Periconceptional Folic Acid Supplementation and the Risk of Spontaneous Abortion among Women Who Prepared to Conceive: Impact of Supplementation Initiation Timing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. National Free Pre-Conception Health Examination Project
2.3. Exposure, Outcomes, and Covariates
2.4. Statistical Analysis
2.5. Ethics Approval and Consent to Participate
3. Results
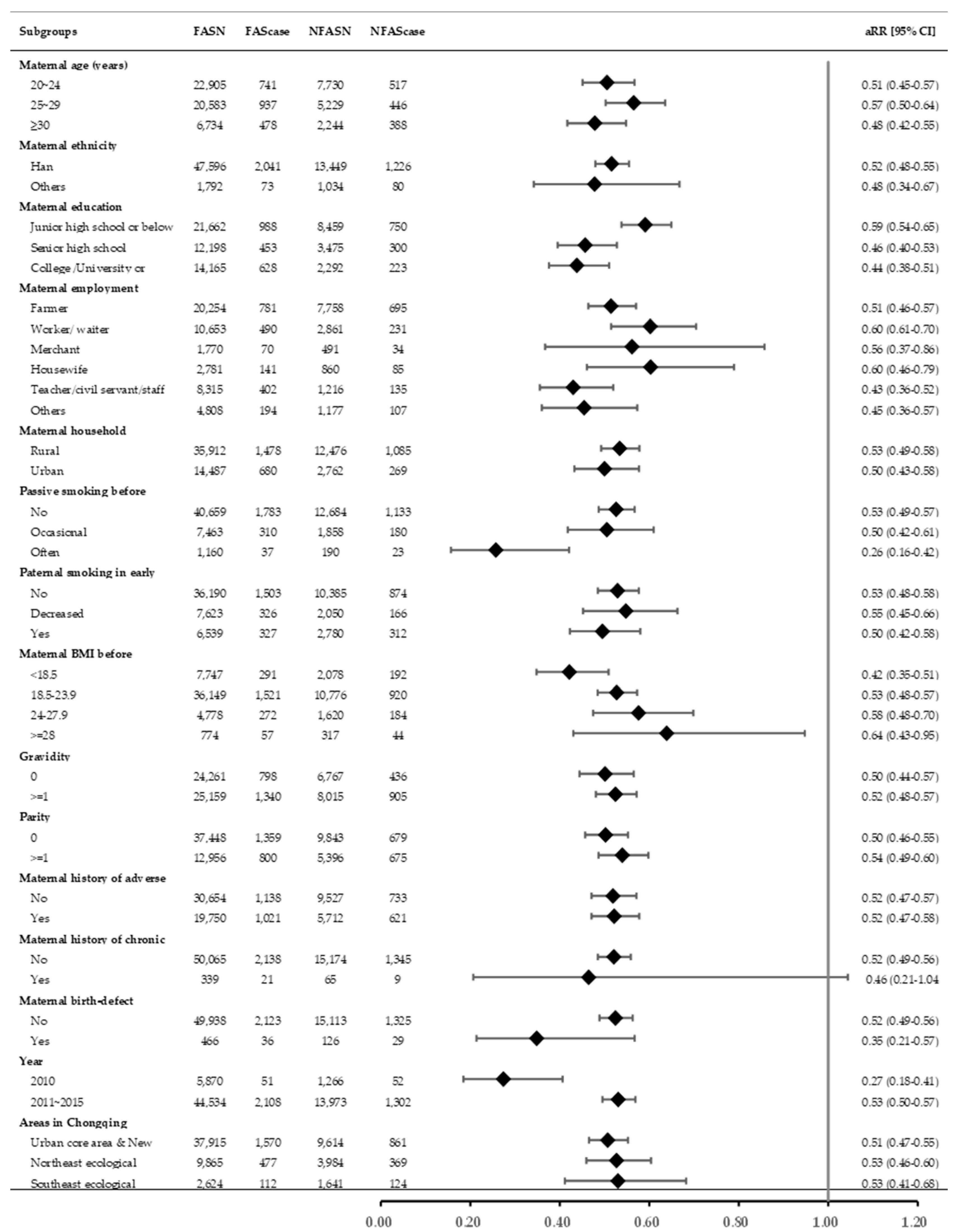
3.1. Maternal Characteristics
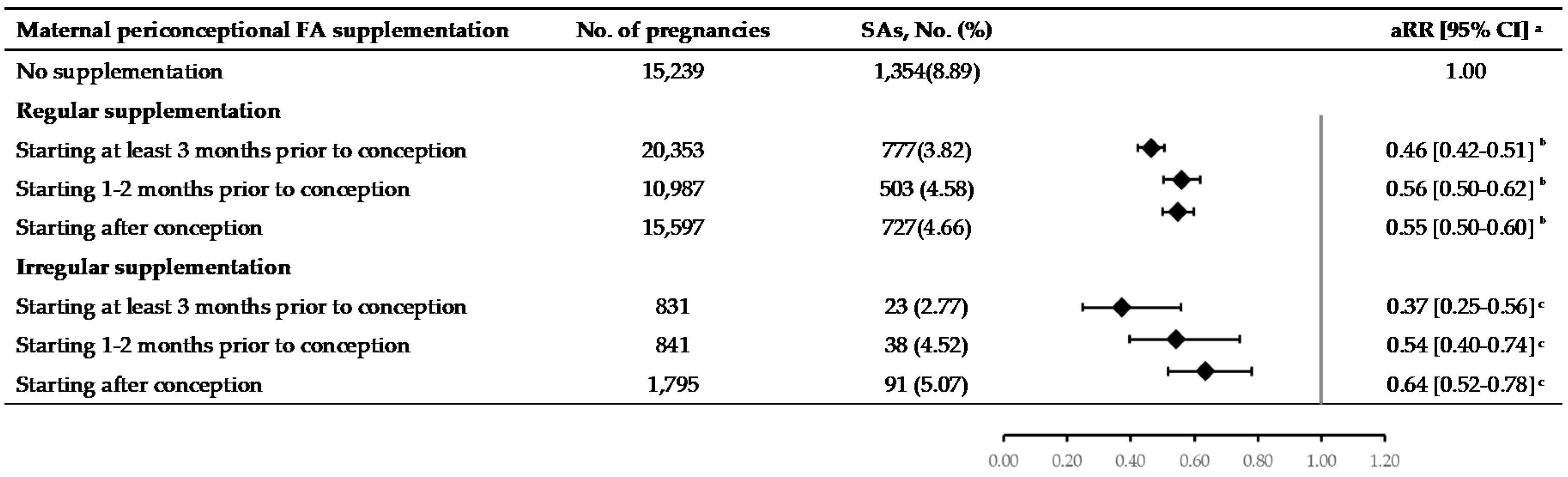
3.2. The Association between Maternal FA Supplementation during Periconceptional Period and the Risk of SA
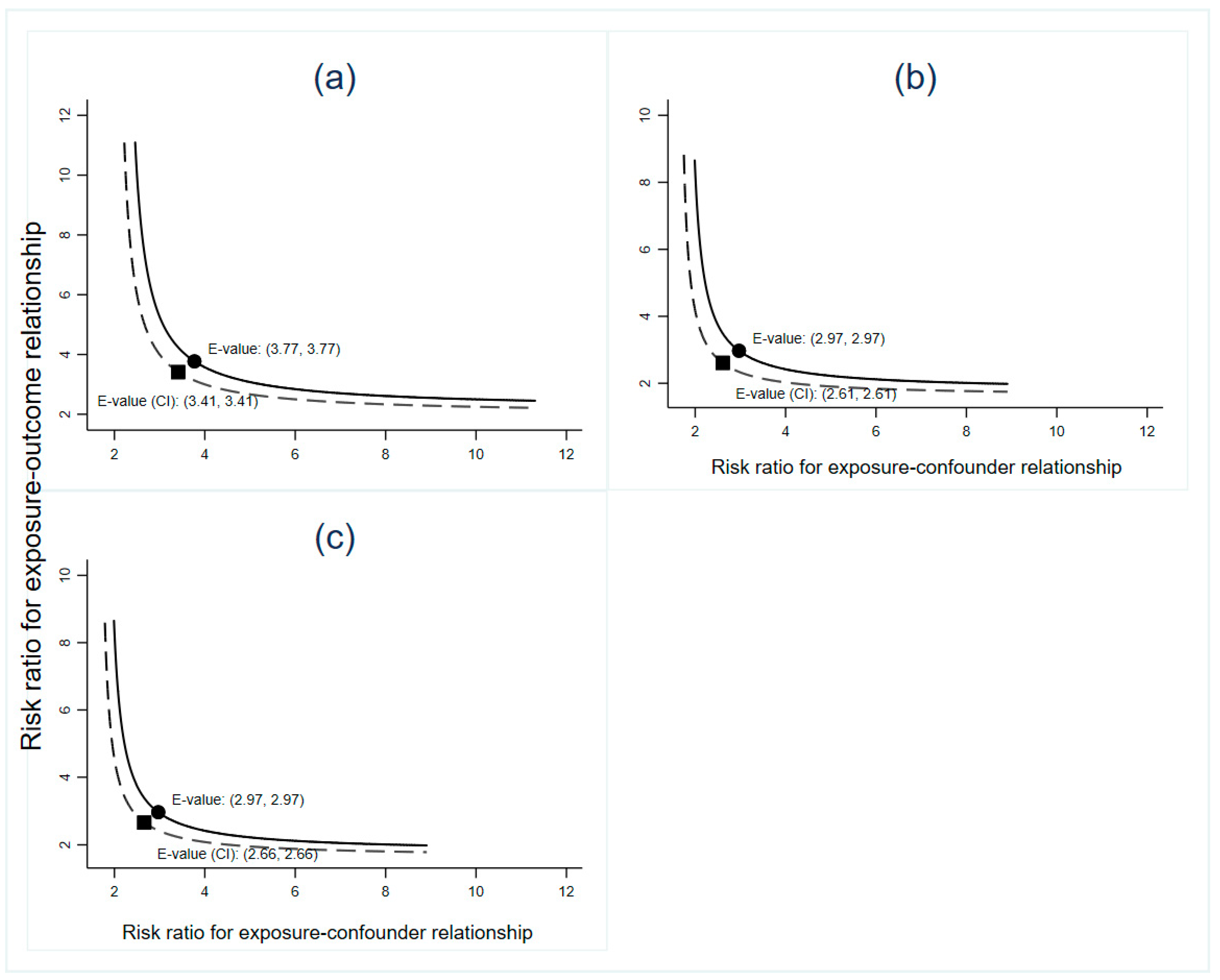
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griebel, C.P.; Halvorsen, J.; Golemon, T.B.; Day, A.A. Management of spontaneous abortion. Am. Fam. Physician 2005, 72, 1243–1250. [Google Scholar] [PubMed]
- Agenor, A.; Bhattacharya, S. Infertility and miscarriage: Common pathways in manifestation and management. Women’s Health 2015, 11, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Brown, S. Miscarriage and its associations. Semin. Reprod. Med. 2008, 26, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.J.; Weinberg, C.R.; O’Connor, J.F.; Baird, D.D.; Schlatterer, J.P.; Canfield, R.E.; Armstrong, E.G.; Nisula, B.C. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988, 319, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Polly, I.; Kalder, M.; Kostev, K. Prevalence of depression, anxiety, and adjustment disorders in women with spontaneous abortion in Germany—A retrospective cohort study. Psychiatry Res. 2017, 258, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Goddijn, M.; Leschot, N.J. Genetic aspects of miscarriage. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C. Biochemical role of folate in cellular metabolism. Clin. Res. Regul. Aff. 2001, 18, 161–180. [Google Scholar] [CrossRef]
- Wald, N.J.; Morris, J.K.; Blakemore, C. Public health failure in the prevention of neural tube defects: Time to abandon the tolerable upper intake level of folate. Public Health Rev. 2018, 39, 2. [Google Scholar] [CrossRef]
- Van Gool, J.D.; Hirche, H.; Lax, H.; De Schaepdrijver, L. Folic acid and primary prevention of neural tube defects: A review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef]
- Wilson, R.D.; Genetics, C.; Wilson, R.D.; Audibert, F.; Brock, J.A.; Carroll, J.; Cartier, L.; Gagnon, A.; Johnson, J.A.; Langlois, S.; et al. Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies. J. Obstet. Gynaecol. Can. 2015, 37, 534–552. [Google Scholar] [CrossRef]
- Chen, C.P. Chromosomal abnormalities associated with neural tube defects (I): Full aneuploidy. Taiwan J. Obstet. Gynecol. 2007, 46, 325–335. [Google Scholar] [CrossRef]
- Hook, E.B.; Czeizel, A.E. Can terathanasia explain the protective effect of folic-acid supplementation on birth defects? Lancet 1997, 350, 513–515. [Google Scholar] [CrossRef]
- Gindler, J.; Li, Z.; Berry, R.J.; Zheng, J.; Correa, A.; Sun, X.; Wong, L.; Cheng, L.; Erickson, J.D.; Wang, Y.; et al. Folic acid supplements during pregnancy and risk of miscarriage. Lancet 2001, 358, 796–800. [Google Scholar] [CrossRef]
- Vila-Nova, C.; Wehby, G.L.; Queiros, F.C.; Chakraborty, H.; Felix, T.M.; Goco, N.; Moore, J.; Gewehr, E.V.; Lins, L.; Affonso, C.M.; et al. Periconceptional use of folic acid and risk of miscarriage—Findings of the Oral Cleft Prevention Program in Brazil. J. Perinat. Med. 2013, 41, 461–466. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Pan, A.; Hu, F.B.; Ma, X. Folic acid supplementation, birth defects, and adverse pregnancy outcomes in Chinese women: A population-based mega-cohort study. Lancet 2016, 388, S91. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L.; Zhang, Y.; Jin, L.; Li, Z.; Zhang, L.; Meng, Q.; Ye, R.; Wang, L.; Ren, A. Plasma folate levels in early to mid pregnancy after a nation-wide folic acid supplementation program in areas with high and low prevalence of neural tube defects in China. Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 501–508. [Google Scholar] [CrossRef]
- Zheng, J.S.; Guan, Y.; Zhao, Y.; Zhao, W.; Tang, X.; Chen, H.; Xu, M.; Wu, L.; Zhu, S.; Liu, H.; et al. Pre-conceptional intake of folic acid supplements is inversely associated with risk of preterm birth and small-for-gestational-age birth: A prospective cohort study. Br. J. Nutr. 2016, 115, 509–516. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Zhang, S.; Liu, M.; Wang, Q.; Shen, H.; Zhang, Y. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: A population-based cohort study. Lancet Glob. Health 2017, 5, e624–e632. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Project Management Plan on Folic Acid Supplement in Neural Tube Defects. 2009. Available online: http://www.nhc.gov.cn/wjw/gfxwj/201304/02c3c3d51117464aa054c08de04b0468.shtml (accessed on 29 June 2009).
- Zhang, J.P. Pregnancy complication. In Obstetrics and Gynecology, 9th ed.; Xie, X., Kong, B.H., Duan, T., Eds.; People’s Medical Publishing House: Beijing, China, 2018; pp. 70–74, 140–146. [Google Scholar]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- Wald, N. Folic acid and the prevention of neural tube defects. Ann. N. Y. Acad. Sci. 1993, 678, 112–129. [Google Scholar] [CrossRef]
- Wilson, R.D.; Sogc Genetics, C.; Special, C. Prenatal screening, diagnosis, and pregnancy management of fetal neural tube defects. J. Obstet. Gynaecol. Can. 2014, 36, 927–939. [Google Scholar] [CrossRef]
- Liu, B.; Gao, E. Risk Factors for Spontaneous Abortion of Chinese Married Women at Reproductive Age. China Public Health 2002, 18, 890–892. [Google Scholar]
- Wang, L. Association between Periconception Environmental Risk Factors and Spontaneous Abortion among Reproductive Aged Couples in Rural China. Ph.D. Thesis, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China, 2018. [Google Scholar]
- Crider, K.S.; Devine, O.; Hao, L.; Dowling, N.F.; Li, S.; Molloy, A.M.; Li, Z.; Zhu, J.; Berry, R.J. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 2014, 349, g4554. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, L.; Jin, L.; Li, Z.; Ren, A. Plasma folate levels and associated factors in women planning to become pregnant in a population with high prevalence of neural tube defects. Birth Defects Res. 2017, 109, 1039–1047. [Google Scholar] [CrossRef]
- Stern, J.; Salih Joelsson, L.; Tyden, T.; Berglund, A.; Ekstrand, M.; Hegaard, H.; Aarts, C.; Rosenblad, A.; Larsson, M.; Kristiansson, P. Is pregnancy planning associated with background characteristics and pregnancy-planning behavior? Acta Obstet. Gynecol. Scand. 2016, 95, 182–189. [Google Scholar] [CrossRef]
- da Rosa, E.B.; Silveira, D.B.; Correia, J.D.; Grapiglia, C.G.; de Moraes, S.A.G.; Nunes, M.R.; Zen, T.D.; Oliveira, C.A.; Correia, E.P.E.; Alcay, C.T.; et al. Periconceptional folic acid supplementation in Southern Brazil: Why are not we doing it right? Am. J. Med. Genet. A 2019, 179, 20–28. [Google Scholar] [CrossRef]
- Kurzawinska, G.; Magielda, J.; Romala, A.; Bartkowiak-Wieczorek, J.; Barlik, M.; Drews, K.; Ozarowski, M.; Seremak-Mrozikiewicz, A. Demographic factors determining folic acid supplementation in pregnant and childbearing age women. Ginekol. Polska 2018, 89, 211–216. [Google Scholar] [CrossRef]
- McGuire, M.; Cleary, B.; Sahm, L.; Murphy, D.J. Prevalence and predictors of periconceptional folic acid uptake--prospective cohort study in an Irish urban obstetric population. Hum. Reprod. 2010, 25, 535–543. [Google Scholar] [CrossRef]
- Xing, X.Y.; Tao, F.B.; Hao, J.H.; Huang, K.; Huang, Z.H.; Zhu, X.M.; Xiao, L.M.; Cheng, D.J.; Su, P.Y.; Zhu, P.; et al. Periconceptional folic acid supplementation among women attending antenatal clinic in Anhui, China: Data from a population-based cohort study. Midwifery 2012, 28, 291–297. [Google Scholar] [CrossRef]
- Tuenter, A.; Bautista Nino, P.K.; Vitezova, A.; Pantavos, A.; Bramer, W.M.; Franco, O.H.; Felix, J.F. Folate, vitamin B12, and homocysteine in smoking-exposed pregnant women: A systematic review. Matern. Child Nutr. 2019, 15, e12675. [Google Scholar] [CrossRef]
- Bakker, R.; Timmermans, S.; Steegers, E.A.; Hofman, A.; Jaddoe, V.W. Folic acid supplements modify the adverse effects of maternal smoking on fetal growth and neonatal complications. J. Nutr. 2011, 141, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Lambrot, R.; Xu, C.; Saint-Phar, S.; Chountalos, G.; Cohen, T.; Paquet, M.; Suderman, M.; Hallett, M.; Kimmins, S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 2013, 4, 2889. [Google Scholar] [CrossRef] [PubMed]
- Hollis, N.D.; Allen, E.G.; Oliver, T.R.; Tinker, S.W.; Druschel, C.; Hobbs, C.A.; O’Leary, L.A.; Romitti, P.A.; Royle, M.H.; Torfs, C.P.; et al. Preconception folic acid supplementation and risk for chromosome 21 nondisjunction: A report from the National Down Syndrome Project. Am. J. Med. Genet. A 2013, 161, 438–444. [Google Scholar] [CrossRef]
- Kharb, S.; Aggarwal, D.; Bala, J.; Nanda, S. Evaluation of Homocysteine, Vitamin B12 and Folic Acid Levels During all the Trimesters in Pregnant and Preeclamptic Womens. Curr. Hypertens. Rev. 2016, 12, 234–238. [Google Scholar] [CrossRef] [PubMed]
| Pregnancy Outcomes | Starting at Least 3 Months before Pregnancy (N = 21,184), No. (%) | Starting 1–2 Months before Pregnancy (N = 11,828), No. (%) | Starting after Conception (N = 17,392), No. (%) | No FA Supplementation (N = 15,239), No. (%) | Total (N = 65,643), No. (%) |
|---|---|---|---|---|---|
| Live birth | 19,988 (94.35) | 11,046 (93.39) | 16,122 (92.70) | 12,806 (84.03) | 59,962 (91.35) |
| Spontaneous abortion | 800 (3.78) | 541 (4.57) | 818 (4.70) | 1354 (8.89) | 3513 (5.35) |
| Induced abortion/Stillbirth | 396 (1.87) | 241 (2.04) | 452 (2.60) | 1079 (7.08) | 2168 (3.30) |
| Maternal Periconceptional FA Supplementation | No. of Pregnancies | SAs, No. (%) | cRR [95% CI] | aRR [95% CI] a |
|---|---|---|---|---|
| No supplementation | 15,239 | 1354 (8.89) | 1.00 | 1.00 |
| Having supplementation | 50,404 | 2159 (4.28) | 0.48 [0.45–0.51] | 0.52 [0.48–0.56] |
| Starting at least 3 months prior to conception | 21,184 | 800 (3.78) | 0.43 [0.39–0.46] | 0.46 [0.42–0.50] |
| Starting 1–2 months prior to conception | 11,828 | 541 (4.57) | 0.51 [0.47–0.57] | 0.56 [0.50–0.62] |
| Starting after conception | 17,392 | 818 (4.70) | 0.53 [0.49–0.58] | 0.56 [0.51–0.61] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.-Y.; Yang, L.; Li, M.; Liu, J.; Zhu, Q.-X.; He, Y.; Zhou, W.-J. Periconceptional Folic Acid Supplementation and the Risk of Spontaneous Abortion among Women Who Prepared to Conceive: Impact of Supplementation Initiation Timing. Nutrients 2020, 12, 2264. https://doi.org/10.3390/nu12082264
Mao Y-Y, Yang L, Li M, Liu J, Zhu Q-X, He Y, Zhou W-J. Periconceptional Folic Acid Supplementation and the Risk of Spontaneous Abortion among Women Who Prepared to Conceive: Impact of Supplementation Initiation Timing. Nutrients. 2020; 12(8):2264. https://doi.org/10.3390/nu12082264
Chicago/Turabian StyleMao, Yan-Yan, Liu Yang, Min Li, Jun Liu, Qian-Xi Zhu, Yang He, and Wei-Jin Zhou. 2020. "Periconceptional Folic Acid Supplementation and the Risk of Spontaneous Abortion among Women Who Prepared to Conceive: Impact of Supplementation Initiation Timing" Nutrients 12, no. 8: 2264. https://doi.org/10.3390/nu12082264
APA StyleMao, Y.-Y., Yang, L., Li, M., Liu, J., Zhu, Q.-X., He, Y., & Zhou, W.-J. (2020). Periconceptional Folic Acid Supplementation and the Risk of Spontaneous Abortion among Women Who Prepared to Conceive: Impact of Supplementation Initiation Timing. Nutrients, 12(8), 2264. https://doi.org/10.3390/nu12082264





