Euglena Gracilis and β-Glucan Paramylon Induce Ca2+ Signaling in Intestinal Tract Epithelial, Immune, and Neural Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dorsal Root Ganglia (DRG) Cells
2.3. Test Substances
2.4. Intravital and In Vitro Imaging
2.5. In Vivo Stimulation Assay
2.6. Statistical Analysis
3. Results
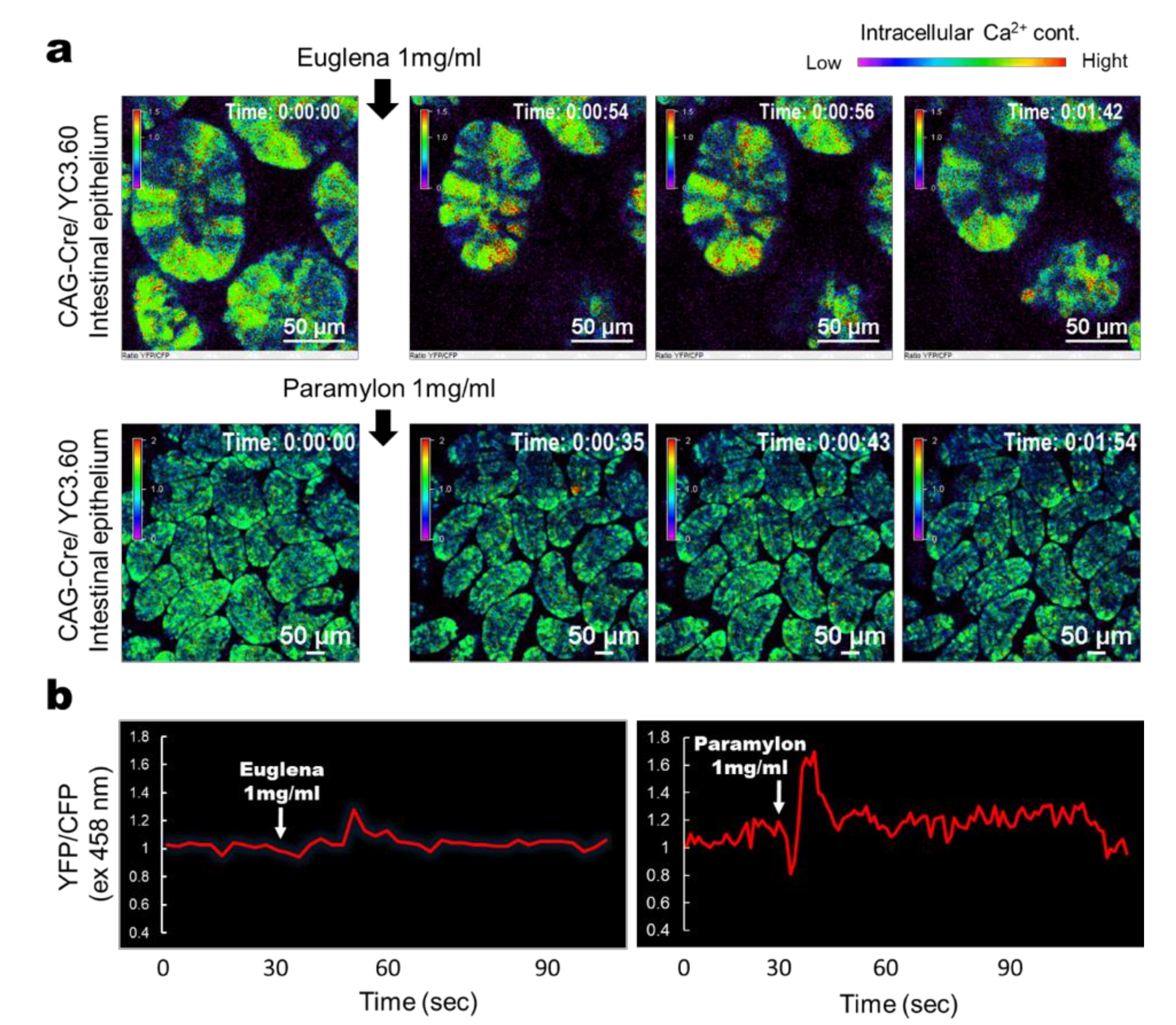
3.1. Euglena and Paramylon Induce Ca2+ Signaling in the IECs of Mice With Ubiquitous Yc3.60 Expression
3.2. Euglena and Paramylon Induce Ca2+ Signaling in DCs
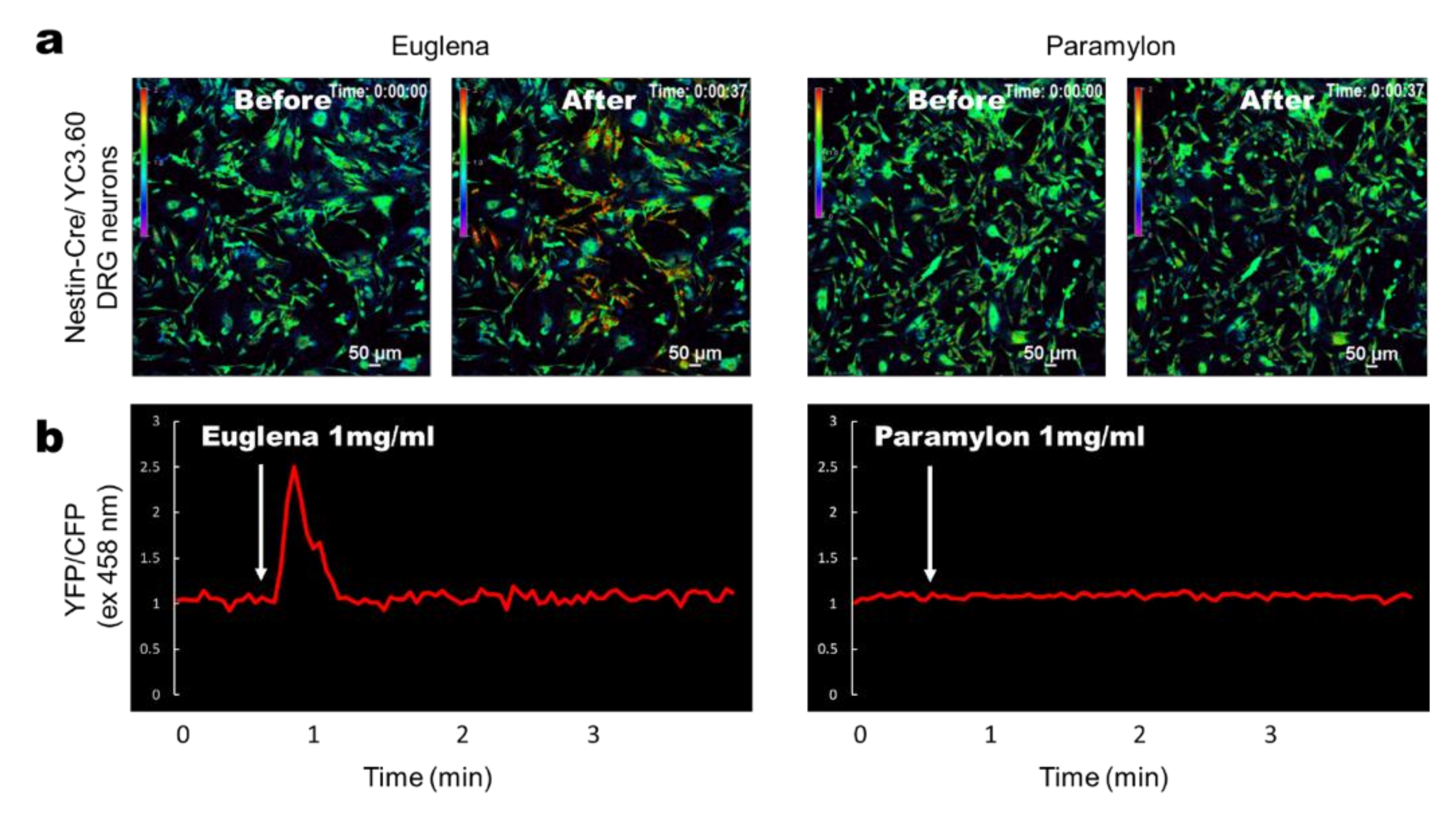
3.3. Euglena Elicits In Vitro Ca2+ Signaling in DRG-Derived Neurons From YC3.60flox/Nestin-Cre Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitsuoka, T. Development of Functional Foods. Biosci. Microbiota Food Health 2014, 33, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Rivera, L.R.; Cho, H.J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef]
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Fölsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Makizaki, Y.; Maeda, A.; Oikawa, Y.; Tamura, S.; Tanaka, Y.; Nakajima, S.; Yamamura, H. Alleviation of low-fiber diet-induced constipation by probiotic Bifidobacterium bifidum G9-1 is based on correction of gut microbiota dysbiosis. Biosci. Microbiota Food Health 2019, 38, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Shiomi, S.; Ushikubo, S.; Inai, R.; Matsuo, T. Effect of a fermented brown rice extract on the gastrointestinal function in methotrexate-treated rats. Biosci. Biotechnol. Biochem. 2013, 77, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Ogasa, S.; Kuwahara, T.; Bando, Y.; Hagiwara, M.; Arimochi, H.; Nakanishi, S.; Iwasaki, T.; Ohnishi, Y. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig. Dis. Sci. 2008, 53, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Kamiya, T.; Liu, Y.; Kadoki, M.; Kakuta, S.; Oshima, K.; Hattori, M.; Takeshita, K.; Kanai, T.; Saijo, S.; et al. Inhibition of dectin-1 signaling ameliorates colitis by inducing lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe 2015, 18, 183–197. [Google Scholar] [CrossRef]
- Inoue, A.; Kodama, N.; Nanba, H. Effect of maitake (Grifola frondosa) D-Fraction on the control of the T lymph node Th-1/Th-2 proportion. Biol. Pharm. Bull. 2002, 25, 536–540. [Google Scholar] [CrossRef][Green Version]
- Jesenak, M.; Banovcin, P.; Rennerova, Z.; Majtan, J. β-Glucans in the treatment and prevention of allergic diseases. Allergol. Immunopathol. (Madr.) 2014, 42, 149–156. [Google Scholar] [CrossRef]
- Sarinho, E.; Medeiros, D.; Schor, D.; Rego Silva, A.; Sales, V.; Motta, M.E.; Costa, A.; Azoubel, A.; Rizzo, J.A. Production of interleukin-10 in asthmatic children after Beta-1-3-glucan. Allergol. Immunopathol. 2009, 37, 188–192. [Google Scholar] [CrossRef]
- Schwartzbach, S.D.; Shigeoka, S. Euglena: Biochemistry, Cell and Molecular Biology; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Kondo, Y.; Kato, A.; Hojo, H.; Nozoe, S.; Takeuchi, M.; Ochi, K. Cytokine-Related Immunopotentiating Activities of Paramylon, a (3-1-≫3)-d-Glucan from Euglena gracilis. J. Pharmacobiodyn. 1992, 15, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 494, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Barsanti, L.; Evangelista, V.; Frassanito, A.M.; Longo, V.; Pucci, L.; Penno, G.; Gualtieri, P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2017, 5, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Ogushi, M.; Nakashima, A.; Nakano, Y.; Suzuki, K. Accelerated wound healing on the skin using a film dressing with β-glucan paramylon. In Vivo 2018, 32, 799–805. [Google Scholar] [CrossRef]
- Aoe, S.; Yamanaka, C.; Nishioka, M.; Onaka, N.; Nishida, N.; Takahashi, M. Effects of paramylon extracted from Euglena gracilis EOD-1 on parameters related to metabolic syndrome in diet-induced obese mice. Nutrients 2019, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Sakanoi, Y.; Shuang, E.; Yamamoto, K.; Ota, T.; Seki, K.; Imai, M.; Ota, R.; Asayama, Y.; Nakashima, A.; Suzuki, K.; et al. Simultaneous intake of Euglena gracilis and vegetables synergistically exerts an anti-inflammatory effect and attenuates visceral fat accumulation by affecting gut microbiota in mice. Nutrients 2018, 10, 1417. [Google Scholar] [CrossRef]
- Okouchi, R.; Shuang, E.; Yamamoto, K.; Ota, T.; Seki, K.; Imai, M.; Ota, R.; Asayama, Y.; Nakashima, A.; Suzuki, K.; et al. Simultaneous intake of Euglena gracilis and vegetables exerts synergistic anti-obesity and anti-inflammatory effects by modulating the gut microbiota in diet-induced obese mice. Nutrients 2019, 11, 204. [Google Scholar] [CrossRef]
- Sugimoto, R.; Ishibashi-Ohgo, N.; Atsuji, K.; Miwa, Y.; Iwata, O.; Nakashima, A.; Suzuki, K. Euglena extract suppresses adipocyte-differentiation in human adipose-derived stem cells. PLoS ONE 2018, 13, 1–14. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition: A new receptor for β-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 8–17. [Google Scholar] [CrossRef]
- Cohen-Kedar, S.; Baram, L.; Elad, H.; Brazowski, E.; Guzner-Gur, H.; Dotan, I. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur. J. Immunol. 2014, 44, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, S.; Kanaya, T.; Fukuda, S.; Nakato, G.; Hanazato, M.; Wu, X.R.; Yamamoto, N.; Ohno, H. Uromodulin-SlpA binding dictates Lactobacillus acidophilus uptake by intestinal epithelial M cells. Int. Immunol. 2017, 29, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yamada, S.; Tominaga, T.; Ichikawa, M.; Miyawaki, A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 10554–10559. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Usami, T.; Kikuta, J.; Ishii, M.; Sasano, T.; Sugiyama, K.; Furukawa, T.; Nakasho, E.; Takayanagi, H.; Tedder, T.F.; et al. Intravital imaging of Ca2+ signals in lymphocytes of Ca2+ biosensor transgenic mice: Indication of autoimmune diseases before the pathological onset. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Kurosaki, T.; Shinohara, H.; Baba, Y.B. Cell Signaling and Fate Decision. Annu. Rev. Immunol. 2010, 28, 21–55. [Google Scholar] [CrossRef]
- Nathanson, M.H. Cellular and subcellular calcium signaling in gastrointestinal epithelium. Gastroenterology 1994, 106, 1349–1364. [Google Scholar] [CrossRef]
- Silva, A.J.; Paylor, R.; Wehner, J.M.; Tonegawa, S. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science 1992, 257, 206–211. [Google Scholar] [CrossRef]
- Feske, S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007, 7, 690–702. [Google Scholar] [CrossRef]
- Adachi, T.; Yoshikawa, S.; Tezuka, H.; Tsuji, N.M.; Ohteki, T.; Karasuyama, H.; Kumazawa, T. Propolis induces Ca2+ signaling in immune cells. Biosci. Microbiota Food Health 2019, 38, 141–149. [Google Scholar] [CrossRef]
- Adachi, T.; Kakuta, S.; Aihara, Y.; Kamiya, T.; Watanabe, Y.; Osakabe, N.; Hazato, N.; Miyawaki, A.; Yoshikawa, S.; Usami, T.; et al. Visualization of probiotic-mediated Ca2+ signaling in intestinal epithelial cells in vivo. Front. Immunol. 2016, 7, 601. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [PubMed]
- Horii, Y.; Nakakita, Y.; Misonou, Y.; Nakamura, T.; Nagai, K. The serotonin receptor mediates changes in autonomic neurotransmission and gastrointestinal transit induced by heat-killed Lactobacillus brevis SBC8803. Benef. Microbes 2015, 6, 817–822. [Google Scholar] [CrossRef]
- Nishimura, Y.; Fukuda, Y.; Okonogi, T.; Yoshikawa, S.; Karasuyama, H.; Osakabe, N.; Ikegaya, Y.; Sasaki, T.; Adachi, T. Dual real-time in vivo monitoring system of the brain-gut axis. Biochem. Biophys. Res. Commun. 2020, 524, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, M.; Takao, K.; Hori, A.; Shiraki, T.; Matsumura, K.; Kobayashi, S. Ionic Basis of Cold Receptors Acting as Thermostats. J. Neurosci. 2002, 22, 3994–4001. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Murphy, C.; O’Neill, L.A.J.; Creagh, E.M. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010, 3, 17–28. [Google Scholar] [CrossRef]
- Raka, F.; Farr, S.; Kelly, J.; Stoianov, A.; Adeli, K. Metabolic control via nutrient-sensing mechanisms: Role of taste receptors and the gut-brain neuroendocrine axis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E559–E572. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Cani, P.; Delzenne, N. The Role of the Gut Microbiota in Energy Metabolism and Metabolic Disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef]
- Shibakami, M.; Shibata, K.; Akashi, A.; Onaka, N.; Takezaki, J.; Tsubouchi, G.; Yoshikawa, H. Correction to: Creation of Straight-Chain Cationic Polysaccharide-Based Bile Salt Sequestrants Made from Euglenoid β-1,3-Glucan as Potential Antidiabetic Agents. Pharm. Res. 2019, 36, 31. [Google Scholar] [CrossRef]
- Gerbe, F.; Legraverend, C.; Jay, P. The intestinal epithelium tuft cells: Specification and function. Cell. Mol. Life Sci. 2012, 69, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakashima, A.; Igarashi, M.; Saito, K.; Konno, M.; Yamazaki, N.; Takimoto, H. Euglena gracilis Z and its carbohydrate storage substance relieve arthritis symptoms by modulating Th17 immunity. PLoS ONE 2018, 13, e0191462. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Nishioka, M.; Onaka, N.; Takahashi, M.; Yamanaka, D.; Adachi, Y.; Ohno, N. Effects of Euglena gracilis EOD-1 Ingestion on Salivary IgA Reactivity and Health-Related Quality of Life in Humans. Nutrients 2019, 11, 1144. [Google Scholar] [CrossRef]
- Ujita, M.; Nagayama, H.; Kanie, S.; Koike, S.; Ikeyama, Y.; Ozaki, T.; Okumura, H. Carbohydrate binding specificity of recombinant human macrophage β-glucan receptor dectin-1. Biosci. Biotechnol. Biochem. 2009, 73, 237–240. [Google Scholar] [CrossRef]
- Kankkunen, P.; Teirilä, L.; Rintahaka, J.; Alenius, H.; Wolff, H.; Matikainen, S. (1,3)-β-Glucans Activate Both Dectin-1 and NLRP3 Inflammasome in Human Macrophages. J. Immunol. 2010, 184, 6335–6342. [Google Scholar] [CrossRef]
- Evans, M.; Falcone, P.H.; Crowley, D.C.; Sulley, A.M.; Campbell, M.; Zakaria, N.; Lasrado, J.A.; Fritz, E.P.; Herrlinger, K.A. Effect of a Euglena gracilis Fermentate on Immune Function in Healthy, Active Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2926. [Google Scholar] [CrossRef]
- Ishiguro, S.; Upreti, D.; Robben, N.; Burghart, R.; Loyd, M.; Ogun, D.; Le, T.; Delzeit, J.; Nakashima, A.; Thakkar, R.; et al. Water extract from Euglena gracilis prevents lung carcinoma growth in mice by attenuation of the myeloid-derived cell population. Biomed. Pharmacother. 2020, 127, 110166. [Google Scholar] [CrossRef]
- Riera, C.E.; Dillin, A. Emerging Role of Sensory Perception in Aging and Metabolism. Trends Endocrinol. Metab. 2016, 27, 294–303. [Google Scholar] [CrossRef]
- Christianson, J.A.; Davis, B.M. The role of visceral afferents in disease. In Translational Pain Research: From Mouse to Man; CRC Press: Bosca Raton, FL, USA, 2009; pp. 51–76. [Google Scholar]
- Assas, B.M.; Pennock, J.I.; Miyan, J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef]
- Riera, C.E.; Huising, M.O.; Follett, P.; Leblanc, M.; Halloran, J.; Van Andel, R.; De Magalhaes Filho, C.D.; Merkwirth, C.; Dillin, A. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 2014, 157, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Benemei, S.; Nicoletti, P.; Capone, J.G.; Geppetti, P. CGRP receptors in the control of pain and inflammation. Curr. Opin. Pharmacol. 2009, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Tsukamoto-Yasui, M.; Hara-Kimura, Y.; Inoue, N.; Nogusa, Y.; Okabe, Y.; Nagashima, K.; Kato, F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J. Appl. Physiol. 2011, 110, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Nomura, T.; Yamada, K.; Mochida, K. Genetic engineering strategies for Euglena gracilis and its industrial contribution to sustainable development goals: A review. Front. Bioeng. Biotechnol. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Van Sadelhoff, J.H.J.; Pardo, P.P.; Wu, J.; Garssen, J.; Van Bergenhenegouwen, J.; Hogenkamp, A.; Hartog, A.; Kraneveld, A.D. The gut-immune-brain axis in autism spectrum disorders; a focus on amino acids. Front. Endocrinol. (Lausanne) 2019, 10, 247. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, K.; Nakashima, A.; Murata, A.; Suzuki, K.; Adachi, T. Euglena Gracilis and β-Glucan Paramylon Induce Ca2+ Signaling in Intestinal Tract Epithelial, Immune, and Neural Cells. Nutrients 2020, 12, 2293. https://doi.org/10.3390/nu12082293
Yasuda K, Nakashima A, Murata A, Suzuki K, Adachi T. Euglena Gracilis and β-Glucan Paramylon Induce Ca2+ Signaling in Intestinal Tract Epithelial, Immune, and Neural Cells. Nutrients. 2020; 12(8):2293. https://doi.org/10.3390/nu12082293
Chicago/Turabian StyleYasuda, Kosuke, Ayaka Nakashima, Ako Murata, Kengo Suzuki, and Takahiro Adachi. 2020. "Euglena Gracilis and β-Glucan Paramylon Induce Ca2+ Signaling in Intestinal Tract Epithelial, Immune, and Neural Cells" Nutrients 12, no. 8: 2293. https://doi.org/10.3390/nu12082293
APA StyleYasuda, K., Nakashima, A., Murata, A., Suzuki, K., & Adachi, T. (2020). Euglena Gracilis and β-Glucan Paramylon Induce Ca2+ Signaling in Intestinal Tract Epithelial, Immune, and Neural Cells. Nutrients, 12(8), 2293. https://doi.org/10.3390/nu12082293



