Association of Serum Calcium and Phosphate Concentrations with Glucose Metabolism Markers: The Furukawa Nutrition and Health Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. Assessment of Dietary Intakes
2.3. Blood Measurements
2.4. Assessment of Other Health-Related Parameters
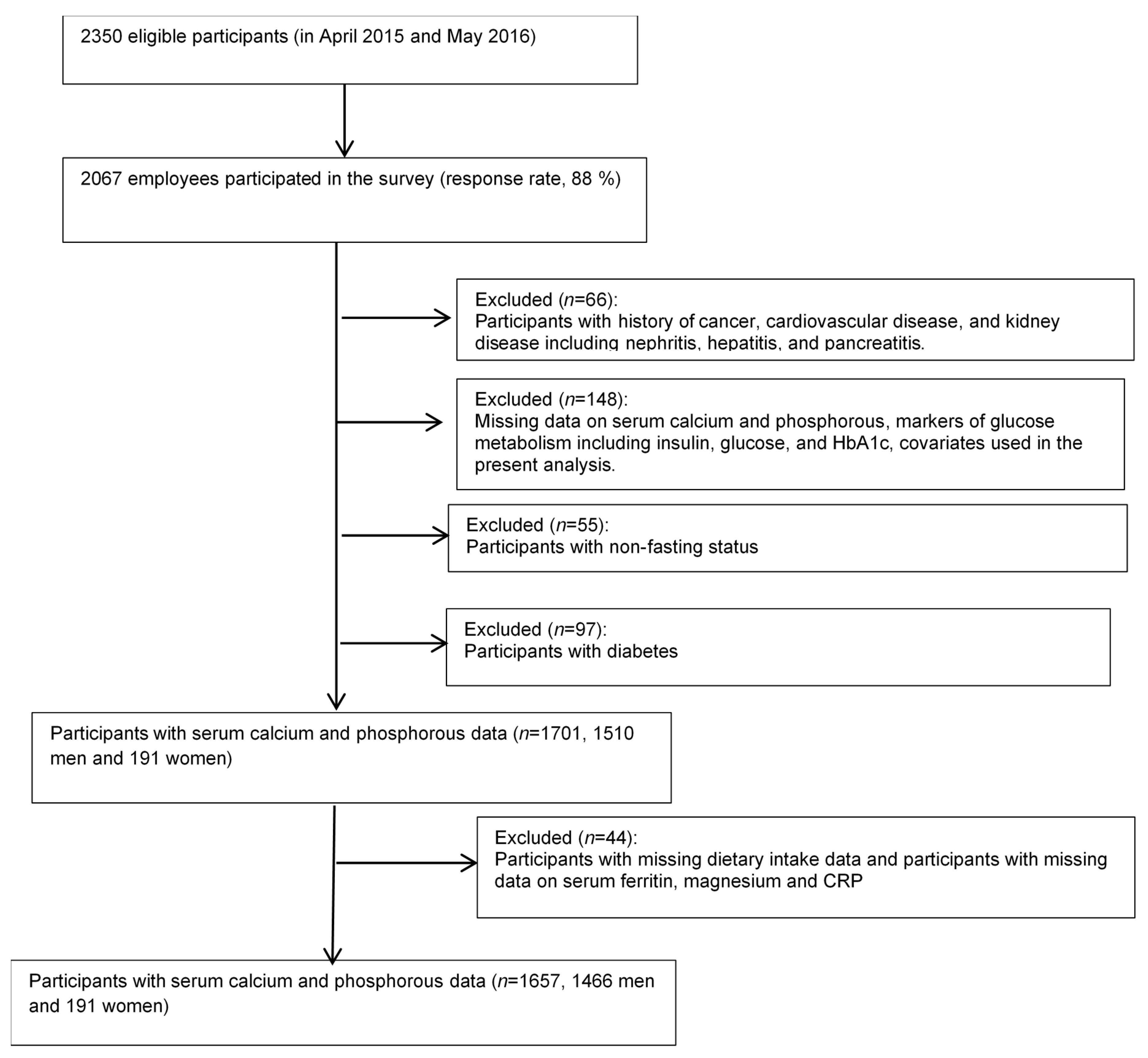
2.5. Participants
2.6. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pr. 2019, 157, 107843. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed. 2019. Available online: https://diabetesatlas.org/en/# (accessed on 5 August 2020).
- Wareham, N.J.; Byrne, C.D.; Carr, C.; Day, N.E.; Boucher, B.J.; Hales, C. Glucose intolerance is associated with altered calcium homeostasis: A possible link between increased serum calcium concentration and cardiovascular disease mortality. Metabolism 1997, 46, 1171–1177. [Google Scholar] [CrossRef]
- Jorde, R.; Schirmer, H.; Njølstad, I.; Løchen, M.-L.; Mathiesen, E.B.; Kamycheva, E.; Figenschau, Y.; Grimnes, G. Serum calcium and the calcium-sensing receptor polymorphism rs17251221 in relation to coronary heart disease, type 2 diabetes, cancer and mortality: The Tromsø Study. Eur. J. Epidemiol. 2013, 28, 569–578. [Google Scholar] [CrossRef]
- Lorenzo, C.; Hanley, A.J.; Rewers, M.J.; Haffner, S.M. Calcium and phosphate concentrations and future development of type 2 diabetes: The Insulin Resistance Atherosclerosis Study. Diabetologia 2014, 57, 1366–1374. [Google Scholar] [CrossRef]
- Rooney, M.R.; Pankow, J.S.; Sibley, S.D.; Selvin, E.; Reis, J.P.; Michos, E.D.; Lutsey, P.L. Serum calcium and incident type 2 diabetes: The Atherosclerosis Risk in Communities (ARIC) study. Am. J. Clin. Nutr. 2016, 104, 1023–1029. [Google Scholar] [CrossRef]
- Suh, S.; Bae, J.C.; Jin, S.-M.; Jee, J.H.; Park, M.K.; Kim, D.K.; Kim, J.H. Serum calcium changes and risk of type 2 diabetes mellitus in Asian population. Diabetes Res. Clin. Pr. 2017, 133, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hawley, C. Calcium × phosphate product. Nephrology 2006, 11, S206–S208. [Google Scholar] [CrossRef]
- Fraser, A.; Williams, D.M.; Lawlor, D.A. Associations of Serum 25-Hydroxyvitamin D, Parathyroid Hormone and Calcium with Cardiovascular Risk Factors: Analysis of 3 NHANES Cycles (2001–2006). PLoS ONE 2010, 5, e13882. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Vasdev, S.; Martin, G.; Gadag, V.; Zhang, H. Altered calcium homeostasis is correlated with abnormalities of fasting serum glucose, insulin resistance, and beta-cell function in the Newfoundland population. Diabetes 2005, 54, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
- Shridhar, K.; Kinra, S.; Gupta, R.; Khandelwal, S.D.P.; Cox, S.E.; Dhillon, P.K. Serum Calcium Concentrations, Chronic Inflammation and Glucose Metabolism: A Cross-Sectional Analysis in the Andhra Pradesh Children and Parents Study (APCaPS). Curr. Dev. Nutr. 2018, 3, nzy085. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Collins, A.J.; Ishani, A.; Kalra, P.A. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, M.; Okaniwa, S.; Nakayama, T. Reduced Serum Phosphorus Levels Were Associated with Metabolic Syndrome in Men But Not in Women: A Cross-Sectional Study among the Japanese Population. Ann. Nutr. Metab. 2017, 71, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Haap, M.; Heller, E.; Thamer, C.; Tschritter, O.; Stefan, N.; Fritsche, A. Association of serum phosphate levels with glucose tolerance, insulin sensitivity and insulin secretion in non-diabetic subjects. Eur. J. Clin. Nutr. 2006, 60, 734–739. [Google Scholar] [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Fukushima, M.; Suzuki, H.; Seino, Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res. Clin. Pr. 2004, 66, S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Eguchi, M.; Nanri, A.; Kochi, T.; Kashino, I.; Kuwahara, K.; Hu, H.; Miki, T.; Kabe, I.; Mizoue, T. Association of dietary and serum magnesium with glucose metabolism markers: The Furukawa Nutrition and Health Study. Clin. Nutr. ESPEN 2018, 24, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Science and Technology Agency. Standard Tables of Food Composition in Japan, 5th ed.; Printing Bureau of the Ministry of Finance: Tokyo, Japan, 2005.
- Leary, N.O.; Pembroke, J.T.; Duggan, P.F. Single Stable Reagent (Arsenazo III) for Optically Robust Measurement of Calcium in Serum and Plasma. Clin. Chem. 1992, 38, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.A.; Bodourian, S.H.; Nicholson, J.F. Enzymatic Method for Determination of Inorganic Phosphate in Serum and Urine with a Centrifugal Analyzer. Clin. Chem. 1974, 20, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Prinyawiwatkul, W.; Xu, Z. Insulin: A review of analytical methods. Analyst 2019, 144, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.M.; Nanri, A.; Yi, S.; Kurotani, K.; Akter, S.; Foo, L.H.; Nishi, N.; Sato, M.; Hayabuchi, H.; Mizoue, T. Serum ferritin is associated with markers of insulin resistance in Japanese men but not in women. Metabolism 2013, 62, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Shiraishi, T.; Wada, M. Association between C-reactive Protein and Insulin Resistance in a Japanese Population: The Minoh Study. Intern. Med. 2005, 44, 542–547. [Google Scholar] [CrossRef]
- Dos Santos, L.C.; Cintra, I.D.P.; Fisberg, M.; Martini, L.A. Calcium intake and its relationship with adiposity and insulin resistance in post-pubertal adolescents. J. Hum. Nutr. Diet 2008, 21, 109–116. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; Lima, A.G.A.; Braz, A.F.; Melo, S.R.D.S.; Morais, J.B.S.; Severo, J.S.; De Oliveira, A.R.S.; Cruz, K.J.C.; Cruz, K.J.C. Role of vitamin D in insulin resistance in obese individuals. Nutrire 2017, 42, 17. [Google Scholar] [CrossRef]
- Hagstrom, E.; Hellman, P.; Lundgren, E.; Lind, L.; Arnlov, J. Serum calcium is independently associated with insulin sensitivity measured with euglycaemic–hyperinsulinaemic clamp in a community-based cohort. Diabetologia 2006, 50, 317–324. [Google Scholar] [CrossRef]
- Ahren, B. Insulin Secretion and Insulin Sensitivity in Relation to Fasting Glucose in Healthy Subjects. Diabetes Care 2007, 30, 644–648. [Google Scholar] [CrossRef]
- Begum, N.; Leitner, W.; Reusch, J.E.; Sussman, K.E.; Draznin, B. GLUT-4 phosphorylation and its intrinsic activity. Mechanism of Ca(2+)-induced inhibition of insulin-stimulated glucose transport. J. Biol. Chem. 1993, 268, 3352–3356. [Google Scholar]
- Byyny, R.L.; Verde, M.L.; Mitchell, W.; Draznin, B.; Lloyd, S. Cytosolic Calcium and Insulin Resistance in Elderly Patients With Essential Hypertension. Am. J. Hypertens 1992, 5, 459–464. [Google Scholar] [CrossRef]
- Arruda, A.P.; Hotamisligil, G.S. Calcium Homeostasis and Organelle Function in the Pathogenesis of Obesity and Diabetes. Cell Metab. 2015, 22, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.H.; Tsirigotis, D.N.; Befroy, D.E.; Caballero, D.; Jurczak, M.J.; Rahimi, Y.; Cline, G.W.; Dufour, S.; Birkenfeld, A.L.; Rothman, D.L.; et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J. 2016, 30, 3378–3387. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, S.; Neufurth, M.; Kokkinopoulou, M.; Feng, Q.; Schröder, H.C.; Wang, X. Polyphosphate as a donor of high-energy phosphate for the synthesis of ADP and ATP. J. Cell Sci. 2017, 130, 2747–2756. [Google Scholar] [CrossRef]
- Bódis, K.; Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur. J. Clin. Investig. 2018, 48, e13017. [Google Scholar] [CrossRef]
- Wollheim, C.B.; Sharp, G.W. Regulation of insulin release by calcium. Physiol. Rev. 1981, 61, 914–973. [Google Scholar] [CrossRef]
- Squires, P. Non-Ca2+-homeostatic functions of the extracellular Ca2+-sensing receptor (CaR) in endocrine tissues. J. Endocrinol. 2000, 165, 173–177. [Google Scholar] [CrossRef][Green Version]
- Gray, E.; Muller, D.; Squires, P.E.; Asare-Anane, H.; Huang, G.-C.; Amiel, S.; Persaud, S.J.; Jones, P.M. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: Involvement of protein kinases. J. Endocrinol. 2006, 190, 703–710. [Google Scholar] [CrossRef]
- Jones, P.M.; Kitsou-Mylona, I.; Gray, E.; Squires, P.E.; Persaud, S.J. Expression and function of the extracellular calcium-sensing receptor in pancreatic beta-cells. Arch. Physiol. Biochem. 2007, 113, 98–103. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Besarab, A.; Caro, J.F. Increased absolute calcium binding to albumin in hypoalbuminaemia. J. Clin. Pathol. 1981, 34, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
| Quartiles of Serum Calcium (mg/dL) | Quartiles of Serum Phosphate (mg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 (High) | Trend p a | Q1 (Low) | Q2 | Q3 | Q4 (High) | Trend p a | |
| Number of participants | 574 | 462 | 371 | 324 | 443 | 423 | 452 | 413 | ||
| Median | 9.3 | 9.6 | 9.7 | 10.0 | 2.6 | 3.1 | 3.7 | 4.7 | ||
| Range | 8.6–9.4 | 9.5–9.6 | 9.7–9.8 | 9.9–11.1 | 1.4–2.8 | 2.9–3.3 | 3.4–4.1 | 4.2–7.3 | ||
| Age (years) | 45.7 ± 8.3 | 44.7 ± 8.5 | 44.8 ± 8.8 | 43.2 ± 10.4 | <0.01 | 47.1 ± 9.2 | 44.9 ± 9.4 | 43.8 ± 8.6 | 43.2 ± 7.5 | <0.01 |
| Sex (men, %) | 84.5 | 89.6 | 89.5 | 95.1 | <0.01 | 91.2 | 84.6 | 90.9 | 88.6 | 0.88 |
| Leisure-time physical activity (METs-h/week) | 8.4 ± 15.9 | 8.9 ± 18.2 | 9.3 ± 19.4 | 9.3 ± 19.0 | 0.44 | 7.7 ± 13.6 | 9.1 ± 17.6 | 10.6 ± 22.8 | 8.1 ± 16.0 | 0.30 |
| Work-related physical activity (METs-h/day) | 15.9 ± 18.0 | 14.8 ± 16.8 | 15.5 ± 17.9 | 13.8 ± 15.3 | 0.10 | 15.3 ± 16.9 | 15.4 ± 18.4 | 15.0 ± 17.4 | 15.0 ± 16.0 | 0.67 |
| BMI (kg/m2) | 23.6 ± 3.4 | 23.6 ± 3.4 | 23.8 ± 3.6 | 23.6 ± 3.6 | 0.92 | 24.0 ± 3.8 | 23.4 ± 3.4 | 23.5 ± 3.3 | 23.6 ± 3.5 | 0.28 |
| Current smoker (%) | 31.7 | 29.4 | 28.6 | 27.2 | 0.13 | 27.3 | 26.5 | 32.7 | 31.7 | 0.06 |
| Current alcohol drinker (≥1 day/week, %) | 49.8 | 56.1 | 56.3 | 55.6 | 0.05 | 54.2 | 52.7 | 52.0 | 57.1 | 0.34 |
| Night and rotating shift work (yes, %) | 18.3 | 18.2 | 19.9 | 16.7 | 0.71 | 17.2 | 14.9 | 19.9 | 21.3 | 0.03 |
| CRP (mg/dL) | 0.09 ± 0.26 | 0.09 ± 0.29 | 0.07 ± 0.18 | 0.08 ± 0.15 | 0.25 | 0.09 ± 0.27 | 0.07 ± 0.20 | 0.08 ± 0.22 | 0.09 ± 0.24 | 0.60 |
| Serum ferritin (ng/mL) | 145 ± 114 | 165 ± 122 | 169 ± 113 | 204 ± 174 | <0.01 | 180 ± 163 | 155 ± 104 | 163 ± 119 | 169 ± 131 | 0.67 |
| Serum magnesium (mg/dL) | 2.21 ± 0.14 | 2.20 ± 0.14 | 2.23 ± 0.14 | 2.22 ± 0.14 | 0.20 | 2.19 ± 0.14 | 2.21 ± 0.14 | 2.23 ± 0.14 | 2.24 ± 0.14 | <0.01 |
| Calcium intake (mg/1000 kcal/day) | 235 ± 95 | 239 ± 91 | 239 ± 90 | 236 ± 89 | 0.70 | 238 ± 92 | 243 ± 97 | 236 ± 87 | 232 ± 90 | 0.19 |
| Phosphorous intake (mg/1000 kcal/day) | 507 ± 102 | 516 ± 110 | 519 ± 102 | 512 ± 102 | 0.26 | 514 ± 98 | 519 ± 110 | 514 ± 100 | 505 ± 106 | 0.08 |
| Vitamin D intake (mg/1000 kcal/day) | 5.7 ± 3.2 | 6.2 ± 3.8 | 6.3 ± 3.9 | 6.1 ± 3.4 | 0.04 | 6.2 ± 3.5 | 6.3 ± 3.8 | 6.0 ± 3.4 | 5.8 ± 3.6 | 0.09 |
| Quartiles of Serum Calcium (mg/dL) | Trend p a | ||||
|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 (High) | ||
| Number of subjects | 574 | 462 | 371 | 324 | |
| Median (range) | 9.3 (8.6–9.4) | 9.6 (9.5–9.6) | 9.7 (9.7–9.8) | 10.0 (9.9–11.1) | |
| Fasting insulin (μU/mL) | |||||
| Model 1 b | 3.62 (3.45–3.80) | 3.80 (3.60–4.01) | 4.06 (3.83–4.31) | 4.41 (4.14–4.70) | <0.001 |
| Model 2 c | 3.59 (3.44–3.74) | 3.84 (3.67–4.01) | 4.01 (3.81–4.22) | 4.48 (4.24–4.73) | <0.001 |
| Model 3 d | 3.58 (3.44–3.72) | 3.90 (3.74–4.08) | 4.04 (3.85–4.24) | 4.50 (4.27–4.74) | <0.001 |
| Fasting glucose (mg/dL) | |||||
| Model 1 b | 89.3 (88.5–90.1) | 90.1 (89.2–90.9) | 90.2 (89.3–91.2) | 92.1 (91.0–93.1) | <0.001 |
| Model 2 c | 89.3 (88.6–90.1) | 90.1 (89.3–90.9) | 90.1 (89.2–91.0) | 92.2 (91.2–93.2) | <0.001 |
| Model 3 d | 88.6 (88.0–89.3) | 89.4 (88.7–90.1) | 89.8 (89.1–90.6) | 91.4 (90.5–92.3) | <0.001 |
| HOMA-IR | |||||
| Model 1 b | 0.80 (0.76–0.84) | 0.84 (0.80–0.89) | 0.90 (0.85–0.96) | 1.00 (0.94–1.07) | <0.001 |
| Model 2 c | 0.79 (0.76–0.83) | 0.85 (0.81–0.89) | 0.89 (0.85–0.94) | 1.02 (0.96–1.08) | <0.001 |
| Model 3 d | 0.78 (0.75–0.82) | 0.86 (0.82–0.90) | 0.90 (0.85–0.94) | 1.01 (0.96–1.07) | <0.01 |
| HOMA-β | |||||
| Model 1 b | 51.7 (49.4–54.2) | 52.9 (50.2–55.7) | 55.2 (52.1–58.6) | 57.2 (53.7–60.9) | 0.01 |
| Model 2 c | 51.2 (49.1–53.5) | 53.3 (50.9–55.9) | 54.9 (52.1–57.9) | 57.9 (54.7–61.2) | 0.001 |
| Model 3 d | 52.1 (50.1–54.3) | 55.1 (52.6–57.6) | 56.2 (53.4–59.1) | 59.4 (56.3–62.7) | <0.001 |
| HbA1c (%) | |||||
| Model 1 b | 5.25 (5.23–5.27) | 5.29 (5.27–5.32) | 5.30 (5.28–5.33) | 5.28 (5.25–5.31) | 0.057 |
| Model 2 c | 5.25 (5.22–5.27) | 5.30 (5.27–5.32) | 5.30 (5.27–5.33) | 5.28 (5.25–5.31) | 0.02 |
| Model 3 d | 5.24 (5.22–5.27) | 5.30 (5.27–5.32) | 5.30 (5.28–5.33) | 5.29 (5.26–5.32) | 0.01 |
| Quartiles of Serum Phosphate (mg/dL) | Trend p a | ||||
|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 (High) | ||
| Number of subjects | 443 | 423 | 452 | 413 | |
| Median (range) | 2.6 (1.4–2.8) | 3.1 (2.9–3.3) | 3.7 (3.4–4.1) | 4.7 (4.2–7.3) | |
| Fasting insulin (μU/mL) | |||||
| Model 1 b | 4.67 (4.42–4.93) | 3.97 (3.76–4.20) | 3.70 (3.50–3.90) | 3.35 (3.17–3.54) | <0.001 |
| Model 2 c | 4.52 (4.32–4.74) | 4.03 (3.85–4.23) | 3.73 (3.57–3.91) | 3.37 (3.21–3.54) | <0.001 |
| Model 3 d | 4.59 (4.39–4.80) | 4.14 (3.97–4.32) | 3.66 (3.49–3.83) | 3.35 (3.20–3.51) | <0.001 |
| Fasting glucose (mg/dL) | |||||
| Model 1 b | 93.1 (92.2–94.0) | 90.3 (89.4–91.2) | 89.7 (88.9–90.6) | 87.7 (86.8–88.6) | <0.001 |
| Model 2 c | 92.9 (92.0–93.8) | 90.5 (89.7–91.4) | 89.8 (89.0–90.7) | 87.5 (86.7–88.4) | <0.001 |
| Model 3 d | 91.8 (91.1–92.6) | 89.5 (88.9–92.6) | 89.4 (88.7–90.2) | 87.6 (86.9–88.3) | <0.001 |
| HOMA-IR | |||||
| Model 1 b | 1.07 (1.01–1.14) | 0.88 (0.83–0.94) | 0.82 (0.77–0.87) | 0.72 (0.68–0.77) | <0.001 |
| Model 2 c | 1.03 (0.99–1.09) | 0.90 (0.86–0.95) | 0.83 (0.79–0.87) | 0.73 (0.69–0.77) | <0.001 |
| Model 3 d | 1.04 (0.99–1.09) | 0.92 (0.88–0.96) | 0.81 (0.77–0.85) | 0.72 (0.69–0.76) | <0.001 |
| HOMA-β | |||||
| Model 1 b | 54.0 (52.6–55.5) | 53.6 (52.2–55.1) | 53.6 (52.2–55.0) | 53.8 (52.4–55.3) | <0.001 |
| Model 2 c | 57.2 (54.5–60.1) | 55.4 (52.7–58.2) | 51.7 (49.3–54.3) | 50.8 (48.3–53.5) | <0.001 |
| Model 3 d | 59.5 (56.8–62.4) | 58.2 (55.7–60.9) | 51.5 (49.0–54.0) | 50.9 (48.5–53.4) | <0.001 |
| HbA1c (%) | |||||
| Model 1 b | 5.29 (5.26–5.31) | 5.28 (5.25-5.30) | 5.31 (5.29–5.34) | 5.25 (5.22–5.27) | 0.07 |
| Model 2 c | 5.28 (5.25–5.30) | 5.28 (5.26–5.31) | 5.31 (5.28–5.33) | 5.25 (5.22–5.27) | 0.11 |
| Model 3 d | 5.28 (5.25–5.30) | 5.29 (5.26–5.31) | 5.30 (5.28–5.33) | 5.25 (5.22–5.27) | 0.08 |
| Quartiles of Serum-Calcium-and-Phosphate Product | Trend p a | ||||
|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 (High) | ||
| Number of subjects | 432 | 424 | 422 | 423 | |
| Median (range) | 24.4 (13.7–27.3) | 29.7 (27.3–32.2) | 35.4 (32.3–39.6) | 45.1 (39.6–73.7) | |
| Fasting insulin (μU/mL) | |||||
| Model 1 b | 4.52 (4.28–4.77) | 3.93 (3.72–4.15) | 3.62 (3.43–3.82) | 3.45 (3.27–3.65) | <0.001 |
| Model 2 c | 4.42 (4.22–4.63) | 4.01 (3.82–4.20) | 3.67 (3.50–3.85) | 3.41 (3.26–3.58) | <0.001 |
| Model 3 d | 4.61 (4.40–4.82) | 4.09 (3.91–4.28) | 3.71 (3.55–3.88) | 3.38 (3.23–3.54) | <0.001 |
| Fasting glucose (mg/dL) | |||||
| Model 1 b | 91.4 (90.6–92.1) | 89.5 (88.7–90.2) | 89.5 (88.8–90.3) | 87.8 (87.1–88.6) | <0.001 |
| Model 2 c | 91.3 (90.5–92.0) | 89.7 (88.9–90.4) | 89.7 (88.9–90.4) | 87.6 (86.9–88.4) | <0.001 |
| Model 3 d | 91.6 (90.9–92.4) | 89.7 (88.9–90.4) | 89.6 (88.9–90.4) | 87.5 (86.8–88.2) | <0.001 |
| HOMA-IR | |||||
| Model 1 b | 1.02 (0.96–1.08) | 0.87 (0.82–0.92) | 0.80 (0.75–0.85) | 0.75 (0.71–0.79) | <0.001 |
| Model 2 c | 1.00 (0.95–1.05) | 0.89 (0.84–0.93) | 0.81 (0.77–0.85) | 0.74 (0.70–0.78) | <0.001 |
| Model 3 d | 1.04 (0.99–1.09) | 0.91 (0.86–0.95) | 0.82 (0.78–0.86) | 0.73 (0.69–0.77) | <0.001 |
| HOMA-β | |||||
| Model 1 b | 59.4 (56.3–62.7) | 55.4 (52.5–58.5) | 50.7 (48.1–53.5) | 52.1 (49.3–54.9) | <0.001 |
| Model 2 c | 58.4 (55.6–61.2) | 56.2 (53.5–58.9) | 51.2 (48.8–53.7) | 51.8 (49.4–54.4) | <0.001 |
| Model 3 d | 59.6 (56.8–62.5) | 57.4 (54.8–60.2) | 51.8 (49.4–54.2) | 52.1 (49.7–54.6) | <0.001 |
| HbA1c (%) | |||||
| Model 1 b | 5.29 (5.26–5.31) | 5.27 (5.24–5.30) | 5.31 (5.28–5.33) | 5.26 (5.23–5.28) | 0.38 |
| Model 2 c | 5.27 (5.25–5.30) | 5.28 (5.25–5.30) | 5.31 (5.29–5.34) | 5.26 (5.23–5.28) | 0.54 |
| Model 3 d | 5.27 (5.24–5.30) | 5.28 (5.26–5.31) | 5.31 (5.28–5.33) | 5.25 (5.22–5.28) | 0.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, S.; Eguchi, M.; Kochi, T.; Kabe, I.; Nanri, A.; Mizoue, T. Association of Serum Calcium and Phosphate Concentrations with Glucose Metabolism Markers: The Furukawa Nutrition and Health Study. Nutrients 2020, 12, 2344. https://doi.org/10.3390/nu12082344
Akter S, Eguchi M, Kochi T, Kabe I, Nanri A, Mizoue T. Association of Serum Calcium and Phosphate Concentrations with Glucose Metabolism Markers: The Furukawa Nutrition and Health Study. Nutrients. 2020; 12(8):2344. https://doi.org/10.3390/nu12082344
Chicago/Turabian StyleAkter, Shamima, Masafumi Eguchi, Takeshi Kochi, Isamu Kabe, Akiko Nanri, and Tetsuya Mizoue. 2020. "Association of Serum Calcium and Phosphate Concentrations with Glucose Metabolism Markers: The Furukawa Nutrition and Health Study" Nutrients 12, no. 8: 2344. https://doi.org/10.3390/nu12082344
APA StyleAkter, S., Eguchi, M., Kochi, T., Kabe, I., Nanri, A., & Mizoue, T. (2020). Association of Serum Calcium and Phosphate Concentrations with Glucose Metabolism Markers: The Furukawa Nutrition and Health Study. Nutrients, 12(8), 2344. https://doi.org/10.3390/nu12082344




