Exclusive Breastfeeding Predicts Higher Hearing-Language Development in Girls of Preschool Age
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Definition of Feeding Type
2.3. Measurement of Children’s and Parents’ Cognitive Scores
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Exclusively and Nonexclusively Breastfed Children
3.2. Relationship between Exclusive Breastfeeding and Children’s Cognitive Development
3.3. Impact of Parental Intelligence and Age on the Relationship between Exclusive Breastfeeding and Children’s Cognitive Development
3.4. Impact of Maternal and Children’s Body Weight on the Relationship between Exclusive Breastfeeding and Children’s Cognitive Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Eilander, A.; Hundscheid, D.C.; Osendarp, S.J.; Transler, C.; Zock, P.L. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: A review of human studies. Prostaglandins Leukot. Essent. Fatty Acids 2007, 76, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Vehling, L.; Chan, D.; Klopp, A.; Nickel, N.C.; McGavock, J.M.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Infant Feeding and Weight Gain: Separating Breast Milk from Breastfeeding and Formula from Food. Pediatrics 2018, 142, e20181092. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Reilly, J.J.; Child Health Information, T. Breastfeeding and lowering the risk of childhood obesity. Lancet 2002, 359, 2003–2004. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T. Breast feeding and the risk of obesity and related metabolic diseases in the child. Metab. Syndr. Relat. Disord. 2005, 3, 222–232. [Google Scholar] [CrossRef]
- Oddy, W.H.; Li, J.; Whitehouse, A.J.; Zubrick, S.R.; Malacova, E. Breastfeeding duration and academic achievement at 10 years. Pediatrics 2011, 127, e137–e145. [Google Scholar] [CrossRef]
- Anderson, J.W.; Johnstone, B.M.; Remley, D.T. Breast-feeding and cognitive development: A meta-analysis. Am. J. Clin. Nutr. 1999, 70, 525–535. [Google Scholar] [CrossRef]
- Horwood, L.J.; Fergusson, D.M. Breastfeeding and later cognitive and academic outcomes. Pediatrics 1998, 101, E9. [Google Scholar] [CrossRef]
- Brion, M.J.; Lawlor, D.A.; Matijasevich, A.; Horta, B.; Anselmi, L.; Araujo, C.L.; Menezes, A.M.; Victora, C.G.; Smith, G.D. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int. J. Epidemiol. 2011, 40, 670–680. [Google Scholar] [CrossRef]
- Jenkins, J.M.; Foster, E.M. The effects of breastfeeding exclusivity on early childhood outcomes. Am. J. Public Health 2014, 104 (Suppl. S1), S128–S135. [Google Scholar] [CrossRef]
- Raisler, J.; Alexander, C.; O’Campo, P. Breast-feeding and infant illness: A dose-response relationship? Am. J. Public Health 1999, 89, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Rifas-Shiman, S.L.; Kleinman, K.P.; Guthrie, L.B.; Bellinger, D.C.; Taveras, E.M.; Gillman, M.W.; Oken, E. Infant feeding and childhood cognition at ages 3 and 7 years: Effects of breastfeeding duration and exclusivity. JAMA Pediatr. 2013, 167, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, E.L.; Michaelsen, K.F.; Sanders, S.A.; Reinisch, J.M. The association between duration of breastfeeding and adult intelligence. JAMA 2002, 287, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Der, G.; Batty, G.D.; Deary, I.J. Effect of breast feeding on intelligence in children: Prospective study, sibling pairs analysis, and meta-analysis. BMJ 2006, 333, 945. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Chiodo, L.M.; Jacobson, J.L. Breastfeeding effects on intelligence quotient in 4- and 11-year-old children. Pediatrics 1999, 103, e71. [Google Scholar] [CrossRef]
- Veena, S.R.; Krishnaveni, G.V.; Srinivasan, K.; Wills, A.K.; Hill, J.C.; Kurpad, A.V.; Muthayya, S.; Karat, S.C.; Nalinakshi, M.; Fall, C.H. Infant feeding practice and childhood cognitive performance in South India. Arch. Dis. Child. 2010, 95, 347–354. [Google Scholar] [CrossRef]
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Breastfeeding and intelligence: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 14–19. [Google Scholar] [CrossRef]
- Casas, M.; Chatzi, L.; Carsin, A.E.; Amiano, P.; Guxens, M.; Kogevinas, M.; Koutra, K.; Lertxundi, N.; Murcia, M.; Rebagliato, M.; et al. Maternal pre-pregnancy overweight and obesity, and child neuropsychological development: Two Southern European birth cohort studies. Int. J. Epidemiol. 2013, 42, 506–517. [Google Scholar] [CrossRef]
- Hinkle, S.N.; Schieve, L.A.; Stein, A.D.; Swan, D.W.; Ramakrishnan, U.; Sharma, A.J. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int. J. Obes. (Lond.) 2012, 36, 1312–1319. [Google Scholar] [CrossRef]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef]
- Sutcliffe, A.G.; Barnes, J.; Belsky, J.; Gardiner, J.; Melhuish, E. The health and development of children born to older mothers in the United Kingdom: Observational study using longitudinal cohort data. BMJ 2012, 345, e5116. [Google Scholar] [CrossRef] [PubMed]
- Falster, K.; Hanly, M.; Banks, E.; Lynch, J.; Chambers, G.; Brownell, M.; Eades, S.; Jorm, L. Maternal age and offspring developmental vulnerability at age five: A population-based cohort study of Australian children. PLoS Med. 2018, 15, e1002558. [Google Scholar] [CrossRef] [PubMed]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Hines, M. Sex-related variation in human behavior and the brain. Trends Cogn. Sci. 2010, 14, 448–456. [Google Scholar] [CrossRef]
- Galante, L.; Lagstrom, H.; Vickers, M.H.; Reynolds, C.M.; Rautava, S.; Milan, A.M.; Cameron-Smith, D.; Pundir, S. Sexually Dimorphic Associations between Maternal Factors and Human Milk Hormonal Concentrations. Nutrients 2020, 12, 152. [Google Scholar] [CrossRef]
- Ivens, J.; Martin, N. A common metric for the Griffiths Scales. Arch. Dis. Child. 2002, 87, 109–110. [Google Scholar] [CrossRef]
- Lanting, C.I.; Fidler, V.; Huisman, M.; Touwen, B.C.; Boersma, E.R. Neurological differences between 9-year-old children fed breast-milk or formula-milk as babies. Lancet 1994, 344, 1319–1322. [Google Scholar] [CrossRef]
- Smith, V.L.; Gerber, S.E. Infant feeding and phonologic development. Int. J. Pediatr. Otorhinolaryngol. 1993, 28, 41–49. [Google Scholar] [CrossRef]
- Gale, C.R.; Marriott, L.D.; Martyn, C.N.; Limond, J.; Inskip, H.M.; Godfrey, K.M.; Law, C.M.; Cooper, C.; West, C.; Robinson, S.M.; et al. Breastfeeding, the use of docosahexaenoic acid-fortified formulas in infancy and neuropsychological function in childhood. Arch. Dis. Child. 2010, 95, 174–179. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Perera, F.; Jankowski, J.; Butscher, M.; Mroz, E.; Flak, E.; Kaim, I.; Lisowska-Miszczyk, I.; Skarupa, A.; Sowa, A. Effect of exclusive breastfeeding on the development of children’s cognitive function in the Krakow prospective birth cohort study. Eur. J. Pediatr. 2012, 171, 151–158. [Google Scholar] [CrossRef]
- Angelsen, N.K.; Vik, T.; Jacobsen, G.; Bakketeig, L.S. Breast feeding and cognitive development at age 1 and 5 years. Arch. Dis. Child. 2001, 85, 183–188. [Google Scholar] [CrossRef]
- Miranda, A.; Sousa, N. Maternal hormonal milieu influence on fetal brain development. Brain Behav. 2018, 8, e00920. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M. Sex differences in the developing brain as a source of inherent risk. Dialogues Clin. Neurosci. 2016, 18, 361–372. [Google Scholar] [PubMed]
- Keenan, K.; Bartlett, T.Q.; Nijland, M.; Rodriguez, J.S.; Nathanielsz, P.W.; Zurcher, N.R. Poor nutrition during pregnancy and lactation negatively affects neurodevelopment of the offspring: Evidence from a translational primate model. Am. J. Clin. Nutr. 2013, 98, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Hinde, K.; Skibiel, A.L.; Foster, A.B.; Del Rosso, L.; Mendoza, S.P.; Capitanio, J.P. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav. Ecol. 2015, 26, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef]
- Une, K.; Takei, Y.A.; Tomita, N.; Asamura, T.; Ohrui, T.; Furukawa, K.; Arai, H. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur. J. Neurol. 2011, 18, 1006–1009. [Google Scholar] [CrossRef]
- Bauer, C.C.; Moreno, B.; Gonzalez-Santos, L.; Concha, L.; Barquera, S.; Barrios, F.A. Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: A magnetic resonance imaging study in Mexican children. Pediatr. Obes. 2015, 10, 196–204. [Google Scholar] [CrossRef]
- Guxens, M.; Mendez, M.A.; Julvez, J.; Plana, E.; Forns, J.; Basagana, X.; Torrent, M.; Sunyer, J. Cognitive function and overweight in preschool children. Am. J. Epidemiol. 2009, 170, 438–446. [Google Scholar] [CrossRef][Green Version]
- Li, R.; Jewell, S.; Grummer-Strawn, L. Maternal obesity and breast-feeding practices. Am. J. Clin. Nutr. 2003, 77, 931–936. [Google Scholar] [CrossRef]
- Lovelady, C.A. Is maternal obesity a cause of poor lactation performance. Nutr. Rev. 2005, 63, 352–355. [Google Scholar] [CrossRef]
- Cawley, J.; Spiess, C.K. Obesity and skill attainment in early childhood. Econ. Hum. Biol. 2008, 6, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Mond, J.M.; Stich, H.; Hay, P.J.; Kraemer, A.; Baune, B.T. Associations between obesity and developmental functioning in pre-school children: A population-based study. Int. J. Obes. (Lond.) 2007, 31, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
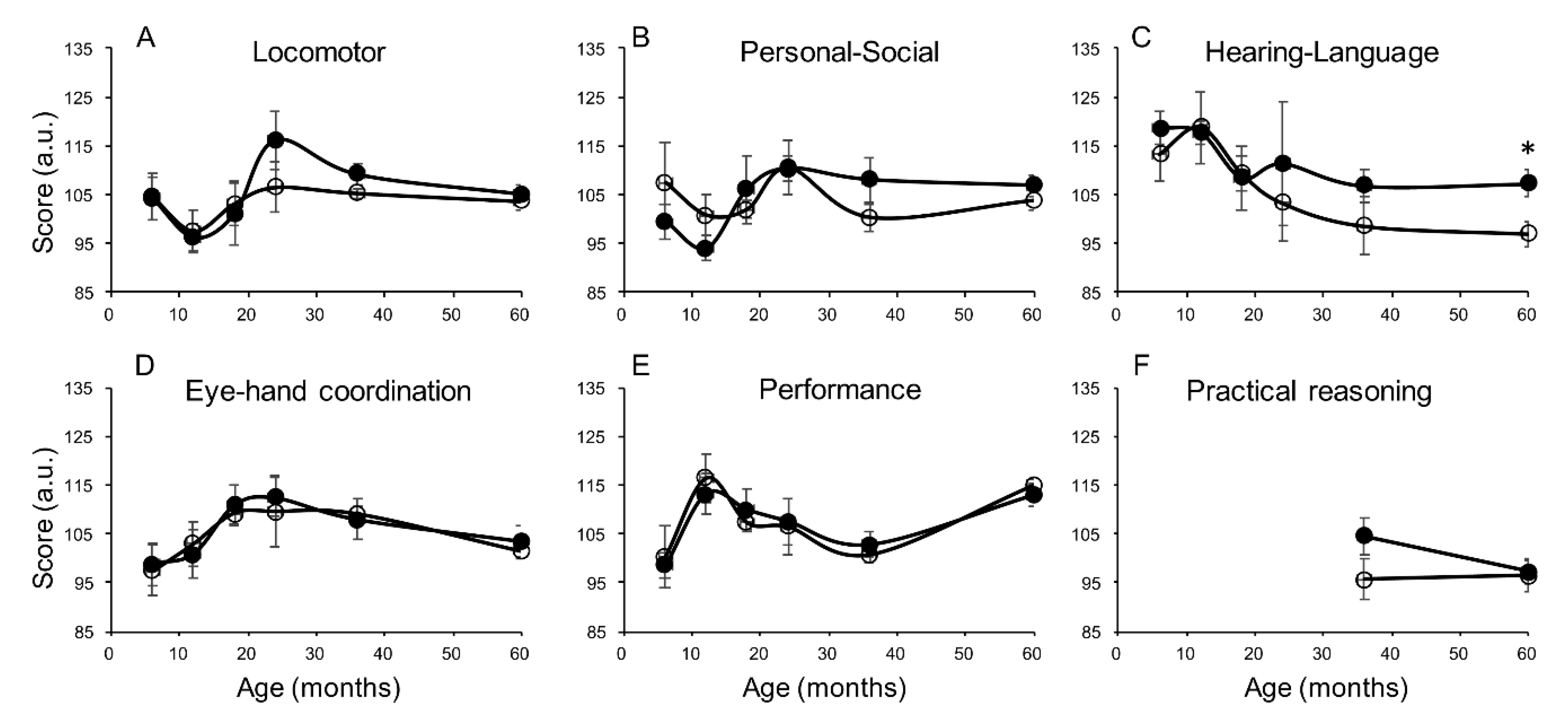
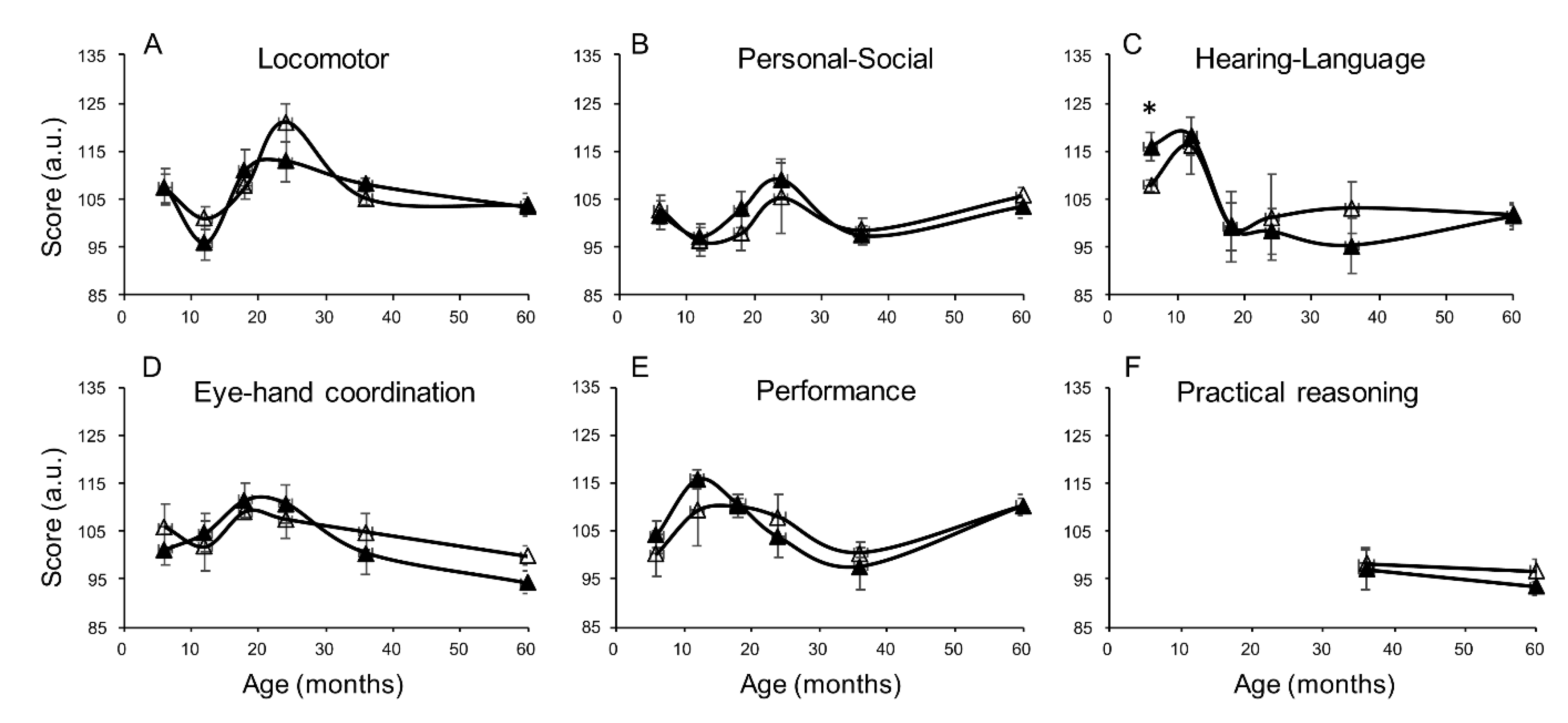
| Variable | N | Exclusive Breastfeeding | Nonexclusive Breastfeeding | p-Value |
|---|---|---|---|---|
| Mixed/formula feeding (N) | 80 | 26/14 | <0.0005 | |
| Sex (Females/Males) | 80 | 16/24 | 24/16 | n.s. |
| Delivery type (Vaginal/C-section) | 80 | 24/16 | 28/12 | n.s. |
| Gestational diabetes (N) | 80 | 12 | 10 | n.s. |
| Gestational age (days) | 80 | 277 ± 1 | 277 ± 2 | n.s. |
| Weaning age (months) | 77 | 5.6 ± 0.1 | 5.3 ± 0.1 | n.s. |
| Mother age (years) | 80 | 33.2 ± 0.7 | 33.7 ± 0.7 | n.s. |
| Father age (years) | 80 | 35.3 ± 0.7 | 37.5 ± 0.8 | 0.04 |
| Mother pregravidic BMI (kg/m2) | 80 | 23.1 ± 06 | 26.8 ± 1.0 | 0.002 |
| Mother gravidic BMI (kg/m2) | 75 | 28.4 ± 0.6 | 31.6 ± 1.1 | 0.016 |
| Mother IQ (RPM score) | 58 | 112.0 ± 1.9 | 113.1 ± 2.4 | n.s. |
| Father IQ (RPM score) | 36 | 114.2 ± 2.3 | 117.9 ± 2.9 | n.s. |
| Birth weight (kg) | 80 | 3.36 ± 0.05 | 3.33 ± 0.07 | n.s. |
| Birth BMI (kg/m2) | 80 | 13.5 ± 0.2 | 13.3 ± 0.2 | n.s. |
| Birth PI (kg/m3) | 80 | 27.0 ± 0.35 | 26.65 ± 0.41 | n.s. |
| Weight at 3 months (kg) | 73 | 6.09 ± 0.14 | 5.94 ± 0.12 | n.s. |
| Weight at 6 months (kg) | 72 | 7.64 ± 0.15 | 7.88 ± 0.15 | n.s. |
| Weight at 12 months (kg) | 71 | 10.03 ± 0.23 | 10.13 ± 0.20 | n.s. |
| Weight at 18 months (kg) | 60 | 11.24 ± 0.28 | 11.46 ± 0.22 | n.s. |
| Weight at 24 months (kg) | 53 | 12.73 ± 0.40 | 12.91 ± 0.36 | n.s. |
| Weight at 36 months (kg) | 54 | 15.14 ± 050 | 15.44 ± 0.38 | n.s. |
| Weight at 60 months (kg) | 66 | 20.18 ± 0.67 | 20.77 ± 0.58 | n.s. |
| BMI at 3 months (kg/m2) | 72 | 16.5 ± 0.3 | 16.3 ± 0.2 | n.s. |
| BMI at 6 months (kg/m2) | 72 | 16.5 ± 0.5 | 17.2 ± 0.3 | n.s. |
| BMI at 12 months (kg/m2) | 69 | 17.2 ± 0.3 | 17.1 ± 0.2 | n.s. |
| BMI at 18 months (kg/m2) | 57 | 16.6 ± 0.3 | 16.7 ± 0.3 | n.s. |
| BMI at 24 months (kg/m2) | 51 | 16.6 ± 0.4 | 16.5 ± 0.3 | n.s. |
| BMI at 36 months (kg/m2) | 52 | 16.5 ± 0.4 | 16.6 ± 0.3 | n.s. |
| BMI at 60 months (kg/m2) | 66 | 16.8 ± 0.5 | 17.1 ± 0.4 | n.s. |
| Weight gain 0–3 months (kg) | 73 | 2.71 ± 0.11 | 2.61 ± 0.10 | n.s. |
| Weight gain 3–6 months (kg) | 69 | 1.57 ± 0.07 | 1.93 ± 0.10 | 0.003 |
| Weight gain 0–6 months (kg) | 72 | 4.28 ± 0.12 | 4.54 ± 0.13 | n.s. |
| Weight gain 0–12 months (kg) | 71 | 6.67±0.20 | 6.81 ± 0.19 | n.s. |
| Weight gain 0–60 months (kg) | 66 | 16.84 ± 0.65 | 17.41 ± 0.56 | n.s. |
| Weight gain 12–24 months (kg) | 51 | 2.84 ± 0.18 | 2.80 ± 0.30 | n.s. |
| Weight gain 24–36 months (kg) | 46 | 2.07 ± 0.22 | 2.52 ± 0.21 | n.s. |
| Weight gain 36–60 months (kg) | 51 | 5.26 ± 0.43 | 5.48 ± 0.46 | n.s. |
| Cranial circ. at 3 months (cm) | 48 | 40.1 ± 0.2 | 40.2 ± 0.3 | n.s. |
| Cranial circ. at 6 months (cm) | 49 | 42.7 ± 0.2 | 43.0 ± 0.3 | n.s. |
| Cranial circ. at 12 months (cm) | 53 | 46.2 ± 0.2 | 45.9 ± 0.3 | n.s. |
| Cranial circ. at 18 months (cm) | 24 | 47.5 ± 0.4 | 47.5 ± 0.4 | n.s. |
| Cranial circ. at 24 months (cm) | 13 | 49.2 ± 0.6 | 49.5 ± 0.5 | n.s. |
| Cranial circ. at 36 months (cm) | 24 | 50.4 ± 0.5 | 50.9 ± 0.4 | n.s. |
| Cranial circ. at 60 months (cm) | 66 | 51.3 ± 0.2 | 51.4 ± 0.3 | n.s. |
| GDMS Scale | Analysis | Total Population B (95% C.I.) | Girls B (95% C.I.) | Boys B (95% C.I.) |
|---|---|---|---|---|
| Locomotor | Univariate | 0.3 (−3.6, 4.3) | 1.5 (−3.9, 6.9) | −0.6 (−6.6, 5.7) |
| Adjusted * | 1.2 (−4.0, 6.5) | 3.5 (−3.6, 10,6) | −1.9 (−11.0, 7.2) | |
| Personal−social | Univariate | 0.2 (−4.7, 5.1) | 3.1 (−3.1, 9.5) | −2.3 (−10.1, 5.5) |
| Adjusted * | 2.9 (−3.5, 9.3) | 6.2 (−2.1, 14.7) | −2.1 (−12.7, 8.5) | |
| Hearing-language | Univariate | 4.3 (−0.9, 9.6) | 10.5 (2.4, 18.7) ** | −0.47 (−7.7, 6.8) |
| Adjusted * | 4.3 (−2.4, 11.0) | 11.0 (0.9, 21.2) ** | −3.0 (−12.1, 6.1) | |
| Eye-hand coordination | Univariate | −2.7 (−8.2, 2.3) | 1.9 (−7.0, 10.9) | −6.0 (−13.0, 1.0) |
| Adjusted * | −2.7 (−9.7, 4.3) | 0.8 (−11.5, 13.2) | −6.2 (−16.4, 3.9) | |
| Performance | Univariate | −1.3 (−5.1, 2.5) | −2.2 (−7.9, 3.4) | −0.1 (−5.6, 5.5) |
| Adjusted * | −0.8 (−5.8, 4.1) | −2.7 (−10.5, 5.1) | 0.7 (−7.4, 8.9) | |
| Practical reasoning | Univariate | −1.6 (−6.6, 3,4) | 0.8 (−8.2, 9.8) | −3.4 (−9.5, 2.7) |
| Adjusted * | 1.0 (−5.0, 7.0) | 4.2 (−6.9, 15.2) | −4.0 (−11.0, 2.9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzzardi, M.A.; Granziera, F.; Sanguinetti, E.; Ditaranto, F.; Muratori, F.; Iozzo, P. Exclusive Breastfeeding Predicts Higher Hearing-Language Development in Girls of Preschool Age. Nutrients 2020, 12, 2320. https://doi.org/10.3390/nu12082320
Guzzardi MA, Granziera F, Sanguinetti E, Ditaranto F, Muratori F, Iozzo P. Exclusive Breastfeeding Predicts Higher Hearing-Language Development in Girls of Preschool Age. Nutrients. 2020; 12(8):2320. https://doi.org/10.3390/nu12082320
Chicago/Turabian StyleGuzzardi, Maria Angela, Federico Granziera, Elena Sanguinetti, Francesca Ditaranto, Filippo Muratori, and Patricia Iozzo. 2020. "Exclusive Breastfeeding Predicts Higher Hearing-Language Development in Girls of Preschool Age" Nutrients 12, no. 8: 2320. https://doi.org/10.3390/nu12082320
APA StyleGuzzardi, M. A., Granziera, F., Sanguinetti, E., Ditaranto, F., Muratori, F., & Iozzo, P. (2020). Exclusive Breastfeeding Predicts Higher Hearing-Language Development in Girls of Preschool Age. Nutrients, 12(8), 2320. https://doi.org/10.3390/nu12082320





