Abstract
“Total” folate in blood has usually been measured to evaluate the folate status of pregnant women. However, folate is composed of many metabolites. The main substrate is 5-methyltetrahydrofolate (5-MTHF), with folic acid (FA) representing a very small component as an unmetabolized species in blood. We longitudinally evaluated 5-MTHF, FA and total homocysteine in maternal and cord blood from Japanese pregnant women. Subjects were 146 pregnant women who participated in the Chiba study of Mother and Child Health (C-MACH) prospective cohort study. Sera were obtained in early and late pregnancy, at delivery, and from cord blood. Species levels were measured by isotope-dilution mass spectrometry. Both 5-MTHF and FA levels were lower than reported levels from pregnant women in populations from countries with mandatory FA fortification. As gestational age progressed, serum 5-MTHF levels decreased, whereas serum FA levels were slightly reduced only at delivery compared to early pregnancy. A significant negative association between serum 5-MTHF and total homocysteine was shown at all examined times, but no associations with FA were evident. At delivery, cord 5-MTHF was significantly higher than maternal levels, while FA again showed no significant correlation. These results suggest that 5-MTHF is actively transported to the fetus through placental transporters and may reflect folate status during pregnancy as a physiologically important species.
1. Introduction
Folate is an essential micronutrient that mediates the transfer of one-carbon units and is involved in the biosynthesis of the thymidylates and purines that constitute nucleic acids, in the metabolism of some amino acids, and in methylation reactions of DNA, histone proteins and neurotransmitters [1]. Folate is essential for cell division, and is thus particularly important for fetal growth and the development of the uteroplacental organs, and folate requirements thus increase during pregnancy [2,3]. Maternal folate deficiency is associated with elevated homocysteine levels in blood and adverse pregnancy outcomes, such as congenital disorders, including neural tube defects (NTD) and pregnancy complications [1,4,5,6]. Folic acid (FA) is used as a supplement in the mandatory or voluntary fortification of certain foods. The FA contained in supplements and fortified foods is metabolized in the body to 5-methyltetrahydrofolate (5-MTHF), through the folate metabolic pathway [7]. In the cytoplasm, 5-MTHF supplies a methyl group to the homocysteine remethylation reaction for methionine synthesis, and this reaction acts to lower blood homocysteine levels [8].
A study of men and women aged between 29 and 86 years in the United States found that blood levels of 5-MTHF and FA were higher after fortification than before this measure was introduced [9]. Intervention studies have shown that when healthy adults continued to take supplements containing 400 µg/day of FA or FA-fortified foods for 5–14 weeks, FA was detected as an unmetabolized species in the blood. This is attributed to dihydrofolate reductase (DHFR), the rate-limiting enzyme in folate metabolism for reducing dihydrofolate to tetrahydrofolate, exceeding its capacity to metabolize FA [10,11,12,13]. One concern is that blood FA may have negative effects on the fetus [14,15]. Previous studies have not been consistent in the reported effects of excessive FA intake and the effects are unclear. [16]. In these studies, “total” folate in blood was measured when examining folate status in pregnant women, while blood FA levels were not measured [17]. Different folate species, such as FA and 5-MTHF, must therefore be measured separately.
Liquid chromatography-tandem mass spectrometry [18] (LC-MS/MS) is able to separately evaluate blood FA and 5-MTHF levels. In epidemiological studies of pregnant women, most reports detected FA in maternal or cord blood [19,20,21,22,23,24]. Studies of folate status in pregnant women in the United States and Germany have shown that serum or plasma 5-MTHF levels were higher in cord blood than in maternal blood [19,25], and maternal blood 5-MTHF levels were shown to correlate positively with cord blood [25], and showed a positive correlation between maternal FA levels and cord blood FA levels [22].
A study of folate status and lifestyles among pregnant women in the United States showed a negative association between 5-MTHF levels and smoking habits during pregnancy, and a positive association with folate intake (dietary folate equivalent µg/day). In a report investigating the association between folate status and preterm delivery in the United States, maternal 5-MTHF levels were negatively associated with a high incidence of preterm delivery [20]. However, these reports only conducted measurements at one time point and were not monitored longitudinally during pregnancy. Previous studies have thus been insufficient to elucidate the dynamics of the molecular species in folate metabolism during pregnancy. Furthermore, FA fortification of cereals is not mandatory in Japan, and the recommendation for folate intake according to Japanese Dietary Reference Intakes (DRIs) is lower than in Western countries [26]. Since the folate measurement data reported by other countries cannot be applied to Japanese populations, the analysis of blood FA and 5-MTHF levels in Japanese subjects will be useful for deciding folate nutritional guidelines in the future.
In this study, we aimed to measure serum 5-MTHF, FA and total homocysteine in Japanese pregnant women, using LC-MS/MS. Additionally, we investigate the longitudinal distribution of the aforementioned species and their changes with gestational age, associations with homocysteine, and relationship to maternal blood and cord blood.
2. Materials and Methods
2.1. Birth Cohort Study
This study was based on the Chiba study of Mother and Child Health (C-MACH), conducted at the Center for Preventive Medical Sciences, Chiba University and the Research Institute for Science and Engineering, Waseda University. C-MACH is a cohort study which aims to explore the effects of genetic and environmental factors, particularly the in-utero environment and the postnatal living environment, on the health of children [27]. This study was approved by the Biomedical Research Ethics Committee of the Graduate School of Medicine, Chiba University (ID: 451, 8 November 2013; ID: 462, 4 December 2013; ID: 502, 28 May 2014), the Ethics Review Committee for Human Genome/Gene Analysis Research, Waseda University (ID: 2013-G002 (3), 13 November 2015), and the Kagawa Nutrition University ethics review committee (ID: 67, 6 July 2016). All subjects provided informed consent for inclusion before participating in the study. The study was conducted in accordance with the Declaration of Helsinki.
2.2. Study Design
The study used a longitudinal design. Blood was collected in four sampling periods: maternal blood in early and late pregnancy (gestational age of 12 and 32 weeks, respectively) and at birth, and umbilical vein blood at birth. A self-administered questionnaire on lifestyle was conducted during early and late pregnancy at the same times as blood collection.
2.3. Subjects
C-MACH recruited healthy pregnant women under 13 weeks of pregnancy who visited Onodera Ladies Clinic and Yamaguchi Women’s Hospital in Chiba prefecture, and Aiwa hospital in Saitama prefecture, between February 2014 and June 2015. Follow-up was terminated if the subject had a miscarriage, stillbirth, withdrawal, or transfer [27]. This study included 146 pregnant women attending Aiwa Hospital, out of 434 pregnant women who participated in C-MACH.
2.4. Mother and Child Information
2.4.1. Lifestyle Data
Information on marital status, parity, smoking habits and alcohol consumption during pregnancy, and household income were obtained from the self-administered questionnaires conducted during early and late pregnancy.
2.4.2. Anthropometric Data
Pre-pregnancy body mass index (kg/m2) was calculated from height and pre-pregnancy weight obtained from the self-administered questionnaire conducted in early pregnancy.
2.4.3. Medical Data of Mother and Infant
Information on maternal age at birth, gestational age, birth weight, birth length and sex were obtained from hospital medical records.
2.4.4. FA Intake
Information of FA containing supplements and fortified foods about brand name, type, duration of use, frequency of intake and amount taken was collected from self-administered questionnaires administered during early and late pregnancy. Based on the method of a previous study [28], the average daily FA intake (µg/day) was calculated using the number of days FA taken, amount of FA products taken per day, and the serving size unit from the FA product label for the 4 weeks prior to the day of blood collection.
2.5. Measurement of Folate Metabolism-Related Substances in Serum
Simultaneous analysis of FA, 5MTHF, and total homocysteine was performed using the isotope-dilution mass spectrometry method [29,30].
2.5.1. Blood Collection
Within 2 h after blood collection, centrifugation was performed at 1700× g for 10 min; 0.5 mL of the supernatant (serum) was dispensed, and stored frozen at −80 °C until measurement.
2.5.2. Sample Preparation
Fifty microliters of serum, 10 μL of internal standard, and 50 μL of 100 mg/mL of tris (2-carboxyethyl) phosphine and 140 μL of 1% (v/v) formic acid in methanol were mixed for 15 min, and the supernatant was centrifuged at 16,200× g for 5 min. Supernatant was passed through a 0.2-μm filter and set in a vial.
2.5.3. Analytical Instruments
The liquid chromatography system was an Agilent 1200 Series (Agilent Technologies Japan, Tokyo, Japan), the ion source was a Turbo Ion Spray (Applied Biosystems SCIEX, Tokyo, Japan), and the triple quadrupole mass spectrometer was a 4000 QTRAP (Applied Biosystems SCIEX, Tokyo, Japan). Various parameters related to the ionization and detection of the standard substance of the measurement component and the corresponding internal standard substance were optimized [m/z 460.2–313.2 (5-MTHF), m/z 442.2–295.2 (FA), m/z 136.0–90.0 (homocysteine), m/z 465.2–313.2 (5-MTHF-13C5), m/z 447.2–295.2 (folic acid-13C5), m/z 140.0–93.9 (homocysteine-d4)]. After setting up these multiple reaction monitoring transitions, simultaneous analysis was performed in Scheduled MRM mode. The measurement time was 13 min, the mobile phase flow rate was 500 μL/min, A: perfluoroheptanoic acid 5 mM aqueous solution and B: acetonitrile gradient, and the separation column used was XSelect HSST3 2.5 μm, 100 × 2.1 (Nihon Waters, Tokyo, Japan).
2.5.4. Measurement and Data Analysis
Sample measurements were performed twice, then the average value was used. Additionally, a calibration curve was created at 8 points every 24 h and quality control was conducted every 12 h. In preliminary validity tests, the coefficient of variations of FA, 5-MTHF, and total homocysteine were 9.9%, 4.7% and 4.1%, respectively, for intra-assay and 3.7%, 8.4% and 2.3% for inter-assay, respectively. Analyst version 1.6.3 analysis software (Applied Biosystems SCIEX, Tokyo, Japan) was used for data processing and quantification. If the peak could not be detected or the signal-to-noise ratio was less than 10, the concentration was converted to 0.
2.6. Statistical Analysis
The distributions of serum 5-MTHF, FA and total homocysteine levels used in the analysis were skewed, so continuous variables are shown as medians and interquartile ranges. The Wilcoxon signed-rank test was used to compare the folate metabolism-related substance levels in maternal serum between each blood sampling period (n = 113), and to compare the FA intake between early and late pregnancy (n = 118). Bonferroni correction was used to adjust for multiple comparisons (p < 0.0167). The difference between maternal blood and cord blood was tested using the Wilcoxon signed rank test (n = 114). Spearman’s rank correlation coefficient was used for the correlation between two variables. The significance level was p < 0.05 (two-tailed test). All statistical analyses were performed using JMP® Pro version 12.2.0 (SAS Institute Japan, Tokyo, Japan).
3. Results
Figure 1 shows the participant flowchart for final number of blood sample analysis at each time point. At the time of recruitment, 146 samples could be measured in early pregnancy, but further serum samples could not be obtained from some subjects, with 131 samples obtained in late pregnancy, 116 at delivery, and 121 from cord blood.
Figure 1.
Flow of participants among pregnant women participating in Chiba study of Mother and Child Health (C-MACH).
Table 1 shows the characteristics of mothers who provided valid responses to the lifestyle questionnaire. All participants in the cohort were Japanese. Mean age (±standard deviation) of the mother at birth was 32.3 ± 4.6 years. Most subjects were married and did not smoke or drink during pregnancy. The proportion of pregnant women who took FA in early pregnancy was 54.6%; this decreased to 32.5% in late pregnancy. FA intake (µg/day) in late pregnancy was significantly lower than in early pregnancy (p < 0.0001, n = 118).

Table 1.
Characteristics of the mothers.
Table 2 shows the characteristics of the neonates. The preterm birth rate was 1.7%. Mean birth weight was 3155 ± 369 g, and the percentage of low birth weight infants was 3.3%.

Table 2.
Characteristics of neonates.
Table 3 shows the distribution of serum 5-MTHF, FA and total homocysteine levels and the difference between blood sampling periods. Maternal 5-MTHF levels significantly decreased and total homocysteine significantly increased from early pregnancy to birth as the pregnancy advanced. Maternal FA levels were significantly decreased at delivery compared to early pregnancy. At birth, cord 5-MTHF levels were much higher than maternal levels, while FA levels did not differ between these samples. Cord total homocysteine levels were lower than those in the mother.

Table 3.
Distribution of serum 5-MTHF, FA and total homocysteine levels and difference between blood sampling periods.
Figure 2 shows the results of correlations between maternal blood and cord blood at delivery. Serum levels of 5-MTHF, FA and total homocysteine showed significant positive correlations between maternal and cord blood.
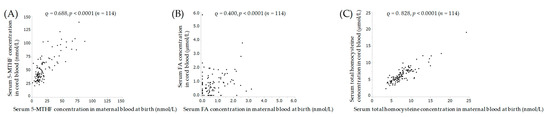
Figure 2.
Correlation of 5-MTHF (A), FA (B) and (C) total homocysteine between maternal and cord blood at birth. Spearman correlation coefficient ρ, and p-value. 5-MTHF, 5-methyltetrahydrofolate; FA, folic acid.
Table 4 shows the correlation between homocysteine and 5-MTHF and FA at each blood sampling period (early pregnancy, late pregnancy, at birth, and cord blood). A significant negative correlation was seen between 5-MTHF to total homocysteine level at all sampling periods, but no significant correlation with FA was identified.

Table 4.
Correlation of 5-MTHF or FA to total homocysteine in each blood sampling period.
4. Discussion
In this study, longitudinal evaluation of 5-MTHF, FA and homocysteine in the serum of maternal and cord blood was performed on Japanese pregnant women. It was found that 5-MTHF levels decreased as gestation progressed, whereas serum FA levels were slightly decreased only at delivery compared to early pregnancy. A cross-sectional analysis showed a significant negative association between 5-MTHF and total homocysteine at all sampling periods, but no relationship between FA and homocysteine. At delivery, cord 5-MTHF levels were much higher than maternal levels, while no significant difference was seen in FA.
Governments such as those in North and South America enforce a policy of mandatory FA fortification for grain products. However, no such policy has yet been adopted in Japan. In the present study, median maternal 5-MTHF levels during pregnancy were 14.1–32.2 nmol/L, and median FA levels were 0.433–0.620 nmol/L. In Germany, median maternal serum 5-MTHF levels (10–90th percentiles) were 15 (4.0–41.9) nmol/L by LC-MS/MS [19], close to the results of our study. Germany does not enforce FA fortification, and pregnant women are encouraged to voluntarily take FA supplements [19]. The results of that study might thus be attributable to a similar environment to Japan. On the other hand, in reports from populations where mandatory FA has been fortified, mean [95% confidence interval (CI)] plasma FA at 13 weeks of gestation was 2.41 (1.99–2.88) nmol/L by LC-MS/MS [23], mean plasma 5-MTHF at 24 weeks of gestation was 39.2 ± 15.5 nmol/L by LC/MS [31] and 36.6 ± 16.3 nmol/L by LC/MS [25], median (95% CI) serum 5-MTHF at 27 weeks’ gestation was 65.3 (24.4–75.5) nmol/L by LC-MS/MS [32], and median (95% CI) serum FA was 0.92 (0.23–1.46) nmol/L by LC-MS/MS [32]. In an exceptional American study by Bodnar, median serum 5-MTHF (25–75th percentile) at gestational age 9.4 weeks for pregnant women with FA fortification was 34.4 (25.2–47.7) nmol/L by LC-MS/MS [20], a value close to that in our study. FA supplements are recommended for pregnant women in early pregnancy in Japan [33]. Furthermore, the proportion of FA intake during early pregnancy in this study was higher than that reported in a previous study of Japanese pregnant women [34,35,36], which may be why concentrations of 5-MTHF in the present study were close to those of Bodnar et al. Serum 5-MTHF and FA in Japanese pregnant women were mostly lower than those in populations from regions with mandatory FA fortification, due to the expected effects of FA exposure, as mentioned in previous studies [9].
Maternal blood 5-MTHF in this study decreased as gestational age progressed, whereas FA levels were slightly decreased only at delivery compared to early pregnancy. This might be due to a decrease in FA intake and rate of intake in late pregnancy compared to early pregnancy. In a previous study of pregnant women, total folate similarly decreased as gestational weeks progressed [37,38,39]. In addition, 5-MTHF is the major folate molecular species, accounting for 82%–93% of folate in blood, whereas FA constitutes only a small amount of total folate [40,41,42]. These results suggest that the longitudinal changes in total folate in previous studies were likely related to 5-MTHF.
This study investigated the relationship of folate metabolism-related substances between mothers and cord blood, and found that both 5-MTHF and FA were significantly positively correlated between maternal and cord blood, cord 5-MTHF levels were much higher than those in maternal blood, while FA levels did not differ between them. Several previous studies have reported that cord blood 5-MTHF was similarly higher than that in maternal blood [19,25]. In addition, maternal FA may not actively accumulate to the fetus [23]. Three folate transporters have been found in placental syncytiotrophoblasts: folate receptor alpha, reduced folate carrier and heme carrier protein 1 [43,44]. The results of these studies thus suggest that FA might be transported to the fetus in a maternal blood-dependent manner, and 5-MTHF might be actively transported from mother to fetus against gradients in the placenta [19,25].
Total blood folate is known to be negatively associated with total homocysteine [8,45,46]. In the elderly (non-pregnant female) population in Germany, plasma 5-MTHF by LC-MS/MS and total homocysteine levels by gas chromatography–mass spectrometry showed a negative correlation [47], consistent with the present results. In our study, the relationship between serum 5-MTHF and total homocysteine levels was examined, and a significant negative correlation was disclosed, but no relationship was apparent between FA and total homocysteine. Therefore, 5-MTHF may reflect folate status during pregnancy.
This study had several limitations. First, the study included only one hospital-based population. Second, compared with the Japan Environment and Children’s Study (JECS) [48], a representative birth cohort study in Japan, maternal age was higher in the present study (mean, 31.2 ± 5.1 years), and smoking and drinking rates during pregnancy were lower than in the JECS, at 18.2% and 45.9%, respectively. Similarly, the distribution of household income was higher than in JECS. Folate status may be higher than a typical Japanese population, because of the influence of household income [49], alcohol consumption [31], and smoking habits [25,31]. Third, this study did not investigate blood during fasting. Previous studies have reported that blood FA and 5-MTHF levels are affected by the fasting state [50]. Fourth, this study did not investigate genetic polymorphisms affecting folate metabolism, such as methylenetetrahydrofolate reductase [51,52] and dihydrofolate reductase [13], which may affect the metabolism of 5-MTHF and FA. Fifth, this study used serum, and homocysteine values in serum are reportedly slightly higher than in plasma [6]. Re-methylation of homocysteine involves two methyl group transferring pathways, through 5-MTHF, using cofactor vitamin B12 and through betaine. In addition, there is a transsulfuration pathway for homocysteine. These related substances were thus not taken into account [6]. Finally, blood levels of 5-MTHF and FA in the blood of study subjects could not be evaluated in this study, because thresholds for excess and deficiency are unknown. In this study, FA was detected in the serum of Japanese pregnant women, and was considered to be unmetabolized FA, but no causal relationship between blood FA levels and negative fetal outcomes has been demonstrated. On the other hand, the benefits of FA in preventing NTD appear incontrovertible [53,54]. Further research is needed to establish optimal blood levels thresholds to balance NTD prevention with excess disease. In the future, it will be important to follow the children of the subjects of this study and to evaluate the relationship between FA overdose and health and disease outcomes in the children, using blood FA concentrations. On the other hand, blood levels of 5-MTHF should be considered as a more sensitive indicator of maternal folate deficiency. The accumulation of this information could provide evidence for appropriate FA use and public health policy.
5. Conclusions
The present results suggested that 5-MTHF was more likely to be transferred to the fetus than FA, correlated negatively with total homocysteine, and represents a physiologically important molecular species in folate metabolism that may reflect folate status during pregnancy. Further research is needed to establish optimal blood levels of 5-MTHF and FA for fetuses.
Author Contributions
Conceptualization, Y.K., H.F. and T.K.; methodology, Y.K., T.K., M.N. and O.H.; validation, Y.K.; formal analysis, Y.K., T.K. and N.Y.; investigation, Y.K., H.F., C.M. and K.S.; resources, C.M., T.K., K.S. (Kenichi Sakurai) and M.N.; data curation, H.F., C.M. and K.S. (Kenichi Sakurai); writing—original draft preparation, Y.K.; writing—review and editing, H.F., T.K., K.S. (Kenichi Sakurai), C.M., K.S. (Kumiko Shoji), M.N., T.O., K.O., N.Y. and Y.Y.; Visualization, Y.K.; supervision, H.F. and Y.Y.; project administration, H.F. and Y.Y.; funding acquisition, K.O., T.K. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Laboratory for Probiotics Research, Juntendo University School of Medicine, which originally received a donation from Amway Japan G.K. and JSPS KAKENHI Grant Numbers 19K11699.
Acknowledgments
We thank the participants in the study and are grateful for the cooperation and support of C-MACH members. We also express our appreciation to the members of Miyagi University for their help in sample measurement.
Conflicts of Interest
The research fund for this research was a donation from Amway Japan G.K. The funding company played no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Fekete, K.; Berti, C.; Cetin, I.; Hermoso, M.; Koletzko, B.V.; Decsi, T. Perinatal folate supply: Relevance in health outcome parameters. Matern. Child Nutr. 2010, 6, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Picciano, M.F. Folate and human reproduction. Am. J. Clin. Nutr. 2006, 83, 993–1016. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.C. In utero physiology: Role of folic acid in nutrient delivery and fetal development. Am. J. Clin. Nutr. 2007, 85, 598S–603S. [Google Scholar] [CrossRef]
- Vollset, S.E.; Refsum, H.; Irgens, L.M.; Emblem, B.M.; Tverdal, A.; Gjessing, H.K.; Monsen, A.L.; Ueland, P.M. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: The Hordaland Homocysteine study. Am. J. Clin. Nutr. 2000, 71, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Herrmann, M.; Obeid, R. Hyperhomocysteinaemia: A critical review of old and new aspects. Curr. Drug Metab. 2007, 8, 17–31. [Google Scholar] [CrossRef]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef]
- Patanwala, I.; King, M.J.; Barrett, D.A.; Rose, J.; Jackson, R.; Hudson, M.; Philo, M.; Dainty, J.R.; Wright, A.J.; Finglas, P.M.; et al. Folic acid handling by the human gut: Implications for food fortification and supplementation. Am. J. Clin. Nutr. 2014, 100, 593–599. [Google Scholar] [CrossRef]
- Finkelstein, J.D. The metabolism of homocysteine: Pathways and regulation. Eur. J. Pediatr. 1998, 157, S40–S44. [Google Scholar] [CrossRef]
- Kalmbach, R.D.; Choumenkovitch, S.F.; Troen, A.M.; D’Agostino, R.; Jacques, P.F.; Selhub, J. Circulating folic acid in plasma: Relation to folic acid fortification. Am. J. Clin. Nutr. 2008, 88, 763–768. [Google Scholar] [CrossRef]
- Kelly, P.; McPartlin, J.; Goggins, M.; Weir, D.G.; Scott, J.M. Unmetabolized folic acid in serum: Acute studies in subjects consuming fortified food and supplements. Am. J. Clin. Nutr. 1997, 65, 1790–1795. [Google Scholar] [CrossRef]
- Fohr, I.P.; Prinz-Langenohl, R.; Bronstrup, A.; Bohlmann, A.M.; Nau, H.; Berthold, H.K.; Pietrzik, K. 5,10-Methylenetetrahydrofolate reductase genotype determines the plasma homocysteine-lowering effect of supplementation with 5-methyltetrahydrofolate or folic acid in healthy young women. Am. J. Clin. Nutr. 2002, 75, 275–282. [Google Scholar] [CrossRef]
- Sweeney, M.R.; McPartlin, J.; Scott, J. Folic acid fortification and public health: Report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 2007, 7, 41. [Google Scholar] [CrossRef]
- Kalmbach, R.D.; Choumenkovitch, S.F.; Troen, A.P.; Jacques, P.F.; D’Agostino, R.; Selhub, J. A 19-base pair deletion polymorphism in dihydrofolate reductase is associated with increased unmetabolized folic acid in plasma and decreased red blood cell folate. J. Nutr. 2008, 138, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Sobczynska-Malefora, A. The adverse effects of an excessive folic acid intake. Eur. J. Clin. Nutr. 2017, 71, 159–163. [Google Scholar] [CrossRef]
- Lamers, Y.; MacFarlane, A.J.; O’Connor, D.L.; Fontaine-Bisson, B. Periconceptional intake of folic acid among low-risk women in Canada: Summary of a workshop aiming to align prenatal folic acid supplement composition with current expert guidelines. Am. J. Clin. Nutr. 2018, 108, 1357–1368. [Google Scholar] [CrossRef]
- Xie, R.H.; Liu, Y.J.; Retnakaran, R.; MacFarlane, A.J.; Hamilton, J.; Smith, G.; Walker, M.C.; Wen, S.W. Maternal folate status and obesity/insulin resistance in the offspring: A systematic review. Int. J. Obes. (Lond.) 2016, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Reeves, K.W.; Bertone-Johnson, E.R. Maternal folate exposure in pregnancy and childhood asthma and allergy: A systematic review. Nutr. Rev. 2014, 72, 55–64. [Google Scholar] [CrossRef] [PubMed]
- De Leenheer, A.P.; Thienpont, L.M. Applications of isotope dilution-mass spectrometry in clinical chemistry, pharmacokinetics, and toxicology. Mass Spectrom. Rev. 1992, 11, 249–307. [Google Scholar] [CrossRef]
- Obeid, R.; Kasoha, M.; Kirsch, S.H.; Munz, W.; Herrmann, W. Concentrations of unmetabolized folic acid and primary folate forms in pregnant women at delivery and in umbilical cord blood. Am. J. Clin. Nutr. 2010, 92, 1416–1422. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Himes, K.P.; Venkataramanan, R.; Chen, J.Y.; Evans, R.W.; Meyer, J.L.; Simhan, H.N. Maternal serum folate species in early pregnancy and risk of preterm birth. Am. J. Clin. Nutr. 2010, 92, 864–871. [Google Scholar] [CrossRef]
- Sweeney, M.R.; McPartlin, J.; Weir, D.G.; Daly, S.; Pentieva, K.; Daly, L.; Scott, J.M. Evidence of unmetabolised folic acid in cord blood of newborn and serum of 4-day-old infants. Br. J. Nutr. 2005, 94, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.R.; Staines, A.; Daly, L.; Traynor, A.; Daly, S.; Bailey, S.W.; Alverson, P.B.; Ayling, J.E.; Scott, J.M. Persistent circulating unmetabolised folic acid in a setting of liberal voluntary folic acid fortification. Implications for further mandatory fortification? BMC Public Health 2009, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.J.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.L.; Kim, Y.I. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef]
- Simhan, H.N.; Himes, K.P.; Venkataramanan, R.; Bodnar, L.M. Maternal serum folate species in early pregnancy and lower genital tract inflammatory milieu. Am. J. Obstet. Gynecol. 2011, 205, 61.e1–61.e7. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D.; Pawlosky, R.J.; Sokol, R.J.; Hannigan, J.H.; Salem, N., Jr. Maternal smoking is associated with decreased 5-methyltetrahydrofolate in cord plasma. Am. J. Clin. Nutr. 2007, 85, 796–802. [Google Scholar] [CrossRef]
- Gomes, S.; Lopes, C.; Pinto, E. Folate and folic acid in the periconceptional period: Recommendations from official health organizations in thirty-six countries worldwide and WHO. Public Health Nutr. 2016, 19, 176–189. [Google Scholar] [CrossRef]
- Sakurai, K.; Miyaso, H.; Eguchi, A.; Matsuno, Y.; Yamamoto, M.; Todaka, E.; Fukuoka, H.; Hata, A.; Mori, C.; Chiba, M.; et al. Chiba study of Mother and Children’s Health (C-MACH): Cohort study with omics analyses. BMJ Open 2016, 6, e010531. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; McDowell, M.A.; Yetley, E.A.; Sempos, C.A.; Burt, V.L.; Radimer, K.L.; Picciano, M.F. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am. J. Clin. Nutr. 2010, 91, 231–237. [Google Scholar] [CrossRef]
- Zheng, X.H.; Jiang, L.Y.; Zhao, L.T.; Zhang, Q.Y.; Ding, L. Simultaneous quantitation of folic acid and 5-methyltetrahydrofolic acid in human plasma by HPLC-MS/MS and its application to a pharmacokinetic study. J. Pharm. Anal. 2015, 5, 269–275. [Google Scholar] [CrossRef]
- Guiraud, S.P.; Montoliu, I.; Da Silva, L.; Dayon, L.; Galindo, A.N.; Corthesy, J.; Kussmann, M.; Martin, F.P. High-throughput and simultaneous quantitative analysis of homocysteine-methionine cycle metabolites and co-factors in blood plasma and cerebrospinal fluid by isotope dilution LC-MS/MS. Anal. Bioanal. Chem. 2017, 409, 295–305. [Google Scholar] [CrossRef]
- Stark, K.D.; Pawlosky, R.J.; Beblo, S.; Murthy, M.; Flanagan, V.P.; Janisse, J.; Buda-Abela, M.; Rockett, H.; Whitty, J.E.; Sokol, R.J.; et al. Status of plasma folate after folic acid fortification of the food supply in pregnant African American women and the influences of diet, smoking, and alcohol consumption. Am. J. Clin. Nutr. 2005, 81, 669–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- West, A.A.; Yan, J.; Perry, C.A.; Jiang, X.; Malysheva, O.V.; Caudill, M.A. Folate-status response to a controlled folate intake in nonpregnant, pregnant, and lactating women. Am. J. Clin. Nutr. 2012, 96, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare. Dietary Reference Intakes for Japanese, 2015; Daiichi Shuppan Publishing Co., Ltd.: Toyko, Japan, 2014.
- Kondo, A.; Morota, N.; Ihara, S.; Saisu, T.; Inoue, K.; Shimokawa, S.; Fujimaki, H.; Matsuo, K.; Shimosuka, Y.; Watanabe, T. Risk factors for the occurrence of spina bifida (a case-control study) and the prevalence rate of spina bifida in Japan. Birth Defects Res. A Clin. Mol. Teratol. 2013, 97, 610–615. [Google Scholar] [CrossRef]
- Obara, T.; Nishigori, H.; Nishigori, T.; Metoki, H.; Ishikuro, M.; Tatsuta, N.; Mizuno, S.; Sakurai, K.; Nishijima, I.; Murai, Y.; et al. Prevalence and determinants of inadequate use of folic acid supplementation in Japanese pregnant women: The Japan Environment and Children’s Study (JECS). J. Matern. Fetal Neonatal Med. 2017, 30, 588–593. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Sasaki, S.; Yamamoto-Hanada, K.; Mezawa, H.; Konishi, M.; Ohya, Y.; Japan, E.; Children’s Study, G. Changes in Dietary Intake in Pregnant Women from Periconception to Pregnancy in the Japan Environment and Children’s Study: A Nationwide Japanese Birth Cohort Study. Matern Child Health J. 2020, 24, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Bruinse, H.W.; van der Berg, H.; Haspels, A.A. Maternal serum folacin levels during and after normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1985, 20, 153–158. [Google Scholar] [CrossRef]
- Qvist, I.; Abdulla, M.; Jagerstad, M.; Svensson, S. Iron, zinc and folate status during pregnancy and two months after delivery. Acta Obstet. Gynecol. Scand. 1986, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bruinse, H.W.; van den Berg, H. Changes of some vitamin levels during and after normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 61, 31–37. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Fazili, Z.; McCoy, L.; Zhang, M.; Gunter, E.W. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin. Chem. 2004, 50, 423–432. [Google Scholar] [CrossRef]
- Kirsch, S.H.; Knapp, J.P.; Herrmann, W.; Obeid, R. Quantification of key folate forms in serum using stable-isotope dilution ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 68–75. [Google Scholar] [CrossRef]
- Fazili, Z.; Sternberg, M.R.; Potischman, N.; Wang, C.Y.; Storandt, R.J.; Yeung, L.; Yamini, S.; Gahche, J.J.; Juan, W.; Qi, Y.P.; et al. Demographic, Physiologic, and Lifestyle Characteristics Observed with Serum Total Folate Differ Among Folate Forms: Cross-Sectional Data from Fasting Samples in the NHANES 2011–2016. J. Nutr. 2020, 150, 851–860. [Google Scholar] [CrossRef]
- Yasuda, S.; Hasui, S.; Yamamoto, C.; Yoshioka, C.; Kobayashi, M.; Itagaki, S.; Hirano, T.; Iseki, K. Placental folate transport during pregnancy. Biosci. Biotechnol. Biochem. 2008, 72, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Solanky, N.; Requena Jimenez, A.; D’Souza, S.W.; Sibley, C.P.; Glazier, J.D. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta 2010, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Byg, K.E.; Hvas, A.M.; Bergholt, T.; Eriksen, L. Erythrocyte folate, plasma folate and plasma homocysteine during normal pregnancy and postpartum: A longitudinal study comprising 404 Danish women. Eur. J. Haematol. 2006, 76, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Fekete, K.; Dullemeijer, C.; Trovato, M.; Souverein, O.W.; Cavelaars, A.; Dhonukshe-Rutten, R.; Massari, M.; Decsi, T.; Van’t Veer, P.; et al. Folate intake and markers of folate status in women of reproductive age, pregnant and lactating women: A meta-analysis. J. Nutr. Metab. 2012, 2012, 470656. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Kirsch, S.H.; Kasoha, M.; Eckert, R.; Herrmann, W. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism 2011, 60, 673–680. [Google Scholar] [CrossRef]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef]
- Caudill, M.A.; Le, T.; Moonie, S.A.; Esfahani, S.T.; Cogger, E.A. Folate status in women of childbearing age residing in Southern California after folic acid fortification. J. Am. Coll. Nutr. 2001, 20, 129–134. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Yetley, E.A.; Lacher, D.A.; Bailey, R.L.; Johnson, C.L. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J. Nutr. 2015, 145, 520–531. [Google Scholar] [CrossRef]
- Tsang, B.L.; Devine, O.J.; Cordero, A.M.; Marchetta, C.M.; Mulinare, J.; Mersereau, P.; Guo, J.; Qi, Y.P.; Berry, R.J.; Rosenthal, J.; et al. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: A systematic review and meta-analysis of trials and observational studies. Am. J. Clin. Nutr. 2015, 101, 1286–1294. [Google Scholar] [CrossRef]
- Colson, N.J.; Naug, H.L.; Nikbakht, E.; Zhang, P.; McCormack, J. The impact of MTHFR 677 C/T genotypes on folate status markers: A meta-analysis of folic acid intervention studies. Eur. J. Nutr. 2017, 56, 247–260. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Pena-Rosas, J.P.; Fernandez-Gaxiola, A.C.; Rayco-Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Centeno Tablante, E.; Pachon, H.; Guetterman, H.M.; Finkelstein, J.L. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst. Rev. 2019, 7, CD012150. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).