Effects of both Pro- and Synbiotics in Liver Surgery and Transplantation with Special Focus on the Gut–Liver Axis—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis
3. Results
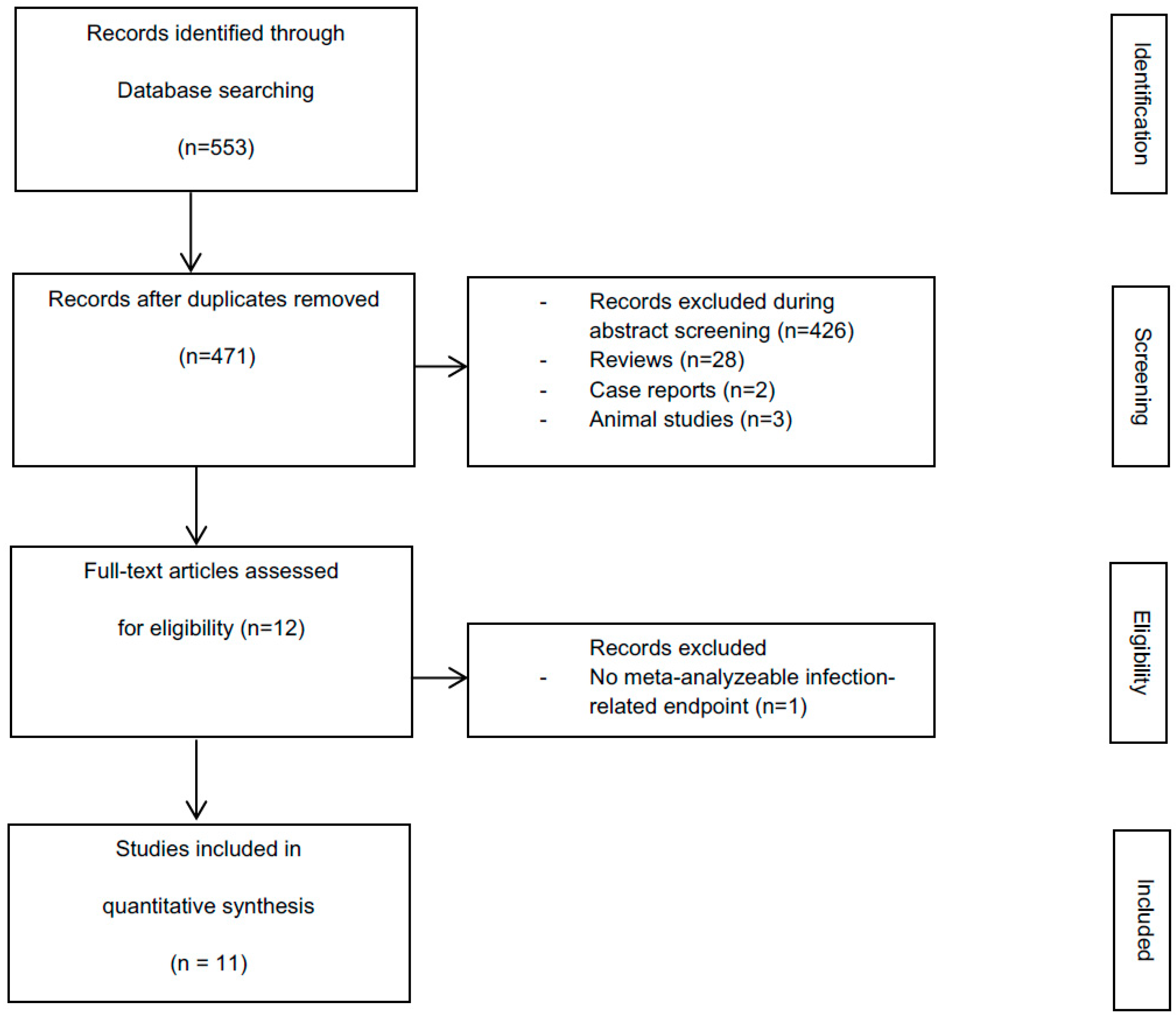
3.1. Literature Search
3.2. Study Characteristics
3.3. Study Quality
3.4. Infection Rate after Liver Transplantation
3.5. Infection Rate after Liver Resection
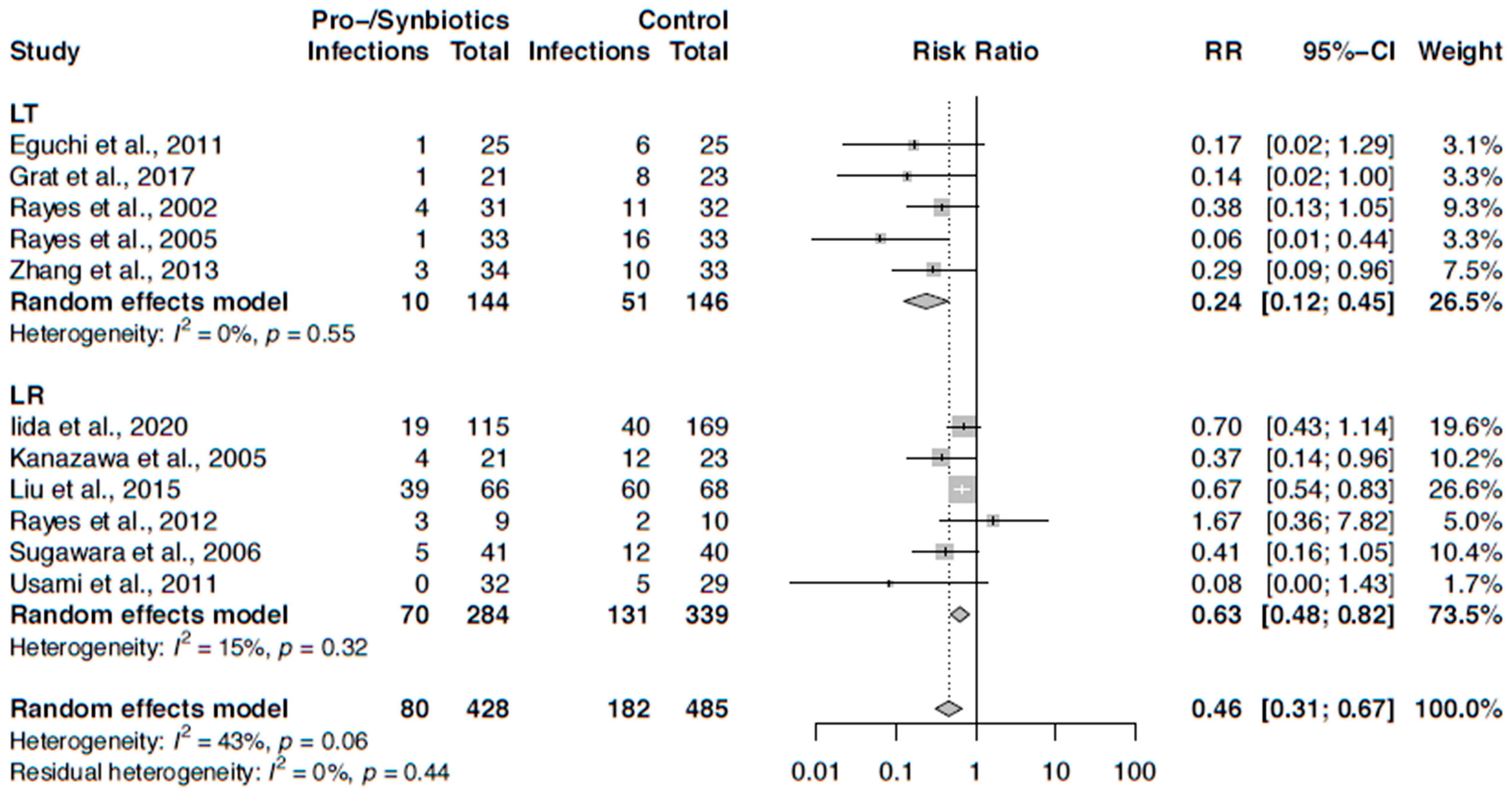
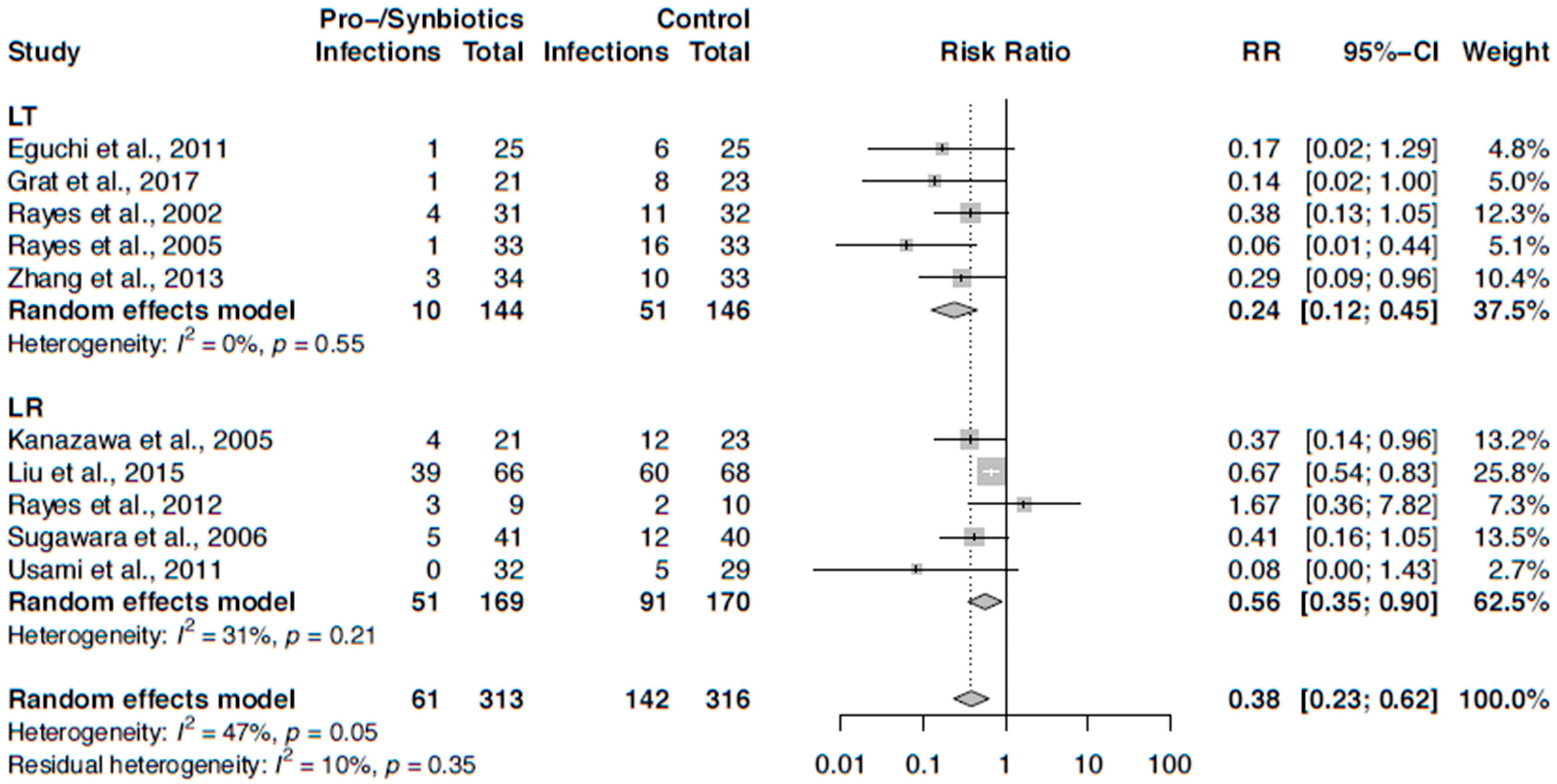
3.6. Meta-Analysis on Infection Rates
3.7. Perioperative Liver Function Parameters in LT
3.8. Perioperative Liver Function Parameters in LR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rayes, N.; Schutz, T.; Lochs, H. Probiotika, Prabiotika und Synbiotika in der Chirurgie und bei kritisch Kranken auf der Intensivstation. Leberresektion, Transplantation. In Probiotika, Präbiotika and Synbiotika, 1st ed.; Bischoff, S.C., Ed.; Georg Thieme Verlag KG: Stuttgart, Germany, 2009; p. 296. [Google Scholar]
- Rahbari, N.N.; Wente, M.; Schemmer, P.; Diener, M.K.; Hoffmann, K.; Motschall, E.; Schmidt, J.; Weitz, J.; Büchler, M.W. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. Br. J. Surg. 2008, 95, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Elbers, H.; Koch, M.; Vogler, P.; Striebel, F.; Bruckner, T.; Mehrabi, A.; Schemmer, P.; Büchler, M.W.; Weitz, J. Randomized clinical trial of stapler versus clamp-crushing transection in elective liver resection. Br. J. Surg. 2014, 101, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Müller-Bütow, V.; Franz, C.; Hinz, U.; Longerich, T.; Büchler, M.W.; Schemmer, P. Factors predictive of survival after stapler hepatectomy of hepatocellular carcinoma: A multivariate, single-center analysis. Anticancer Res. 2014, 34, 767–776. [Google Scholar] [PubMed]
- Bruns, H.; Lozanovski, V.J.; Schultze, D.; Hillebrand, N.; Hinz, U.; Büchler, M.W.; Schemmer, P. Prediction of postoperative mortality in liver transplantation in the era of MELD-based liver allocation: A multivariate analysis. PLoS ONE 2014, 9, e98782. [Google Scholar] [CrossRef]
- Hoffmann, K.; Unsinn, M.; Hinz, U.; Weiss, K.H.; Waldburger, N.; Longerich, T.; Radeleff, B.; Schirmacher, P.; Büchler, M.W.; Schemmer, P. Outcome after a liver resection of benign lesions. HPB (Oxford) 2015, 17, 994–1000. [Google Scholar] [CrossRef][Green Version]
- Schemmer, P.; Friess, H.; Büchler, M.W. Recent advances in surgical therapy for primary and metastatic liver cancer. Ann. Surg. Hepatol. 2002, 7, 124–133. [Google Scholar]
- Nickkholgh, A.; Weitz, J.; Encke, J.; Sauer, P.; Mehrabi, A.; Büchler, M.W.; Schmidt, J.; Schemmer, P. Utilization of extended donor criteria in liver transplantation: A comprehensive review of the literature. Nephrol. Dial. Transplant. 2007, 22, viii29–viii36. [Google Scholar] [CrossRef][Green Version]
- Schemmer, P.; Friess, H.; Dervenis, C.; Schmidt, J.; Weitz, J.; Uhl, W.; Büchler, M.W. The use of endo GIA vascular staplers in liver surgery and their potential benefit: A review. Dig. Surg. 2007, 24, 300–305. [Google Scholar] [CrossRef]
- Schemmer, P.; Bruns, H.; Weitz, J.; Schmidt, J.; Büchler, M.W. Liver transection using vascular stapler: A review. HPB (Oxford) 2008, 10, 249–252. [Google Scholar] [CrossRef]
- Lin, S.; Hoffmann, K.; Schemmer, P. Treatment of hepatocellular carcinoma: A systematic review. Liver Cancer 2012, 1, 144–158. [Google Scholar] [CrossRef]
- Gotthardt, D.N.; Weiss, K.H.; Rupp, C.; Bode, K.; Eckerle, I.; Rudolph, G.; Bergemann, J.; Kloeters-Plachky, P.; Chahoud, F.; Büchler, M.W.; et al. Bacteriobilia and fungibilia are associated with outcome in patients with endoscopic treatment of biliary complications after liver transplantation. Endoscopy 2013, 45, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 2007, 357, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Xourafas, D.; Pawlik, T.M.; Cloyd, J.M. Early Morbidity and Mortality after Minimally Invasive Liver Resection for Hepatocellular Carcinoma: A Propensity-Score Matched Comparison with Open Resection. J. Gastrointest. Surg. 2019, 23, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Su, S.; Li, B.; Fang, C. Efficacy of probiotics and prebiotics in prevention of infectious complications following hepatic resections: Systematic review and meta-analysis. J. Gastrointest. Liver Dis. 2019, 28, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Kaido, T.; Mori, A.; Oike, F.; Mizumoto, M.; Ogura, Y.; Hata, K.; Yoshizawa, A.; Iida, T.; Uemoto, S. Impact of pretransplant nutritional sta- tus in patients undergoing liver transplantation. Hepatogastroenterology 2010, 57, 1489–1492. [Google Scholar]
- Singh, N.; Paterson, D.L.; Gayowski, T.; Wagener, M.M.; Marino, I.R. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transplant. 2000, 6, 54–61. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef]
- Bateman, B.T.; Schmidt, U.; Berman, M.F.; Bittner, E.A. Temporal trends in the epidemiology of severe postoperative sepsis after elective surgery: A large, nationwide sample. Anesthesiology 2010, 112, 917–925. [Google Scholar] [CrossRef]
- Moore, L.J.; Moore, F.A.; Todd, S.R.; Jones, S.L.; Turner, K.L.; Bass, B.L. Sepsis in general surgery: The 2005—National surgical quality improvement program perspective. Arch. Surg. 2010, 145, 695–700. [Google Scholar] [CrossRef]
- Vogel, T.R.; Dombrovskiy, V.Y.; Carson, J.L.; Graham, A.M.; Lowry, S.F. Postoperative sepsis in the United States. Ann. Surg. 2010, 252, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Martinez, E.G.; Gregorio, G.V.; Dans, L.F. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst. Rev. 2010, 2010, CD003048. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Bousvaros, A.; Lee, J.W.; Diaz, A.; Davidson, E.J. Efficacy of probiotic use in acute diarrhea in children: A meta-analysis. Dig. Dis. Sci. 2002, 47, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Mrukowicz, J.Z. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: A systematic review of published randomized, double-blind, placebo-controlled trials. J. Pediatr. Gastroenterol. Nutr. 2001, 33 (Suppl. S2), 17–25. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, C.W.; Feudtner, C.; Garrison, M.M.; Christakis, D.A. Lactobacillus therapy for acute infectious diarrhea in children: A meta-analysis. Pediatrics 2002, 109, 678–684. [Google Scholar] [CrossRef]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med. Infect. Dis. 2007, 5, 97–105. [Google Scholar] [CrossRef]
- Johnston, B.C.; Supina, A.L.; Ospina, M.; Vohra, S. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2007, 2007, CD004827. [Google Scholar]
- Johnston, B.C.; Supina, A.L.; Ospina, M.; Vohra, S. Probiotics for pediatric antibiotic-associated diarrhea: A meta-analysis of randomized placebo-controlled trials. CMAJ 2006, 175, 377–383. [Google Scholar] [CrossRef]
- Szajewska, H.; Ruszczyński, M.; Radzikowski, A. Probiotics in the prevention of antibiotic-associated diarrhea in children: A meta-analysis of randomized controlled trials. J. Pediatr. 2006, 149, 367–372. [Google Scholar] [CrossRef]
- Cremonini, F.; Di Caro, S.; Nista, E.C.; Bartolozzi, F.; Capelli, G.; Gasbarrini, G.; Gasbarrini, A. Meta-analysis: The effect of probiotic administration on antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2002, 16, 1461–1467. [Google Scholar] [CrossRef]
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ 2002, 324, 1361. [Google Scholar] [CrossRef] [PubMed]
- Hawrelak, J.A.; Whitten, D.L.; Myers, S.P. Is Lactobacillus rhamnosus GG effec- tive in preventing the onset of antibiotic-associated diarrhoea: A systematic review. Digestion 2005, 72, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Mrukowicz, J. Meta-analysis: Non-pathogenic yeast Saccharo- myces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2005, 22, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.D.; Pyrsopoulos, N.; Rustgi, V.K. Microbiota and the liver. Liver Transplant. 2018, 24, 539–550. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in dandomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. Available online: http://www.ahri.a/programs/clinical_epidemiology/oxford.asp (accessed on 27 October 2017).
- Grąt, M.; Wronka, K.M.; Lewandowski, Z.; Grąt, K.; Krasnodębski, M.; Stypułkowski, J.; Hołówko, W.; Masior, Ł.; Kosińska, I.; Wasilewicz, M.; et al. Effects of continuous use of probiotics before liver transplantation: A randomized, double-blind, placebo- controlled trial. Clin. Nutr. 2017, 36, 1530–1539. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Hansen, S.; Boucsein, K.; Müller, A.R.; Serke, S.; Bengmark, S.; Neuhaus, P. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: A controlled trial in liver transplant recipients. Transplantation 2002, 74, 123–127. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Theruvath, T.; Schiller, R.A.; Langrehr, J.M.; Jonas, S.; Bengmark, S.; Neuhaus, P. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation—A randomized, double-blind trial. Am. J. Transplant. 2005, 5, 125–130. [Google Scholar] [CrossRef]
- Eguchi, S.; Takatsuki, M.; Hidaka, M.; Soyama, A.; Ichikawa, T.; Kanematsu, T. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: A prospective randomized study. Am. J. Surg. 2011, 201, 498–502. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Wu, J.; Chalson, H.; Merigan, L.; Mitchell, A. Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobiliary Surg. Nutr. 2013, 2, 142–147. [Google Scholar] [PubMed]
- Kanazawa, H.; Nagino, M.; Kamiya, S.; Komatsu, S.; Mayumi, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Tanaka, R.; Nimura, Y. Synbiotics reduce postoperative infectious complications: A randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch. Surg. 2005, 390, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, C.; Huang, M.; Tong, C.; Zhang, X.; Wang, L.; Peng, H.; Lan, P.; Zhang, P.; Huang, N.; et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: A double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Rifatbegovic, Z.; Mesic, D.; Ljuca, F.; Zildzic, M.; Avdagic, M.; Grbic, K.; Agic, M.; Hadziefendic, B. Effect of probiotics on liver function after surgery resection for malignancy in the liver cirrhotic. Med. Arch. 2010, 64, 208–211. [Google Scholar]
- Rayes, N.; Pilarski, T.; Stockmann, M.; Bengmark, S.; Neuhaus, P.; Seehofer, D. Effect of pre- and probiotics on liver regeneration after resection: A randomised, double-blind pilot study. Benef. Microbes 2012, 3, 237–244. [Google Scholar] [CrossRef]
- Usami, M.; Miyoshi, M.; Kanbara, Y.; Aoyama, M.; Sakaki, H.; Shuno, K.; Hirata, K.; Takahashi, M.; Ueno, K.; Tabata, S.; et al. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. JPEN J. Parenter. Enter. Nutr. 2011, 35, 317–328. [Google Scholar] [CrossRef]
- Sugawara, G.; Nagino, M.; Nishio, H.; Ebata, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Nimura, Y. Perioperative Synbiotic Treatment to Prevent Postoperative Infectious Complications in Biliary Cancer Surgery: A Randomized Controlled Trial. Ann. Surg. 2006, 244, 706–714. [Google Scholar] [CrossRef]
- Iida, H.; Sasaki, M.; Maehira, H.; Mori, H.; Yasukawa, D.; Takebayashi, K.; Kurihara, M.; Bamba, S.; Tani, M. The effect of preoperative synbiotic treatment to prevent surgical-site infection in hepatic resection. J. Clin. Biochem. Nutr. 2020, 66, 67–73. [Google Scholar] [CrossRef]
- Sawas, T.; Al Halabi, S.; Hernaez, R.; Carey, W.D.; Cho, W.K. Patients Receiving Prebiotics and Probiotics Before Liver Transplantation Develop Fewer Infections Than Controls: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 1567–1574. [Google Scholar] [CrossRef]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef]
- Faure, E.; Equils, O.; Sieling, P.A.; Thomas, L.; Zhang, F.X.; Kirschning, C.J.; Polentarutti, N.; Muzio, M.; Arditi, M. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-and TLR-in endothelial cells. J. Biol. Chem. 2000, 275, 11058–11063. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuqoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, C.C.; Mabvuure, N.T.; Ali, A.; Kozar, R.A.; Herndon, D.N. The surgically induced stress response. JPEN J. Parenter. Enter. Nutr. 2013, 37 (Suppl. S5), 21S–29S. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.H.; Adiamah, A.; Kushairi, A.; Varadhan, K.K.; Krznaric, Z.; Kulkarni, A.D.; Neal, K.R.; Lobo, D.N. Perioperative Probiotics or Synbiotics in Adults Undergoing Elective Abdominal Surgery: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2020, 271, 1036–1047. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Lara, L.F.; Koulaouzidis, A.; Misera, A.; Maciejewska, D.; Marlicz, W. A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications. J. Clin. Med. 2018, 7, 556. [Google Scholar] [CrossRef]
- Moran, C.P.; Musa, S.A.; Rahman, T.M. The use of probiotics in the surgical patient. Eur. Surg. 2011, 44, 91–98. [Google Scholar] [CrossRef]
- Masuda, T.; Shirabe, K.; Yoshiya, S.; Matono, R.; Morita, K.; Hashimoto, N.; Ikegami, T.; Yoshizumi, T.; Baba, H.; Maehara, Y. Nutrition Support and Infections Associated With Hepatic Resection and Liver Transplantation in Patients With Chronic Liver Disease. J. Parenter. Enter. Nutr. 2012, 37, 318–326. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, Z.P.; Ha, D.K.; Bengmark, S.; Kurtovic, J.; Riordan, S.M. Synbiotic modulation of gut flora: Effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004, 39, 1441–1449. [Google Scholar] [CrossRef]
- Horvath, A.; Leber, B.; Schmerboeck, B.; Tawdrous, M.; Zettel, G.; Hartl, A.; Madl, T.; Stryeck, S.; Fuchs, D.; Lemesch, S.; et al. Randomised clinical trial: The effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 2016, 44, 926–935. [Google Scholar] [CrossRef]
- Yeh, D.C.; Wu, C.C.; Ho, W.M.; Cheng, S.B.; Lu, I.Y.; Liu, T.J.; P’eng, F.K. Bacterial translocation after cirrhotic liver resection: A clinical investigation of patients. J. Surg. Res. 2003, 111, 209–214. [Google Scholar] [CrossRef]
- Viehman, J.A.; Clancy, C.J.; Clarke, L.; Shields, R.K.; Silveira, F.P.; Kwak, E.J.; Vergidis, P.; Hughes, C.; Humar, A.; Nguyen, M.H. Surgical site infections after liver transplantation: Emergence of multidrug-resistant bacteria and implications for prophylaxis and treatment strategies. Transplantation 2016, 100, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, M.R.; Descourouez, J.L.; Siodlak, M.; Tjugum, S.; Rice, J.P.; Fernandez, L.A. Efficacy and Safety of Probiotics and Synbiotics in Liver Transplantation. Pharmacotherapy 2018, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]

| Study (Ref.) | Country | Study Population | Pro-/Synbiotic (n)/ Control (n) | Pro-/Synbiotics Used | Pro-/Synbiotic Content and Pharmaceutical Form | Control Used | Time of Administration | Results Pro-Synbiotic/Control Postoperative Infection Rate | Age (Years) All pt.; Pro-/Synbiotic/ Control | Study Design |
|---|---|---|---|---|---|---|---|---|---|---|
| Liver transplantation (LT) | PA/PE n = 290/343 | |||||||||
| Rayes et al., 2002 [40] | Germany | 63/105 | 31/32 | Synbiotics (Lactobacillus plantarum 299 and inulin with selective bowel decontamination and enteric nutrition) | 109 CFU 2×/day | Placebo/inulin | Just before LT until 12 days post LT (13 days) | 4/31, 11/32 FU POD 13 | 50 ± 2; 50 ± 2/ 50 ± 2 | RCT |
| Rayes et al., 2005 [41] | Germany | 66/66 | 33/33 | Synbiotics (Pediococcus pentosaceus, Leuconostoc mesenteroides, L. paracasei, 1010 L. plantarum 2362, beta-glucan, inulin, pectin, and resistant starch and enteric nutrition) | probiotic: 1010 CFU, prebiotic: 10 g 2×/day; powder | Placebo/fibers | Just after LT until 14 days post LT (13 days) | 1/33, 16/33 FU POD 30 | 51.5 ± 2; 53 ± 2/ 50 ± 2 | RCT |
| Eguchi et al., 2011 [42] | Japan | 50/50 | 25/25 | Synbiotics (Bifidobacte rium breve, Lactobacillus casei, Galactooligo saccharide and enteric nutrition) | 15 mg 20 mg 15 mg 3×/day | No intervention, enteric nutrition | 2 days prior to LDLT until 14 days post LDLT (16 days) | 1/25, 6/25 FU POD 19 | 56.5 ± NR; 56 (33–66)/ 57 (25–68) | RCT |
| Zhang et al., 2013 [43] | Australia | 67/67 | 34/33 | Synbiotics (Lactobacillus acidophilus, plantarum, lactis, casei, rhamnosus, brevis, Bifidobacterium lactis and fibers and enteric nutrition) | 15.5 × 109; 5.0 × 109; 2.0 × 109; 1.5 × 109; 1.5 × 109; 1.5 × 109 CFU; capsules | Enteric nutrition and fibers | Immediately after LT for 7 days at minimum (7 days) | 3/34, 10/33 FU POD 8 | 56.01 ± 10.98; 57 ± 10/ 55 ± 12 | RCT |
| Grat et al., 2017 [39] | Poland | 44/55 | 21/23 | Probiotics (Lactococcus lactis, Lactobacillus casei, Lactobacillus acidophilus and Bifidobacterium bifidum) | 50% 25% 12.5% 12.5% 3 × 109 CFU; capsules | Placebo | Starting at the time of wait-listing for LT until LT Treatment duration for <2 weeks, 2–10 weeks, and >10 weeks in 16.7% (4/24), 37.5% (9/24), and 45.8% (11/24) in the probiotic group and 15.4% (4/26), 53.8% (14/26), and 30.8% (8/26) in the placebo group | 30-day infection rate 0.09 (95%CI 0.01–0.83) 1/21, 8/23 90-day infection rate 0.06 (95%CI 0.01–0.48) 1/21, 11/23 FU POD 90 | 50.95 ± NR; 52 (47 - 58)/50 (35 - 61) | RCT |
| Liver resection (LR) | n = 743/849 | |||||||||
| Kanazawa et al., 2005 [44] | Japan | 44/54 | 21/23 | Bifidobacterium breve strain Yakult, Lactobacillus casei strain Shirota; prebiotic: galactooligo saccharides | 1 × 108/g 1 × 108/g 3 g/day prebiotic: 12 g/day | No intervention, enteric nutrition | Just after LT until 14 days post LT (13 days) | 4/21, 12/23 FU POD 30 | 63.75 ± 9.64; 62.5 ± 9.9/64.9 ± 9.4 | RCT |
| Liu et al., 2015 [45] | China | 134/150 | 66/68 | Lactobacillus plantarum, Lactobacillus acidophilus, Bifidobacterium longum | 2.6 × 1014 CFU 2 g/day | Placebo | 6 days prior to LR until 10 days post LR (16 days) | 39/66, 60/68 FU POD 10 | 62.84 ± 17.17; 65.62 ± 18.18/60.16 ± 16.20 | RCT |
| Rayes et al., 2012 [47] | Germany | 19/33 | 9/10 | Pediacoccus pentosaceus, Leuconostoc mesenteroides Lactobacillus paracasei subspecies paracasei, Lactobacillus plantarum; prebiotic: bioactive fibers: betaglucan, inulin, pectin, and resistant starch | probiotic: 1010 CFU, prebiotic: 10 g 2×/day; powder | Placebo/fibers | 1 day prior to LR until 10 days post LR (11 days) | 3/9, 2/10 FU POD 14 | 60.05 ± 13.89; 61 ± 16/59 ± 11 | RCT |
| Sugawara et al., 2006 [49] | Japan | 81/101 | 41/40 | Lactobacillus casei strain Shirota, Bifidobacterium breve strain Yakult; prebiotic: galactooligosaccharides | Pre-LR: 4 × 1010, 80 mL 1 × 1010, 100 mL prebiotic: 15 g 1×/day Post-LR: 1 × 108/g 1 × 108/g 3 g/day prebiotic: 10 g 1×/day | No intervention, syn biotics administered only post LR | 14 days prior to LR and 14 days post LR (28 days) | 5/41, 12/40 FU POD 30 | 63.15 ± 8.84; 63.1 ± 7.9/ 63.2 ± 9.8 | RCT |
| Usami et al., 2011 [48] | Japan | 61/67 | 32/29 | Lactobacillus casei strain Shirota, Bifidobacterium breve strain Yakult; prebiotic: galactooligosaccharides | 1 × 108/g 1 × 108/g 3 g/day prebiotic: 10 g 1×/day | No intervention | 14 days prior to LR and 11 days post LR (25 days) | 0/32, 5/29 FU POD 30 | 65.42 ± 9.86; 62.1 ± 10.2/ 69.1 ± 8.0 | RCT |
| Rifatbegovic et al., 2010 [46] | Bosnia | 120/120 | 60/60 | Lactobacillus plantarum 2362, L. paracasei subsp paracasei 19, Pediacoccus pentoseceus 5-33:3 and 32-77:1, L. raffinolactis and fibers | NR | No intervention | 3 days prior to LR until 7 days post LR (10 days) | NR FU POD 14 | NR | Prospective non- randomized, NOS: 7 |
| Iida et al., 2020 [50] | Japan | 284/324 | 115/169 | Clostridium butyricum and fibers | 6 g/day 12 g/day | No intervention | 30 days prior to LR until 1 days prior to LR (30 days) | 19/115, 40/169 FU POD 3 | 67.2 ± NR; 68.2 ± 11.6/ 66.2 ± 12.6 | Prospective non- randomized, NOS: 8 |
| Study (Ref.) | Results Pro-Synbiotic/Control Perioperative Liver Function | Assessment of Liver Function | Study Design |
|---|---|---|---|
| Liver Transplantation (LT) | |||
| Rayes et al., 2002 [40] | No difference AST, ALT, GGT, AP | FU POD 13 | RCT |
| Rayes et al., 2005 [41] | No difference AST, ALT, GGT, AP | FU POD 30 | RCT |
| Grat et al., 2017 [39] | -ALT (IU/l) 398.8 ± 307.68/441.05 ± 432.72 -AST (IU/l) 140.4 ± 123.59/105.6 ± 62.92 -Bili (mg/dl) 2.5 ± 1.91/2.9 ± 2.59 -INR 1.05 ± 0.10/1.16 ± 0.18 | FU POD 5 | RCT |
| Liver Resection (LR) | |||
| Liu et al., 2015 [45] | -ALT (U/l) 32.62 ± 18.86/35.68 ± 15.26 -AST (U/l) 28.22 ± 18.86/29.68 ± 16.56 -ALT (U/l) 36.28 ± 18.92/56.20 ± 18.16 -AST (U/l) 36.18 ± 21.52/45.62 ± 22.68 | Prior to LR FU POD 10 | RCT |
| Rayes et al., 2012 [47] | 13C methacetin test (LiMAx (%)) 160 ± 45/135 ± 60 13C methacetin test (LiMAx (%)) 260 ± 85/240 ± 80 -AST (U/l) 110/90 -ALT (U/l) 210/150 -GLDH (U/l) 70/35 -AST (U/l) 80/60 -ALT (U/l) 100/95 -GLDH (U/l) 30/25 -AST (U/l) 65/50 -ALT (U/l) 100/70 -GLDH (U/l) 2 | FU POD 5 FU POD 14 FU POD 5 FU POD 10 FU POD 14 | RCT |
| Usami et al., 2011 [48] | No difference AST, ALT, bilirubin | FU POD 15 | RCT |
| Rifatbegovic et al., 2010 [46] | -ALT (U/l) 50 ± 5/68 ± 7 -Bili (mcmol/l) 17 ± 0.8/31.3 ± 1.5 -Indocyaningreen test 1.15/1.425 | FU POD 14 | Prospective non-randomized, NOS: 7 |
| Iida et al., 2020 [50] | PostLR liver failure (grade) -A: 8/115, 13/169 -B: 1/115, 16/169 -C: 1/115, 4/169 -ALT (IU/l) prior to synbiotics–after synbiotics (n = 115): 23 (16, 31)–20 (13, 34) -AST (IU/l) prior to synbiotics–after synbiotics (n = 115): 27 (22, 43)–26 (20, 34) -Bilirubin (mg/dl) prior to synbiotics–after synbiotics (n = 115): 0.7 (0.5, 0.8)–0.6 (0.4, 0.8) Prothrombin activity (%) prior to synbiotics–after synbiotics (n = 115): 96 (87, 109)–96 (85, 103) | FU POD 3 | Prospective non-randomized, NOS: 8 |
| Study (Ref.) | Gut Microbiota Changes |
|---|---|
| Liver Transplantation (LT) | |
| Eguchi et al., 2011 [42] | No significant changes between the groups Enterococcus sp. evident in both groups in 25% of the immunosuppressed patients |
| Grat et al., 2017 [39] | Probiotic group: Bacteroides sp. (p = 0.008), Enterococcus sp. (p = 0.04) significantly increased in comparison to pre-trial values as compared to control group |
| Liver Resection (LR) | |
| Kanazawa et al., 2005 [44] | Synbiotic group: Lactobacillus and Bifidobacterium increased postoperatively in comparison to controls (p < 0.05) Control group: Enterobacteriaceae, Pseudomonas, Candida increased in comparison to synbiotic group (p < 0.05) Enterococci increased postoperatively in both groups |
| Sugawara et al., 2006 [49] | Pre-and post-operative probiotic group: Bifidobacterium significantly increased after preoperative treatment (p < 0.05) Anaerobic bacteria numbers were unchanged before and after surgery between the two groups |
| Usami et al., 2011 [48] | Bacteroidaceae, Bifidobacterium decreased, Candida increased 1 week postoperatively, resembled preoperative values after 2 weeks No differences concerning liver function |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahn, J.; Pregartner, G.; Schemmer, P. Effects of both Pro- and Synbiotics in Liver Surgery and Transplantation with Special Focus on the Gut–Liver Axis—A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2461. https://doi.org/10.3390/nu12082461
Kahn J, Pregartner G, Schemmer P. Effects of both Pro- and Synbiotics in Liver Surgery and Transplantation with Special Focus on the Gut–Liver Axis—A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(8):2461. https://doi.org/10.3390/nu12082461
Chicago/Turabian StyleKahn, Judith, Gudrun Pregartner, and Peter Schemmer. 2020. "Effects of both Pro- and Synbiotics in Liver Surgery and Transplantation with Special Focus on the Gut–Liver Axis—A Systematic Review and Meta-Analysis" Nutrients 12, no. 8: 2461. https://doi.org/10.3390/nu12082461
APA StyleKahn, J., Pregartner, G., & Schemmer, P. (2020). Effects of both Pro- and Synbiotics in Liver Surgery and Transplantation with Special Focus on the Gut–Liver Axis—A Systematic Review and Meta-Analysis. Nutrients, 12(8), 2461. https://doi.org/10.3390/nu12082461






