Differential Effects of Chronic Ingestion of Refined Sugars versus Natural Sweeteners on Insulin Resistance and Hepatic Steatosis in a Rat Model of Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sweeteners
2.2. Experimental Design
2.3. Indirect Calorimetry
2.4. Oral Glucose Tolerance Test
2.5. Biochemistry
2.6. Liver Histology
2.7. Statistical Analyses
3. Results
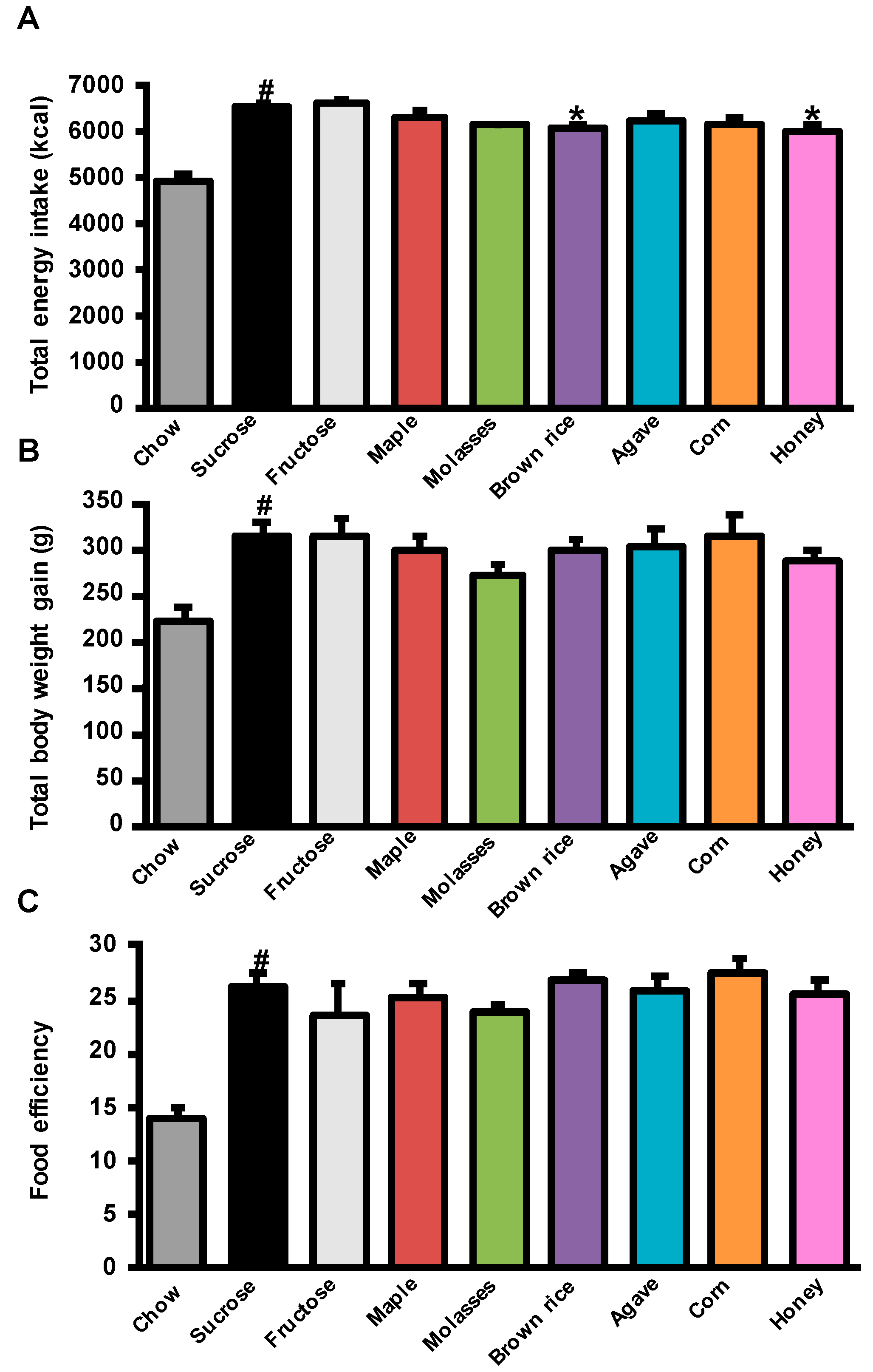
3.1. Effects of Added Refined and Natural Sweeteners on Energy Intake, Body Weight Gain, and Adiposity
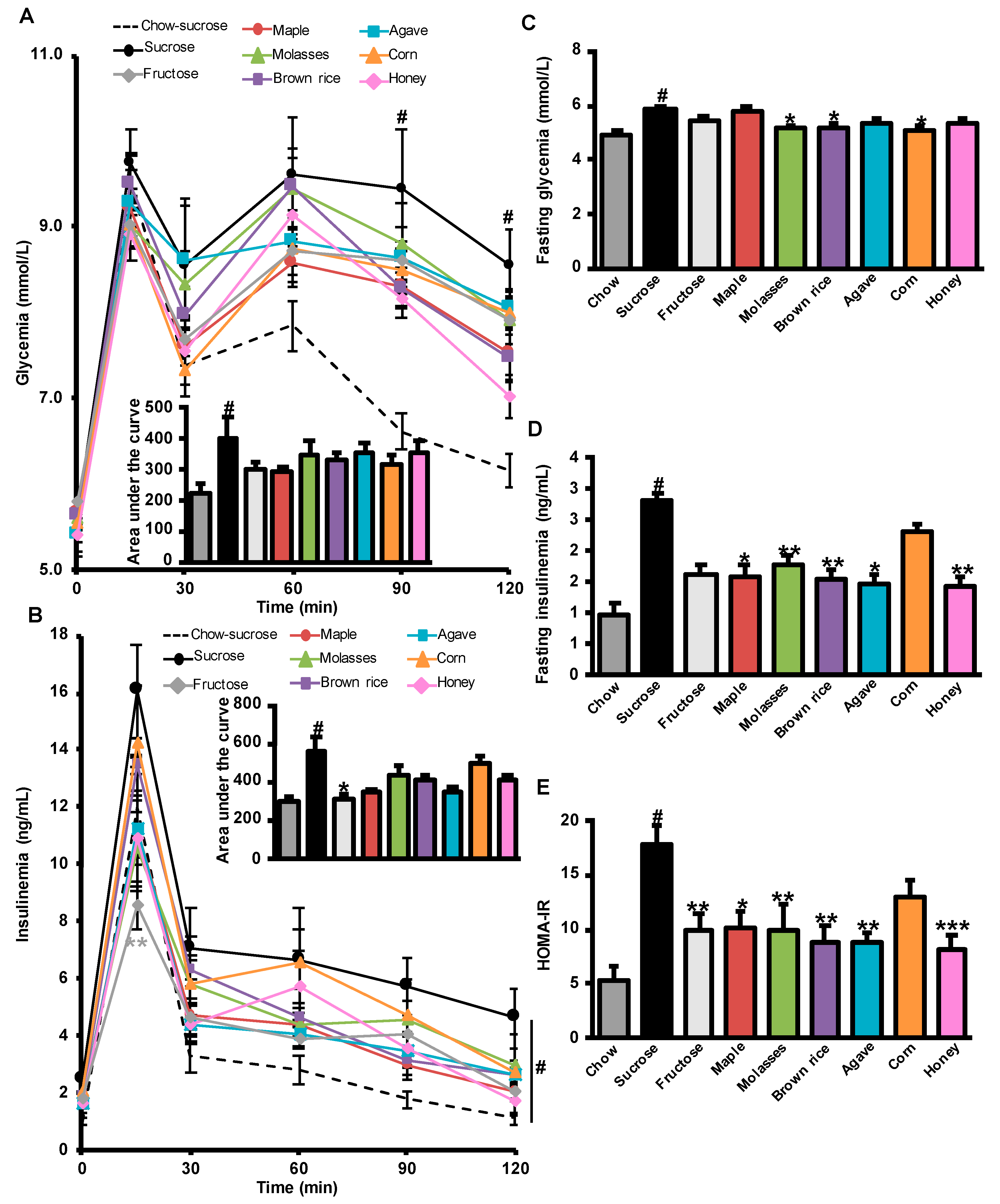
3.2. Effects of Added Refined and Natural Sweeteners on Diet-Induced Insulin Resistance
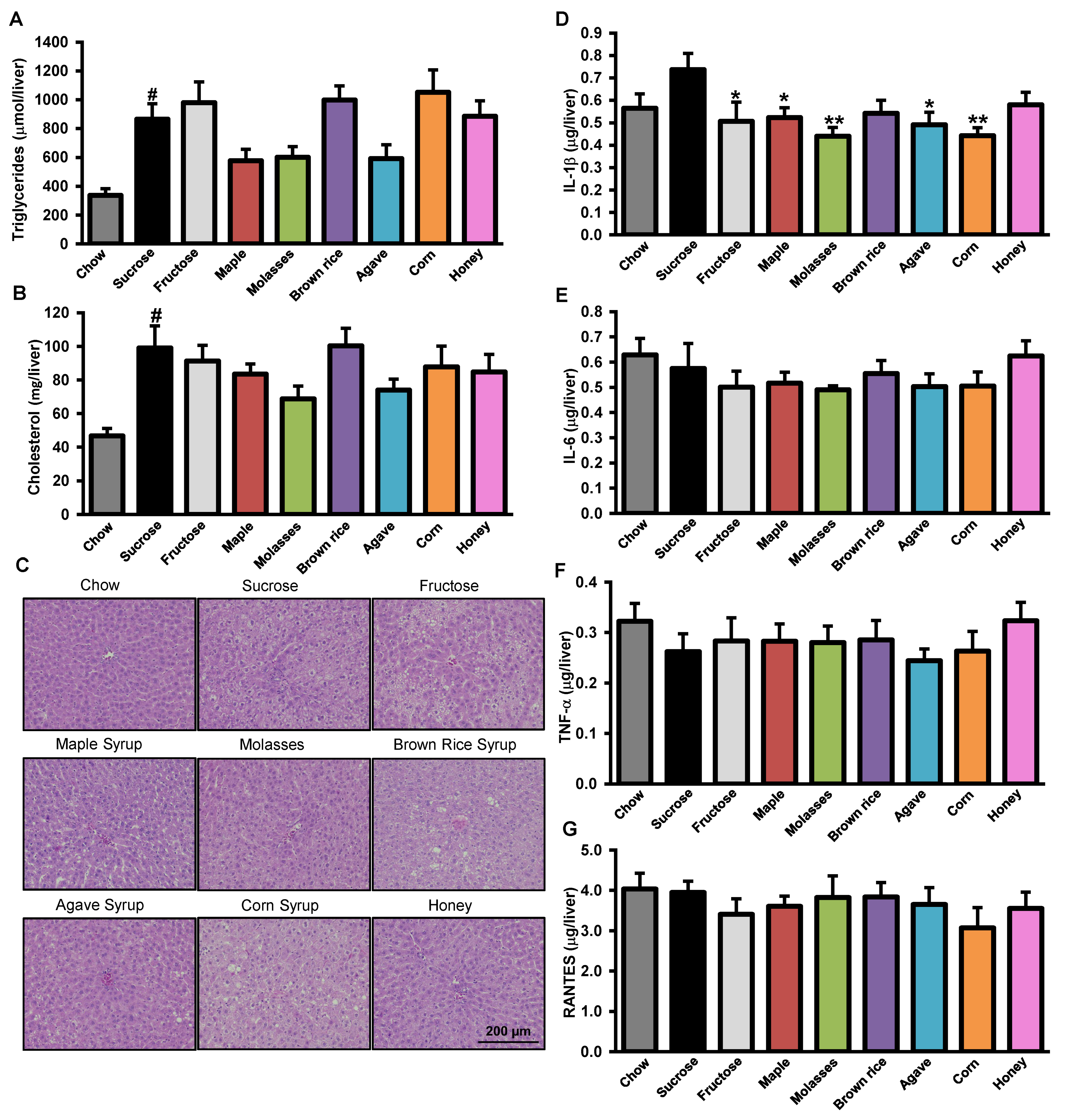
3.3. Effects of Added Refined and Natural Sweeteners on Liver Steatosis and Inflammation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 July 2020).
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. Public health: The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes a meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.; Gunn, P.; Wittekind, A.; Cottrell, R. The effects of sucrose on metabolic health: A systematic review of human intervention studies in healthy adults. Crit. Rev. Food Sci. Nutr. 2013, 53, 591–614. [Google Scholar] [CrossRef]
- Acton, R.B.; Vanderlee, L.; Hobin, E.P.; Hammond, D. Added sugar in the packaged foods and beverages available at a major Canadian retailer in 2015: A descriptive analysis. CMAJ Open 2017, 5, E1–E6. [Google Scholar] [CrossRef]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Havel, P.J. Fructose consumption: Potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr. Opin. Lipidol. 2008, 19, 16. [Google Scholar] [CrossRef]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2010, 299. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 2009, 119. [Google Scholar] [CrossRef]
- Softic, S.; Gupta, M.K.; Wang, G.X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Invest. 2017, 127, 4059–4074. [Google Scholar] [CrossRef] [PubMed]
- Baena, M.; Sanguesa, G.; Davalos, A.; Latasa, M.J.; Sala-Vila, A.; Sanchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Sci. Rep. 2016, 6, 26149. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Mu, W.; Roncal, C.; Sautin, Y.Y.; Abdelmalek, M.; Reungjui, S.; Le, M.; Nakagawa, T.; Lan, H.Y.; Yu, X.; et al. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur. J. Nutr. 2010, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sheludiakova, A.; Rooney, K.; Boakes, R.A. Metabolic and behavioural effects of sucrose and fructose/glucose drinks in the rat. Eur. J. Nutr. 2012, 51, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; Frese, M.; Romero, J.; Cunningham, J.H.; Mills, K.E. Fructose replacement of glucose or sucrose in food or beverages lowers postprandial glucose and insulin without raising triglycerides: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 506–518. [Google Scholar] [CrossRef]
- Evans, R.A.; Frese, M.; Romero, J.; Cunningham, J.H.; Mills, K.E. Chronic fructose substitution for glucose or sucrose in food or beverages has little effect on fasting blood glucose, insulin, or triglycerides: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 519–529. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: Comparison with dextrose and sucrose. J. Med. Food 2004, 7, 100–107. [Google Scholar] [CrossRef]
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.M.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S.M.; Aghasizadeh, R.; et al. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. ScientificWorldJournal 2008, 8, 463–469. [Google Scholar] [CrossRef]
- Chepulis, L.; Starkey, N. The long-term effects of feeding honey compared with sucrose and a sugar-free diet on weight gain, lipid profiles, and DEXA measurements in rats. J. Food Sci. 2008, 73, H1–H7. [Google Scholar] [CrossRef]
- Nemoseck, T.M.; Carmody, E.G.; Furchner-Evanson, A.; Gleason, M.; Li, A.; Potter, H.; Rezende, L.M.; Lane, K.J.; Kern, M. Honey promotes lower weight gain, adiposity, and triglycerides than sucrose in rats. Nutr. Res. 2011, 31, 55–60. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Taga, A. Comparison of the enhancement of plasma glucose levels in type 2 diabetes Otsuka Long-Evans Tokushima Fatty rats by oral administration of sucrose or maple syrup. J. Oleo. Sci. 2013, 62, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yamamoto, T.; Tanabe, W.; Ito, Y.; Kurabuchi, S.; Mitamura, K.; Taga, A. Changes in plasma glucose in Otsuka Long-Evans Tokushima Fatty rats after oral administration of maple syrup. J. Oleo. Sci. 2015, 64, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Holloway, B.; Nemoseck, T.; Cole, S.; Petrisko, Y.; Hong, M.Y.; Kern, M. Effects of agave nectar versus sucrose on weight gain, adiposity, blood glucose, insulin, and lipid responses in mice. J. Med. Food 2014, 17, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and Quantification of Antioxidant Components of Honeys from Various Floral Sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Seeram, N.P. Quebecol, a novel phenolic compound isolated from Canadian maple syrup. J. Funct. Foods 2011, 3, 125–128. [Google Scholar] [CrossRef]
- Ball, D. The chemical composition of maple syrup. J. Chem. Ed. 2007, 84, 1647–1650. [Google Scholar] [CrossRef]
- St-Pierre, P.; Pilon, G.; Dumais, V.; Dion, C.; Dubois, M.-J.; Dubé, P.; Desjardins, Y.; Marette, A. Comparative analysis of maple syrup to other natural sweeteners and evaluation of their metabolic responses in healthy rats. J. Funct. Foods 2014, 11, 460–471. [Google Scholar] [CrossRef]
- Jain, R.; Venkatasubramanian, P. Sugarcane Molasses—A Potential Dietary Supplement in the Management of Iron Deficiency Anemia. J. Diet. Suppl. 2017, 14, 589–598. [Google Scholar] [CrossRef]
- Kleiber, M. Body size and metabolic rate. Physiol. Rev. 1947, 27, 511–541. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Berres, M.L.; Koenen, R.R.; Rueland, A.; Zaldivar, M.M.; Heinrichs, D.; Sahin, H.; Schmitz, P.; Streetz, K.L.; Berg, T.; Gassler, N.; et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J. Clin. Invest. 2010, 120, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Yamashita, H.; Hiemori, M.; Inagaki, E.; Kimoto, M.; Okamoto, M.; Tsuji, H.; Memon, A.N.; Mohammadio, A.; Natori, Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J. Nutr. Sci. Vitam. 2007, 53, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitam. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and Glucose. J. Biol. Chem. 2002, 277, 15252–15260. [Google Scholar] [CrossRef]
- Apostolidis, E.; Li, L.; Lee, C.; Seeram, N.P. In vitro evaluation of phenolic-enriched maple syrup extracts for inhibition of carbohydrate hydrolyzing enzymes relevant to type 2 diabetes management. J. Funct. Foods 2011, 3, 100–106. [Google Scholar] [CrossRef]
- Ahmad, A.; Azim, M.K.; Mesaik, M.A.; Khan, R.A. Natural honey modulates physiological glycemic response compared to simulated honey and D-glucose. J. Food Sci. 2008, 73, H165–H167. [Google Scholar] [CrossRef]
- Munstedt, K.; Sheybani, B.; Hauenschild, A.; Bruggmann, D.; Bretzel, R.G.; Winter, D. Effects of basswood honey, honey-comparable glucose-fructose solution, and oral glucose tolerance test solution on serum insulin, glucose, and C-peptide concentrations in healthy subjects. J. Med. Food 2008, 11, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Van De Wier, B.; Koek, G.H.; Bast, A.; Haenen, G.R.M.M. The Potential of Flavonoids in the Treatment of Non-alcoholic Fatty Liver Disease. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonne, S.; Pilon, G.; Fournier, M.; Lecours, M.A.; Desjardins, Y.; Roy, D.; et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef]
- Kamari, Y.; Shaish, A.; Vax, E.; Shemesh, S.; Kandel-Kfir, M.; Arbel, Y.; Olteanu, S.; Barshack, I.; Dotan, S.; Voronov, E.; et al. Lack of interleukin-1alpha or interleukin-1beta inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J. Hepatol. 2011, 55, 1086–1094. [Google Scholar]
- Stienstra, R.; van Diepen, J.A.; Tack, C.J.; Zaki, M.H.; van de Veerdonk, F.L.; Perera, D.; Neale, G.A.; Hooiveld, G.J.; Hijmans, A.; Vroegrijk, I.; et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 15324–15329. [Google Scholar] [CrossRef]
- Rosas-Villegas, A.; Sanchez-Tapia, M.; Avila-Nava, A.; Ramirez, V.; Tovar, A.R.; Torres, N. Differential Effect of Sucrose and Fructose in Combination with a High Fat Diet on Intestinal Microbiota and Kidney Oxidative Stress. Nutrients 2017, 9, 393. [Google Scholar] [CrossRef]
- Kamei, A.; Watanabe, Y.; Shinozaki, F.; Yasuoka, A.; Kondo, T.; Ishijima, T.; Toyoda, T.; Arai, S.; Abe, K. Administration of a maple syrup extract to mitigate their hepatic inflammation induced by a high-fat diet: A transcriptome analysis. Biosci. Biotechnol. Biochem. 2015, 79, 1893–1897. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Hosseinidoust, Z.; Tufenkji, N. Polyphenolic extract from maple syrup potentiates antibiotic susceptibility and reduces biofilm formation of pathogenic bacteria. Appl. Environ. Microbiol. 2015, 81, 3782–3792. [Google Scholar] [CrossRef]
- Sun, S.Z.; Empie, M.W. Fructose metabolism in humans—what isotopic tracer studies tell us. Nutr. Metab. 2012, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Le, K.A. Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin. Res. Hepatol. Gastroenterol. 2012, 36, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Griffen, S.C.; Bremer, A.A.; Vink, R.G.; Schaefer, E.J.; Nakajima, K.; Schwarz, J.M.; Beysen, C.; Berglund, L.; Keim, N.L.; et al. Metabolic responses to prolonged consumption of glucose- and fructose-sweetened beverages are not associated with postprandial or 24-h glucose and insulin excursions. Am. J. Clin. Nutr. 2011, 94. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, T.J.; Lowndes, J.; Sinnett, S.; Rippe, J.M. Fructose Containing Sugars at Normal Levels of Consumption Do Not Effect Adversely Components of the Metabolic Syndrome and Risk Factors for Cardiovascular Disease. Nutrients 2016, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 2018, 27, 351–361. [Google Scholar] [CrossRef]
- Laughlin, M.R. Normal roles for dietary fructose in carbohydrate metabolism. Nutrients 2014, 6, 3117–3129. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef]
| Body (g) | Liver (g) | Pancreas (g) | iWAT (g) | eWAT (g) | BAT (g) | ||
|---|---|---|---|---|---|---|---|
| Chow | Sucrose | 557.3 ± 20.2 | 14.41 ± 1.02 | 1.58 ± 0.09 | 7.95 ± 1.58 | 11.74 ± 0.86 | 0.56 ± 0.05 |
| HFHS | Sucrose | 650.3 ± 17.9 # | 16.23 ± 0.81 | 1.27 ± 0.06 # | 16.45 ± 1.57 # | 23.84 ± 1.53 # | 0.82 ± 0.03 # |
| Fructose | 652.2 ± 20.9 | 16.40 ± 0.81 | 1.29 ± 0.07 | 12.00 ± 1.17 | 21.68 ± 1.53 | 0.77 ± 0.07 | |
| Maple | 633.9 ± 14.8 | 15.18 ± 0.35 | 1.36 ± 0.06 | 14.56 ± 1.47 | 22.03 ± 1.48 | 0.63 ± 0.03 ** | |
| Molasses | 606.4 ± 13.8 | 14.82 ± 0.52 | 1.45 ± 0.04 | 11.56 ± 1.18 | 18.23 ± 1.25 | 0.66 ± 0.04 * | |
| Brown Rice | 633.3 ± 15.9 | 15.49 ± 0.73 | 1.36 ± 0.07 | 15.02 ± 1.55 | 22.91 ± 1.55 | 0.74 ± 0.02 | |
| Agave | 638.5 ± 17.1 | 15.32 ± 0.57 | 1.33 ± 0.06 | 12.97 ± 1.55 | 23.65 ± 2.33 | 0.73 ± 0.06 | |
| Corn | 639.3 ± 12.8 | 15.14 ± 0.72 | 1.34 ± 0.06 | 13.96 ± 1.46 | 20.78 ± 1.08 | 0.69 ± 0.05 | |
| Honey | 621.7 ± 12.6 | 15.26 ± 0.41 | 1.49 ± 0.06 | 12.01 ± 1.56 | 18.91 ± 1.77 | 0.63 ± 0.05 * |
| O2 24 h (mL/kg0.75/h) | CO2 24 h (mL/kg0.75/h) | EE 24 h (cal/h/g0.75) | RER 24 h | ||
|---|---|---|---|---|---|
| Chow | Sucrose | 811 ± 8 | 772 ± 17 | 22.8 ± 0.4 | 0.95 ± 0.01 |
| HFHS | Sucrose | 770 ± 13 # | 638 ± 13 ### | 21.2 ± 0.4 # | 0.82 ± 0.00 ### |
| Fructose | 811 ± 14 | 670 ± 14 | 22.1 ± 0.4 | 0.83 ± 0.00 | |
| Maple | 793 ± 8 | 647 ± 7 | 21.5 ± 0.2 | 0.82 ± 0.00 | |
| Molasses | 813 ± 19 | 653 ± 13 | 22.1 ± 0.5 | 0.80 ± 0.00 | |
| Brown Rice | 756 ± 14 | 616 ± 16 | 20.6 ± 0.4 | 0.78 ± 0.03 | |
| Agave | 756 ± 44 | 654 ± 10 | 21.6 ± 0.3 | 0.82 ± 0.01 | |
| Corn | 780 ± 12 | 650 ± 17 | 21.2 ± 0.3 | 0.83 ± 0.01 | |
| Honey | 798 ± 39 | 669 ± 21 | 22.3 ± 0.7 | 0.82 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle, M.; St-Pierre, P.; Pilon, G.; Marette, A. Differential Effects of Chronic Ingestion of Refined Sugars versus Natural Sweeteners on Insulin Resistance and Hepatic Steatosis in a Rat Model of Diet-Induced Obesity. Nutrients 2020, 12, 2292. https://doi.org/10.3390/nu12082292
Valle M, St-Pierre P, Pilon G, Marette A. Differential Effects of Chronic Ingestion of Refined Sugars versus Natural Sweeteners on Insulin Resistance and Hepatic Steatosis in a Rat Model of Diet-Induced Obesity. Nutrients. 2020; 12(8):2292. https://doi.org/10.3390/nu12082292
Chicago/Turabian StyleValle, Marion, Philippe St-Pierre, Geneviève Pilon, and André Marette. 2020. "Differential Effects of Chronic Ingestion of Refined Sugars versus Natural Sweeteners on Insulin Resistance and Hepatic Steatosis in a Rat Model of Diet-Induced Obesity" Nutrients 12, no. 8: 2292. https://doi.org/10.3390/nu12082292
APA StyleValle, M., St-Pierre, P., Pilon, G., & Marette, A. (2020). Differential Effects of Chronic Ingestion of Refined Sugars versus Natural Sweeteners on Insulin Resistance and Hepatic Steatosis in a Rat Model of Diet-Induced Obesity. Nutrients, 12(8), 2292. https://doi.org/10.3390/nu12082292





