Lowering Low-Density Lipoprotein Cholesterol Concentration with Plant Stanol Esters to Reduce the Risk of Atherosclerotic Cardiovascular Disease Events at a Population Level: A Critical Discussion
Abstract
1. Introduction
2. Relationship between LDL-C Concentration and ASCVD
2.1. Quantification of Outcomes
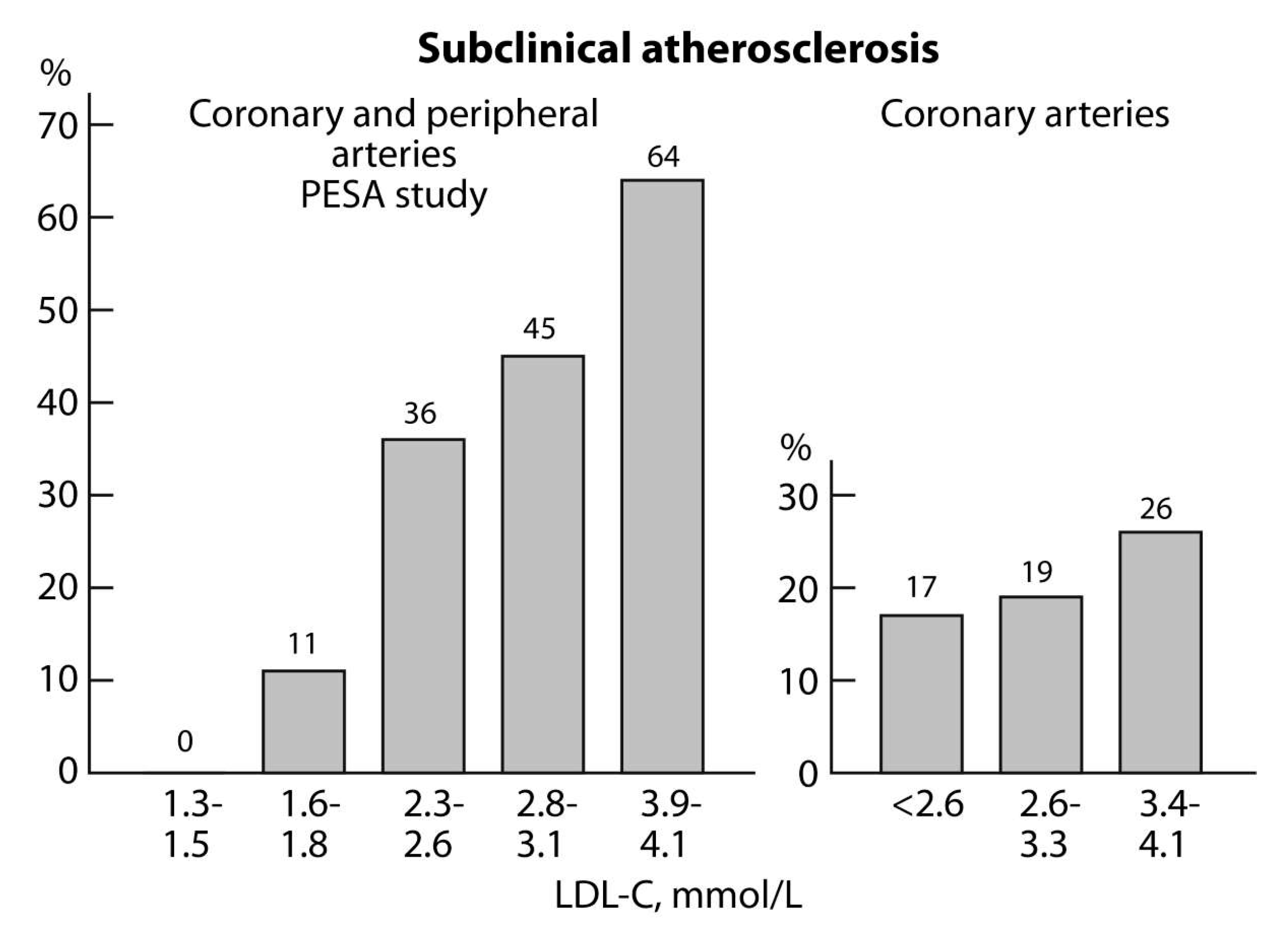
2.2. Subclinical Atherosclerosis
3. Dietary Means to Lower LDL-C Concentrations
3.1. Dietary Saturated Fat and Cholesterol
3.2. Egg Consumption, Serum Cholesterol, and ASCVDs
3.3. Plant Stanol Esters as a Dietary Means to Lower LDL-C Concentrations
3.4. Plant Stanol Esters and Egg Consumption
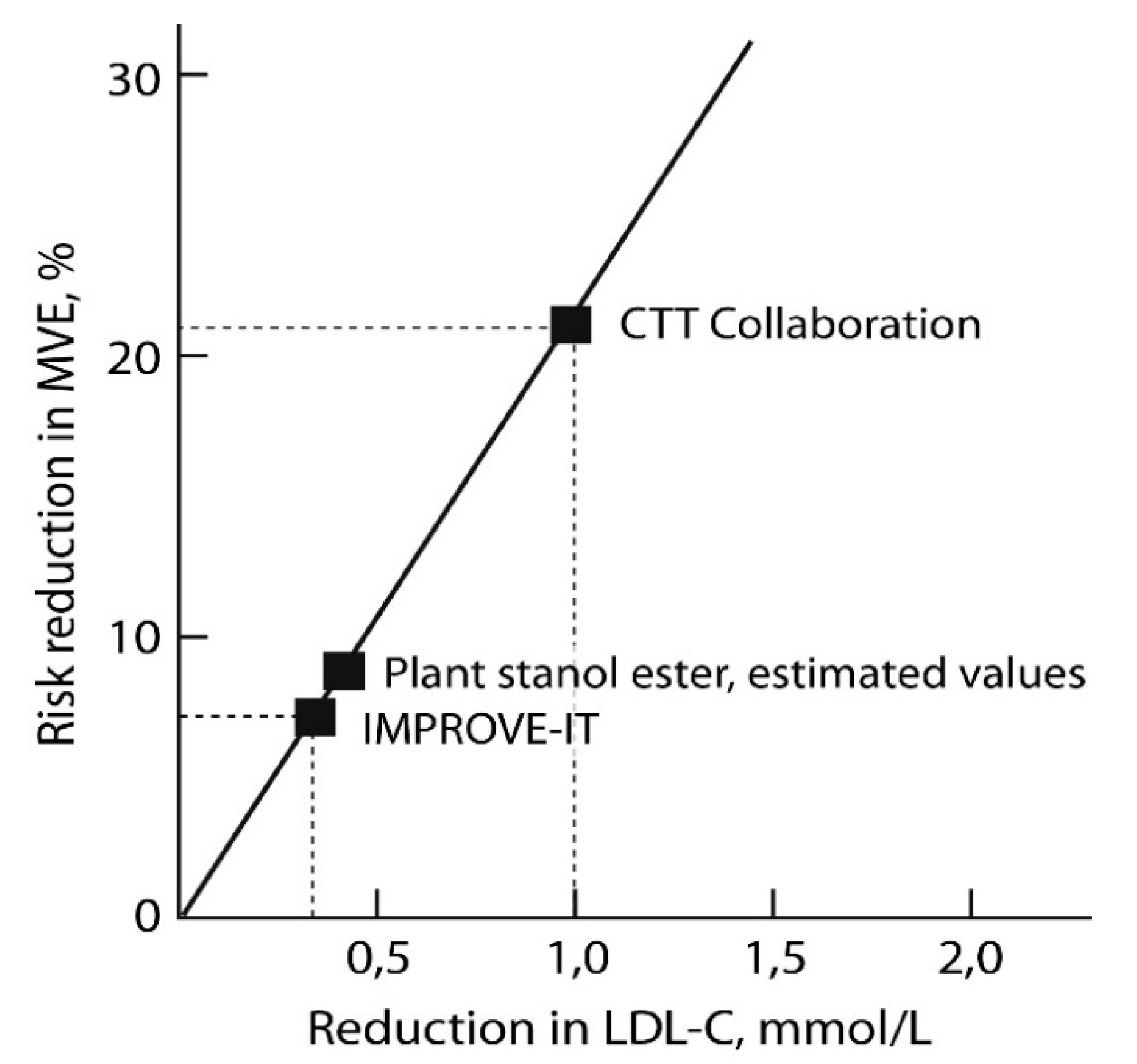
3.5. Plant Stanol Esters and the Risk of ASCVD Events
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Lopez, A.D.; Adair, T. Is the long-term decline in cardiovascular-disease mortality in high-income countries over? Evidence from national vital statistics. Int. J. Epidemiol. 2019, 48, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Brandts, J.; Ray, K.K. Low density lipoprotein cholesterol-lowering strategies and population health. Circulation 2020, 141, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90 056 participants in 14 randomized trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions. A systematic review and meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A., Sr.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease. A Mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- The Myocardial Infarction Genetics Consortium Investigators. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 2014, 371, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Sudhop, T.; Lütjohann, D.; Kodal, A.; Igel, M.; Tribble, D.L.; Shah, S.; Perevozskaya, I.; Von Bergmann, K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 2002, 106, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Friera, L.; Fuster, V.; López-Melgar, B.; Oliva, B.; García-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibáñez, B.; Fernández-Ortiz, A.; et al. Normal LDL-cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991. [Google Scholar] [CrossRef] [PubMed]
- Won, K.-B.; Park, G.-M.; Yang, Y.J.; Ann, S.H.; Kim, Y.G.; Yang, D.H.; Kang, J.W.; Lim, T.H.; Kim, H.K.; Choe, J.; et al. Independent role of low-density lipoprotein cholesterol in subclinical coronary atherosclerosis in the absence of traditional cardiovascular risk factors. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M.; Kovanen, P.T.; Bilheimer, D.W. Cellular pathology of homozygous familial hypercholesterolemia. Am. J. Pathol. 1979, 97, 327–357. [Google Scholar]
- 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid modification to reduce cardiovascular risk. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; on behalf of the American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary cholesterol and cardiovascular risk: A science advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Heggen, E.; Dushanov, S.; Klemsdal, T.O.; Tonstad, S. BMI modifies the effect of dietary fat on atherogenic lipids; a randomized clinical trial. Am. J. Clin. Nutr. 2019, 110, 832–841. [Google Scholar] [CrossRef]
- Maki, K.C. The fat of the matter: Lipoprotein effects of dietary fatty acids vary by body weight status. Am. J. Clin. Nutr. 2019, 110, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Mattson, F.H.; Erickson, B.A.; Kligman, A.M. Effect of dietary cholesterol on serum cholesterol in man. Am. J. Clin. Nutr. 1972, 25, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Ahrens, E.H., Jr.; Davignon, J. The interaction of cholesterol absorption and cholesterol synthesis in man. J. Lipid Res. 1969, 10, 304–315. [Google Scholar] [PubMed]
- Applebaum-Bowden, D.; Haffner, S.M.; Hartsook, E.; Luk, K.H.; Albers, J.J.; Hazzard, W.R. Down-regulation of the low-density lipoprotein receptor by dietary cholesterol. Am. J. Clin. Nutr. 1984, 39, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Kern, F., Jr. Normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day. Mechanisms of adaptation. N. Engl. J. Med. 1991, 324, 896–899. [Google Scholar] [CrossRef]
- Gylling, H.; Miettinen, T.A. Cholesterol absorption and synthesis related to low density lipoprotein metabolism during varying cholesterol intake in men with different apoE phenotypes. J. Lipid Res. 1992, 33, 1361–1371. [Google Scholar]
- Vincent, M.J.; Allen, B.; Palacios, O.M.; Haber, L.T.; Maki, K.C. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am. J. Clin. Nutr. 2019, 109, 7–16. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Cornelis, M.C.; Wilkins, J.T.; Ning, H.; Carnethon, M.R.; Greenland, P.; Mentz, R.J.; Tucker, K.L.; Zhao, L.; et al. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA 2019, 321, 1081–1095. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Mohan, V.; Lear, S.; Swaminathan, S.; Wielgosz, A.; Seron, P.; Avezum, A.; Lopez-Jaramillo, P.; et al. Association of egg intake with blood lipids, cardiovascular disease, and mortality in 177,000 people in 50 countries. Am. J. Clin. Nutr. 2020, 111, 795–803. [Google Scholar] [CrossRef]
- Gylling, H.; Radhakrishnan, R.; Miettinen, T.A. Reduction of serum cholesterol in postmenopausal women with previous myocardial infarction and cholesterol malabsorption induced by dietary sitostanol ester margarine. Women with dietary sitostanol. Circulation 1997, 96, 4226–4231. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Vuoristo, M.; Nissinen, M.; Järvinen, H.J.; Gylling, H. Serum, biliary, and fecal cholesterol and plant sterols in colectomized patients before and during consumption of stanol ester margarine. Am. J. Clin. Nutr. 2000, 71, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E., Jr.; McGill, J.B.; Zeng, C.M.; Covey, D.F.; Stearns, J.; Stenson, W.F.; Spilburg, C.A. Gastrointestinal absorption and plasma kinetics of soy Δ5-phytosterols and phytostanols in humans. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E911–E916. [Google Scholar] [CrossRef]
- Thompson, G.R.; Grundy, S.M. History and development of plant sterol and stanol esters for cholesterol-lowering purposes. Am. J. Cardiol. 2005, 96, 3D–9D. [Google Scholar] [CrossRef] [PubMed]
- Musa-Veloso, K.; Poon, T.H.; Elliot, J.A.; Chung, C. A comparison of the LDL-cholesterol lowering efficacy of plant stanols and plant sterols over a continuous dose range: Results of a meta-analysis of randomized, placebo-controlled trials. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Scientific opinion of the panel on dietetic products, nutrition, and allergies. Scientific Opinion on the substantiation of a health claim related to 3 g/day plant stanols as plant stanol esters and lowering blood LDL-cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 1–15. [Google Scholar]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Gylling, H.; Halonen, J.; Lindholm, H.; Konttinen, J.; Simonen, P.; Nissinen, M.J.; Savolainen, A.; Talvi, A.; Hallikainen, M. The effects of plant stanol ester consumption on arterial stiffness and endothelial function in adults: A randomised controlled clinical trial. BMC Cardiovasc. Dis. 2013, 13, 50. [Google Scholar] [CrossRef]
- Baumgartner, S.; Mensink, R.P.; DeSmet, E.; Konings, M.; Fuentes, S.; De Vos, W.M.; Plat, J. Effects of plant stanol ester consumption on fasting plasma oxy(phyto)sterol concentrations as related to fecal microbiota characteristics. J. Steroid Biochem. Mol. Biol. 2017, 169, 46–53. [Google Scholar] [CrossRef]
- Simonen, P.; Stenman, U.H.; Gylling, H. Serum proprotein convertase subtilisin/kexin type 9 concentration is not increased by plant stanol ester consumption in normo- to moderately hypercholesterolaemic non-obese subjects. The BLOOD FLOW intervention study. Clin. Sci. 2015, 129, 439–446. [Google Scholar] [CrossRef]
- Ruuth, M.; Äikäs, L.; Tigistu-Sahle, F.; Käkelä, R.; Lindholm, H.; Simonen, P.; Kovanen, P.T.; Gylling, H.; Öörni, K. Plant stanol esters reduce LDL (Low-Density Lipoprotein) aggregation by altering LDL surface lipids. The blood flow randomized intervention study. Arterioscler. Thromb. Vasc. Biol. 2020, 40. [Google Scholar] [CrossRef]
- Gylling, H.; Miettinen, T.A. Baseline intestinal absorption and synthesis of cholesterol regulate its response to hypolipidaemic treatments in coronary patients. Atherosclerosis 2002, 160, 477–481. [Google Scholar] [CrossRef]
- Thongtang, N.; Lin, J.; Schaefer, E.J.; Lowe, R.S.; Tomassini, J.E.; Shah, A.K.; Tershakovec, A.M. Effects of ezetimibe added to statin therapy on markers of cholesterol absorption and synthesis and LDL-C lowering in hyperlipidemic patients. Atherosclerosis 2012, 225, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Lakoski, S.G.; Xu, F.; Vega, G.L.; Grundy, S.M.; Chandalia, M.; Lam, C.; Lowe, R.S.; Stepanavage, M.E.; Musliner, T.A.; Cohen, J.C.; et al. Indices of cholesterol metabolism and relative responsiveness to ezetimibe and simvastatin. J. Clin. Endocrinol. Metab. 2010, 95, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Miettinen, T.A. Serum cholesterol and cholesterol and lipoprotein metabolism in hypercholesterolaemic NIDDM patients before and during sitostanol ester-margarine treatment. Diabetologia 1994, 37, 773–780. [Google Scholar] [CrossRef]
- Gylling, H.; Miettinen, T.A. Effects of inhibiting cholesterol absorption and synthesis on cholesterol and lipoprotein metabolism in hypercholesterolemic non-insulin-dependent diabetic men. J. Lipid Res. 1996, 37, 1776–1785. [Google Scholar]
- Bosner, M.S.; Lange, L.G.; Stenson, W.F.; Ostlund, R.E., Jr. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 1999, 40, 302–308. [Google Scholar]
- Tovar, J.; Johansson, M.; Björck, I. A multifunctional diet improves cardiometabolic-related biomarkers independently of weight changes: An 8-week randomized controlled intervention in healthy overweight and obese subjects. Eur. J. Nutr. 2016, 55, 2295–2306. [Google Scholar] [CrossRef]
- Scientific Committee on Food (SCF). General View on the Long-Term Effects of the Intake of Elevated Levels of Phytosterols from Multiple Dietary Sources, with Particular Attention to the Effects on ß-carotene. Opinion Adopted by the Scientific Committee on Food on 26 September 2002. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out143_en.pdf (accessed on 3 July 2002).
- US FDA GRAS Notice no. GRN 000438, Plant Stanol Esters. 2013. Available online: https://www.fda.gov/media/123342/download (accessed on 8 April 2019).
- WHO Food Additives Series, 60, Safety Evaluation of Certain Food Additives/Prepared by the Sixty-Ninth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: http://whqlibdoc.who.int/publications/2009/9789241660600eng.pdf (accessed on 12 August 2009).
- Mensink, R.P.; Ebbing, S.; Lindhout, M.; Plat, J.; Van Heugten, M.M.A. Effects of plant stanol esters supplied in low-fat yoghurt on serum lipids and lipoproteins, non-cholesterol sterols and fat soluble antioxidant concentrations. Atherosclerosis 2002, 160, 205–213. [Google Scholar] [CrossRef]
- Fineli. National Food Composition Database in Finland. Version 20. Available online: https://fineli.fi/fineli/en/index (accessed on 27 June 2019).
- Plat, J.; Mensink, R.P. Effects of plant stanol esters on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase mRNA expression in mononuclear blood cells of healthy men and women. FASEB J. 2002, 16, 258–260. [Google Scholar] [CrossRef]
- Brunner, F.J.; Waldeyer, C.; Ojeda, F.; Salomaa, V.; Kee, F.; Sans, S.; Thorand, B.; Giampaoli, S.; Brambilla, P.; Tunstall-Pedoe, H.; et al. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: Results from the Multinational Cardiovascular Risk Consortium. Lancet 2019, 394, 2173–2183. [Google Scholar] [CrossRef]
- DeMets, D.L.; Psaty, B.M.; Fleming, T.R. When can intermediate outcomes be used as surrogate outcomes? JAMA 2020, 323, 1184–1185. [Google Scholar] [CrossRef] [PubMed]
| Diet | Biliary Cholesterol Flux, (mg/day) | Dietary Cholesterol Flux, (mg/day) | Total Cholesterol Flux, (mg/day) | Absorbed Total Cholesterol, (mg/day) | Difference Versus Home Diet (mg/day) |
|---|---|---|---|---|---|
| Home diet (data from original study [30]) | 971 | 247 | 1218 | 497 | |
| Home diet + 2.7 g plant stanols/day (data from original study [30]) | 971 | 247 | 1218 | 285 | –212 |
| Home diet + 1 egg (200 mg cholesterol) * | 971 | 447 | 1418 | 578 | + 81 |
| Home diet + 1 egg (200 mg cholesterol) + 2.7 g plant stanols/day * | 971 | 447 | 1418 | 332 | –165 |
| Plant Stanol Consumption, g/day | Change in LDL-C, mmol/L | Change in LDL-C (%) | Change in ASCVD Events (%) |
|---|---|---|---|
| 1 | –0.19 | –5.4 | –4.0 |
| 1.5 | –0.27 | –7.4 | –5.7 |
| 2 | –0.33 | –9.2 | –6.9 |
| 3 | –0.42 | –11.8 | –8.8 |
| 4 | –0.48 | –13.7 | –10.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gylling, H.; Strandberg, T.E.; Kovanen, P.T.; Simonen, P. Lowering Low-Density Lipoprotein Cholesterol Concentration with Plant Stanol Esters to Reduce the Risk of Atherosclerotic Cardiovascular Disease Events at a Population Level: A Critical Discussion. Nutrients 2020, 12, 2346. https://doi.org/10.3390/nu12082346
Gylling H, Strandberg TE, Kovanen PT, Simonen P. Lowering Low-Density Lipoprotein Cholesterol Concentration with Plant Stanol Esters to Reduce the Risk of Atherosclerotic Cardiovascular Disease Events at a Population Level: A Critical Discussion. Nutrients. 2020; 12(8):2346. https://doi.org/10.3390/nu12082346
Chicago/Turabian StyleGylling, Helena, Timo E. Strandberg, Petri T. Kovanen, and Piia Simonen. 2020. "Lowering Low-Density Lipoprotein Cholesterol Concentration with Plant Stanol Esters to Reduce the Risk of Atherosclerotic Cardiovascular Disease Events at a Population Level: A Critical Discussion" Nutrients 12, no. 8: 2346. https://doi.org/10.3390/nu12082346
APA StyleGylling, H., Strandberg, T. E., Kovanen, P. T., & Simonen, P. (2020). Lowering Low-Density Lipoprotein Cholesterol Concentration with Plant Stanol Esters to Reduce the Risk of Atherosclerotic Cardiovascular Disease Events at a Population Level: A Critical Discussion. Nutrients, 12(8), 2346. https://doi.org/10.3390/nu12082346





