50. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The cocoa, cognition, and aging (CoCoA) study—A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N.; Kulkarni, S.S. A review on laboratory liver function tests. Pan Afr. Med. J. 2009, 3, 17. [Google Scholar]
- Lin, C.S.; Chang, C.J.; Lu, C.C.; Martel, J.; Ojcius, D.M.; Ko, Y.F.; Young, J.D.; Lai, H.C. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed. J. 2014, 37, 259–268. [Google Scholar] [PubMed]
- Sachdev, P.; Blacker, D.; Blazer, D.; Ganguli, M.; Jeste, D.; Paulsen, J.; Petersen, R. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Ashendorf, L.; Vanderslice-Barr, J.L.; McCaffrey, R.J. Motor tests and cognition in healthy older adults. Appl. Neuropsychol. 2009, 16, 171–176. [Google Scholar] [CrossRef]
- Van der Elst, W.; Van Boxtel, M.P.; Van Breukelen, G.J.; Jolles, J. The Stroop color-word test: Influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment 2006, 13, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, H.; Zatsiorsky, V.; Latash, M. The effects of strength training on finger strength and hand dexterity in healthy elderly individuals. J. Appl. Physiol. 2008, 105, 1166–1178. [Google Scholar] [CrossRef]
- Martins, M.; Neves, L.; Rodrigues, P.; Vasconcelos, O.; Castro, S.L. Orff-Based music training enhances children’s manual dexterity and bimanual coordination. Front. Psychol. 2018, 9, 2616. [Google Scholar] [CrossRef]
- Halbach, M.M.; Spann, C.O.; Egan, G. Effect of sleep deprivation on medical resident and student cognitive function: A prospective study. Am. J. Obstet. Gynecol. 2003, 188, 1198–1201. [Google Scholar] [CrossRef]
- Boggs, D.L.; Cortes-Briones, J.A.; Surti, T.; Luddy, C.; Ranganathan, M.; Cahill, J.D.; Sewell, A.R.; D’Souza, D.C.; Skosnik, P.D. The dose-dependent psychomotor effects of intravenous delta-9-tetrahydrocannabinol (Δ9-THC) in humans. J. Psychopharmacol. 2018, 32, 1308–1318. [Google Scholar] [CrossRef]
- Wilkosc, M.; Szalkowska, A.; Skibinska, M.; Zajac-Lamparska, L.; Maciukiewicz, M.; Araszkiewicz, A. BDNF gene polymorphisms and haplotypes in relation to cognitive performance in Polish healthy subjects. Acta Neurobiol. Exp. (Wars) 2016, 76, 43–52. [Google Scholar]
- Gunstad, J.; Benitez, A.; Smith, J.; Glickman, E.; Spitznagel, M.B.; Alexander, T.; Juvancic-Heltzel, J.; Murray, L. Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. J. Geriatr. Psychiatry Neurol. 2008, 21, 166–170. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.M.; Kelly, Á.M.; Robertson, I.H.; Hussey, J. An investigation into the relationship between cardiorespiratory fitness, cognition and BDNF in young healthy males. Neurosci. Lett. 2019, 704, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Dols, A.; Thesing, C.S.; Bouckaert, F.; Voshaar, R.C.O.; Comijs, H.C.; Stek, M.L. BDNF serum levels are not related to cognitive functioning in older depressed patients and controls. Int. Psychogeriatr. 2015, 27, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Bäckman, L.; Nyberg, L.; Lindenberger, U.; Li, S.-C.; Farde, L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci. Biobehav. Rev. 2006, 30, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, S.; Ding, L.; Liu, N.; Gao, D. Serum levels of glial cell line-derived neurotrophic factor and multiple neurotransmitters: In relation to cognitive performance in Parkinson’s disease with mild cognitive impairment. Int. J. Geriatr. Psychiatry 2020, 35, 153–162. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Hagen, C.E.; Machulda, M.M.; Hollman, J.H.; Roberts, R.O.; Knopman, D.S.; Petersen, R.C.; Mielke, M.M. The association between peripheral total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 and functional and cognitive outcomes in the Mayo Clinic Study of Aging. Neurobiol. Aging 2018, 66, 68–74. [Google Scholar] [CrossRef]
- Tumati, S.; Burger, H.; Martens, S.; van der Schouw, Y.T.; Aleman, A. Association between cognition and serum insulin-like growth factor-1 in middle-aged & older men: An 8 year follow-up study. PLoS ONE 2016, 11, e0154450. [Google Scholar]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J. Neuroimmunol. 2014, 273, 8–21. [Google Scholar] [CrossRef]
- AsghariHanjani, N.; Vafa, M. The role of IGF-1 in obesity, cardiovascular disease, and cancer. Med. J. Islam. Repub. Iran 2019, 33, 56. [Google Scholar] [PubMed]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of aronia berry (poly)phenols on vascular function and gut microbiota: A double-blind randomized controlled trial in adult men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Zeki Al Hazzouri, A.; Vittinghoff, E.; Sidney, S.; Reis, J.P.; Jacobs, D.R., Jr.; Yaffe, K. Intima-Media thickness and cognitive function in stroke-free middle-aged adults: Findings from the coronary artery risk development in young adults study. Stroke 2015, 46, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Urbina, E.M.; Srinivasan, S.R.; Kieltyka, R.L.; Tang, R.; Bond, M.G.; Chen, W.; Berenson, G.S. Correlates of carotid artery stiffness in young adults: The bogalusa heart study. Atherosclerosis 2004, 176, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Jochemsen, H.M.; Muller, M.; Bots, M.L.; Scheltens, P.; Vincken, K.L.; Mali, W.P.; van der Graaf, Y.; Geerlings, M.I. Arterial stiffness and progression of structural brain changes: The SMART-MR study. Neurology 2015, 84, 448–455. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef]
- Watson, A.W.; Haskell-Ramsay, C.F.; Kennedy, D.O.; Cooney, J.M.; Trower, T.; Scheepens, A. Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase-B in healthy young adults. J. Funct. Foods 2015, 17, 524–539. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Case, A.J.; Agraz, D.; Ahmad, I.M.; Zimmerman, M.C. Low-Dose aronia melanocarpa concentrate attenuates paraquat-induced neurotoxicity. Oxid. Med. Cell. Longev. 2016, 2016, 5296271. [Google Scholar] [CrossRef] [PubMed]
- Mattoli, L.; Cangi, F.; Maidecchi, A.; Ghiara, C.; Ragazzi, E.; Tubaro, M.; Stella, L.; Tisato, F.; Traldi, P. Metabolomic fingerprinting of plant extracts. J. Mass Spectrom. 2006, 41, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Sueiro, L.; Yousef, G.G.; Seigler, D.; De Mejia, E.G.; Grace, M.H.; Lila, M.A. Chemopreventive Potential of flavonoid extracts from plantation-bred and wild Aronia melanocarpa (Black chokeberry) fruits. J. Food Sci. 2006, 71, C480–C488. [Google Scholar] [CrossRef]
- Dang, J.S.; Figueroa, I.J.; Helton, W.S. Determining practice effects on a cognitive flexibility assessment. Proc. Hum. Factors Ergon. Soc. Ann. Meet. 2017, 61, 1829–1833. [Google Scholar] [CrossRef]
- Bartels, C.; Wegrzyn, M.; Wiedl, A.; Ackermann, V.; Ehrenreich, H. Practice effects in healthy adults: A longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010, 11, 118. [Google Scholar] [CrossRef]
- Soares, F.C.; de Oliveira, T.C.G.; de Macedo, L.D.eD.; Tomás, A.M.; Picanço-Diniz, D.L.W.; Bento-Torres, J.; Bento-Torres, N.V.O.; Picanço-Diniz, C.W. CANTAB object recognition and language tests to detect aging cognitive decline: An exploratory comparative study. Clin. Interv. Aging 2014, 10, 37–48. [Google Scholar]

| 90 mg Aronia (n = 34) | 150 mg Aronia (n = 35) | Placebo (n = 32) | p-Value | |
|---|---|---|---|---|
| Male Gender (n; %) | 11; 32% | 11; 31% | 14; 44% | 0.510 |
| Age (years) | 53 ± 1 | 53 ± 1 | 53 ± 1 | 0.722 |
| BMI (kg/m2) | 29.5 ± 0.4 | 29.4 ± 0.5 | 29.3 ± 0.5 | 0.988 |
| Pegboard dominant hand score 2 | 94.4 ± 1.6 | 97.3 ± 2.1 | 92.9 ± 2.0 | 0.244 |
| Pegboard non-dominant hand score 2 | 98.1 ± 2.0 | 103.9 ± 2.0 | 100.4 ± 2.1 | 0.125 |
| Total correct—total incorrect 3 | 47.2 ± 4.7 | 54.5 ± 4.9 | 55.4 ± 5.6 | 0.461 |
| Total edited—total incorrect and missed 3 | 49.1 ± 4.7 | 54.8 ± 4.7 | 55.6 ± 5.8 | 0.615 |
| Stroop Interference (sec) 2 | 32.7 ± 2.0 | 31.5 ± 2.7 | 30.7 ± 1.9 | 0.833 |
| Brachial systolic blood pressure (mm Hg) | 130.0 ± 2.6 | 130.1 ± 2.1 | 131.4 ± 2.6 | 0.896 |
| Brachial diastolic blood pressure (mm Hg) | 81.3 ± 1.5 | 81.2 ± 1.3 | 82.2 ± 1.4 | 0.873 |
| Mood (T-Scores) | 90 mg Aronia (n = 34) | 150 mg Aronia (n = 35) | Placebo (n = 32) | Time*Treatment Interaction | Main Effect of Time | Main Effect of Treatment |
|---|---|---|---|---|---|---|
| Afraid | 0.881 a | 0.191 b | 0.301 b | |||
| Baseline | 45.1 ± 0.9 | 45.1 ± 0.9 | 45.0 ± 0.7 | |||
| 6 weeks | 46.1 ± 1.2 | 45.1 ± 0.7 | 47.3 ± 1.1 | |||
| 12 weeks | 46.0 ± 1.2 | 45.9 ± 1.5 | 48.5 ± 1.7 | |||
| 24 weeks | 44.8 ± 0.4 | 45.0 ± 0.5 | 45.9 ± 0.9 | |||
| Angry | 0.700 a | 0.051 b | 0.115 b | |||
| Baseline | 44.0 ± 1.1 | 43.3 ± 0.3 | 44.0 ± 0.6 | |||
| 6 weeks | 43.8 ± 0.3 | 43.5 ± 0.3 | 47.0 ± 1.4 | |||
| 12 weeks | 44.8 ± 0.8 | 44.6 ± 1.3 | 46.3 ± 1.5 | |||
| 24 weeks | 45.2 ± 0.8 | 44.5 ± 0.7 | 45.5 ± 1.4 | |||
| Confused | 0.392 a | 0.463 b | 0.231 b | |||
| Baseline | 45.5 ± 1.5 | 43.7 ± 0.7 | 45.1 ± 1.2 | |||
| 6 weeks | 45.2 ± 0.9 | 43.7 ± 0.4 | 44.9 ± 0.8 | |||
| 12 weeks | 44.2 ± 0.5 | 44.6 ± 1.3 | 47.3 ± 1.8 | |||
| 24 weeks | 45.8 ± 1.9 | 44.5 ± 0.7 | 46.0 ± 1.5 | |||
| Energetic | 0.306 a | 0.183 b | 0.632 b | |||
| Baseline | 44.5 ± 2.0 | 45.1 ± 2.2 | 47.1 ± 2.0 | |||
| 6 weeks | 44.6 ± 2.3 | 44.7 ± 2.1 | 40.9 ± 2.3 | |||
| 12 weeks | 47.2 ± 2.2 | 44.1 ± 2.1 | 46.6 ± 2.8 | |||
| 24 weeks | 44.8 ± 1.9 | 43.9 ± 2.1 | 44.6 ± 2.4 | |||
| Happy | 0.119 a | 0.112 b | 0.366 b | |||
| Baseline | 41.9 ± 2.2 | 43.3 ± 2.3 | 42.9 ± 2.8 | |||
| 6 weeks | 41.0 ± 2.6 | 40.7 ± 2.5 | 36.8 ± 2.4 | |||
| 12 weeks | 46.5 ± 2.1 | 41.0 ± 2.7 | 39.5 ± 2.7 | |||
| 24 weeks | 41.0 ± 2.3 | 42.0 ± 2.4 | 41.8 ± 2.5 | |||
| Sad | 0.976 a | 0.600 b | 0.069 b | |||
| Baseline | 44.3 ± 1.0 | 43.7 ± 0.7 | 43.4 ± 0.5 | |||
| 6 weeks | 45.9 ± 1.3 | 43.7 ± 0.6 | 45.8 ± 1.3 | |||
| 12 weeks | 45.7 ± 1.2 | 44.4 ± 1.5 | 47.1 ± 1.7 | |||
| 24 weeks | 45.8 ± 1.1 | 44.1 ± 0.7 | 45.2 ± 1.4 | |||
| Tense | 0.974 a | 0.318 b | 0.156 b | |||
| Baseline | 45.4 ± 1.2 | 45.6 ± 1.3 | 44.0 ± 0.9 | |||
| 6 weeks | 43.9 ± 1.1 | 43.6 ± 0.7 | 45.3 ± 1.2 | |||
| 12 weeks | 43.9 ± 0.9 | 43.9 ± 1.3 | 44.6 ± 1.5 | |||
| 24 weeks | 43.4 ± 1.1 | 42.7 ± 0.6 | 44.6 ± 1.4 | |||
| Tired | 0.169 a | 0.379 b | 0.318 b | |||
| Baseline | 43.8 ± 1.1 | 44.4 ± 1.4 | 41.1 ± 1.1 | |||
| 6 weeks | 44.1 ± 1.5 | 45.0 ± 1.6 | 44.5 ± 1.4 | |||
| 12 weeks | 47.2 ± 1.6 | 45.0 ± 1.6 | 44.5 ± 1.3 | |||
| 24 weeks | 45.4 ± 1.5 | 43.5 ± 1.3 | 45.6 ± 1.8 |
| 90 mg Aronia (n = 34) | 150 mg Aronia (n = 35) | Placebo (n = 32) | Time*Treatment Interaction | Main Effect of Time | Main Effect of Treatment | |
|---|---|---|---|---|---|---|
| eP (kPa) | 0.810 a | 0.908 b | 0.165 b | |||
| Baseline | 75.6 ± 4.8 | 62.4 ± 3.6 | 65.9 ± 3.0 | |||
| 6 weeks | 65.7 ± 3.4 | 62.1 ± 2.0 | 63.8 ± 3.0 | |||
| 12 weeks | 65.4 ± 3.7 | 67.2 ± 3.8 | 60.3 ± 2.9 | |||
| 24 weeks | 67.0 ± 3.3 | 64.1 ± 2.4 | 60.0 ± 3.3 | |||
| cIMT Mean (mm) | 0.723 a | 0.326 b | 0.328 b | |||
| Baseline | 0.64 ± 0.01 | 0.63 ± 0.02 | 0.64 ± 0.01 | |||
| 6 weeks | 0.62 ± 0.02 | 0.64 ± 0.01 | 0.64 ± 0.02 | |||
| 12 weeks | 0.63 ± 0.02 | 0.64 ± 0.01 | ||||
| 24 weeks | 0.62 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 | |||
| ABI | 0.612 a | 0.973 b | ||||
| Baseline | 1.23 ± 0.02 | 1.21 ± 0.01 | 1.23 ± 0.02 | |||
| 6 weeks | 1.24 ± 0.02 | 1.23 ± 0.02 | 1.25 ± 0.02 | |||
| 12 weeks | 1.26 ± 0.02 | 1.24 ± 0.02 | 1.23 ± 0.02 | |||
| 24 weeks | 1.27 ± 0.02 | 1.25 ± 0.02 | 1.26 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; de Groot, E.; Plat, J. The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals. Nutrients 2020, 12, 2475. https://doi.org/10.3390/nu12082475
Ahles S, Stevens YR, Joris PJ, Vauzour D, Adam J, de Groot E, Plat J. The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals. Nutrients. 2020; 12(8):2475. https://doi.org/10.3390/nu12082475
Chicago/Turabian StyleAhles, Sanne, Yala R. Stevens, Peter J. Joris, David Vauzour, Jos Adam, Eric de Groot, and Jogchum Plat. 2020. "The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals" Nutrients 12, no. 8: 2475. https://doi.org/10.3390/nu12082475
APA StyleAhles, S., Stevens, Y. R., Joris, P. J., Vauzour, D., Adam, J., de Groot, E., & Plat, J. (2020). The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals. Nutrients, 12(8), 2475. https://doi.org/10.3390/nu12082475









