Acute Effects of a Polyphenol-Rich Leaf Extract of Mangifera indica L. (Zynamite) on Cognitive Function in Healthy Adults: A Double-Blind, Placebo-Controlled Crossover Study
Abstract
1. Introduction
2. Methods
2.1. Design
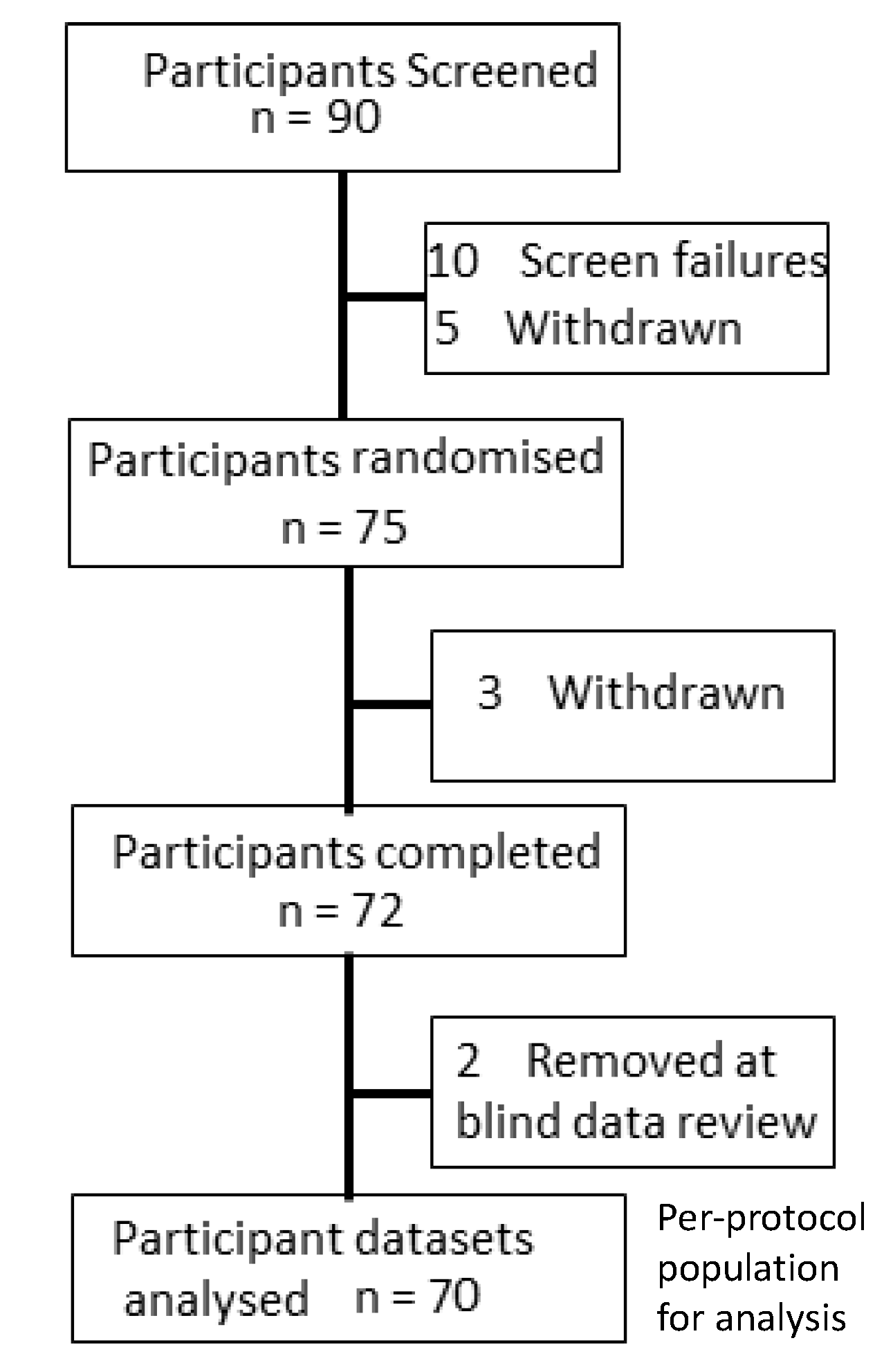
2.2. Participants
2.3. Treatments
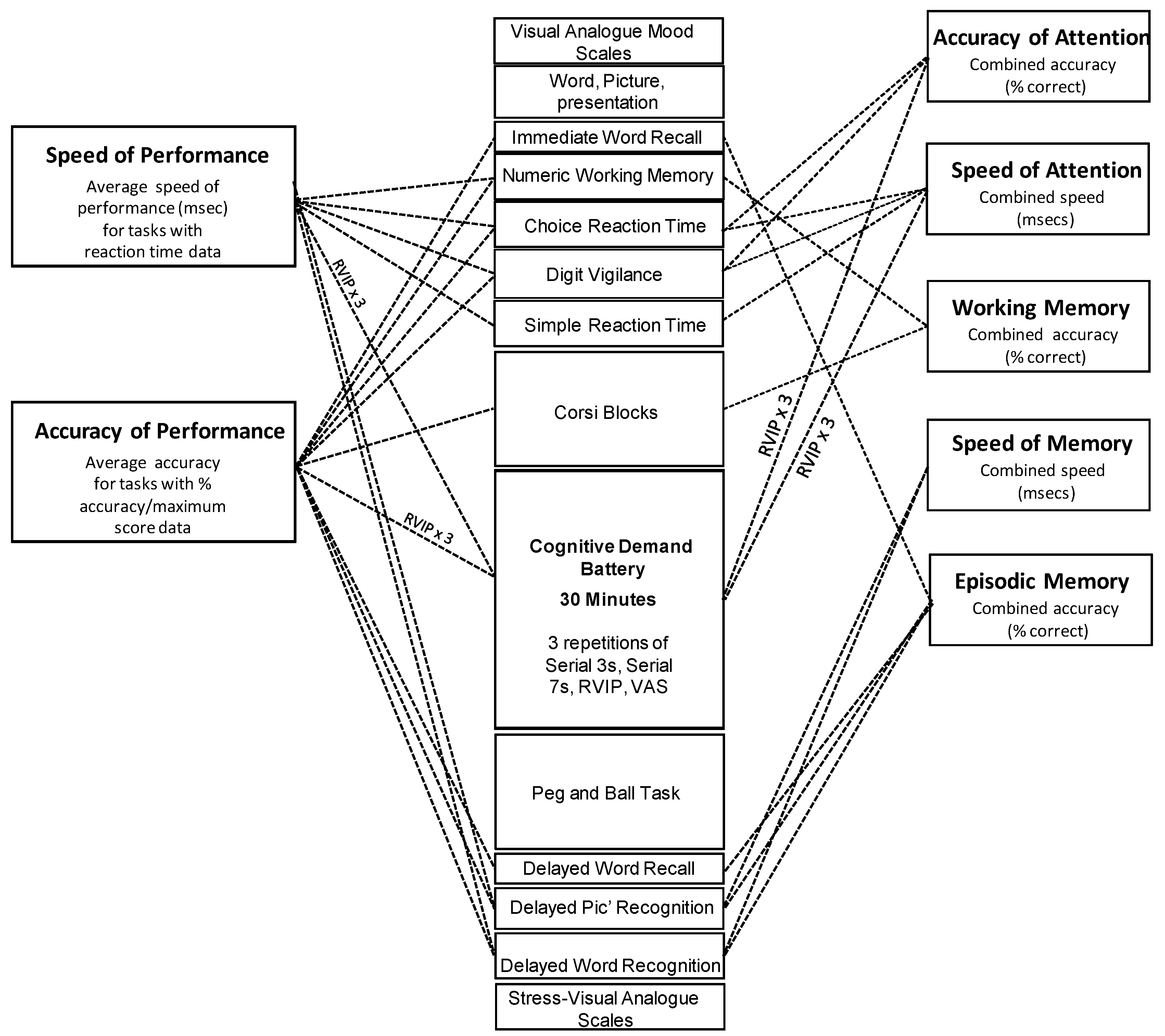
2.4. Psychological Measures
2.4.1. Cognitive Tasks
2.4.2. Mood and Psychological State
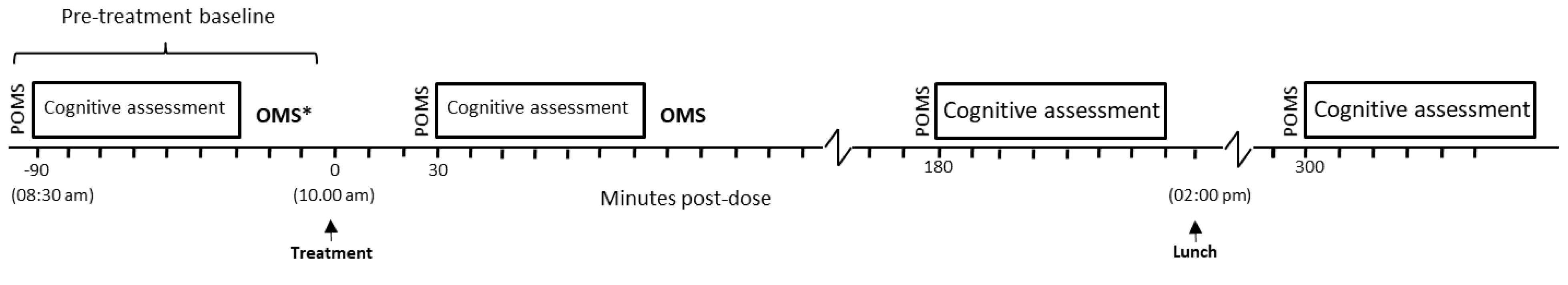
2.5. Procedure
2.6. Analysis
3. Results
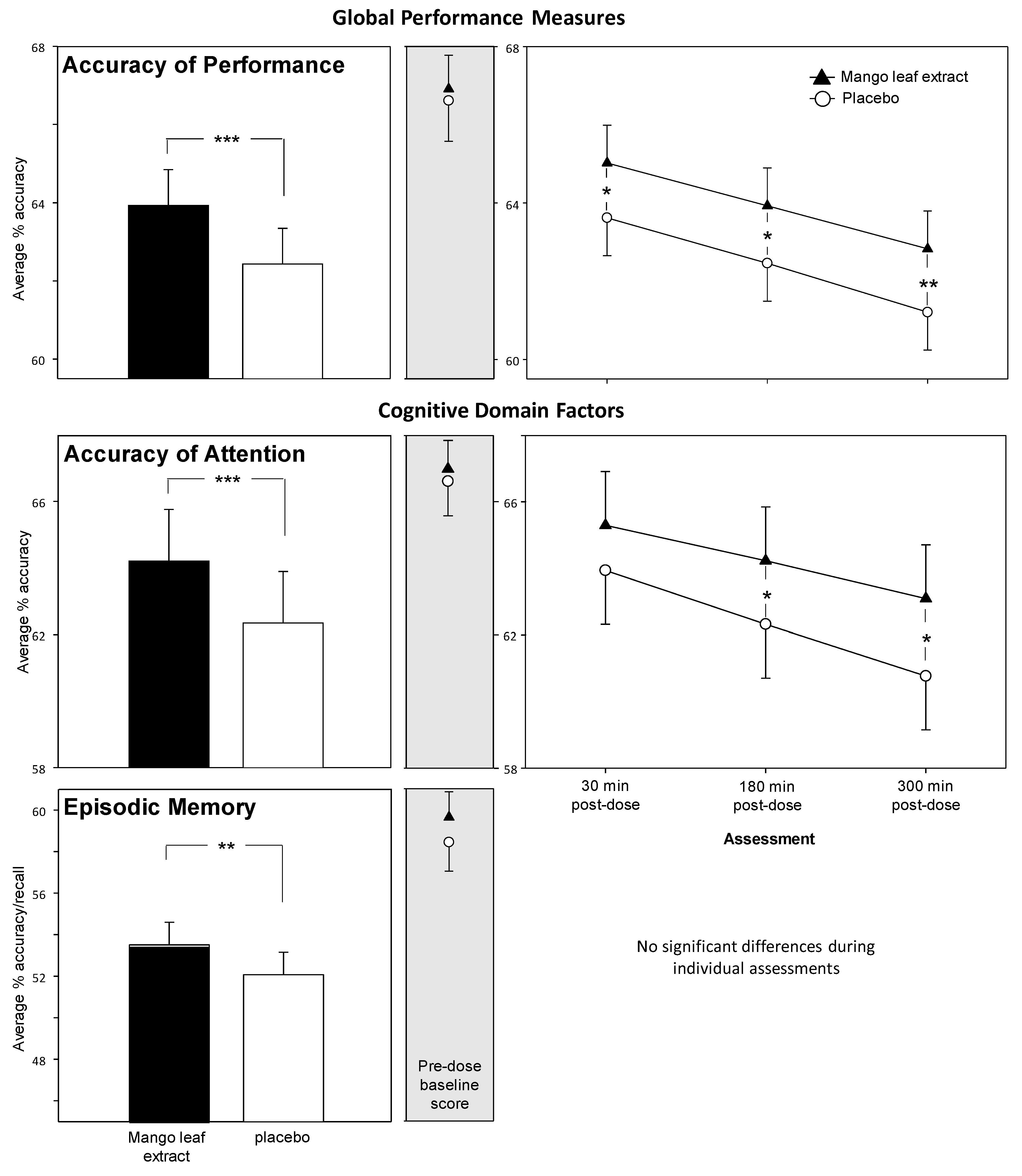
3.1. Cognitive Task Global and Factor Outcomes
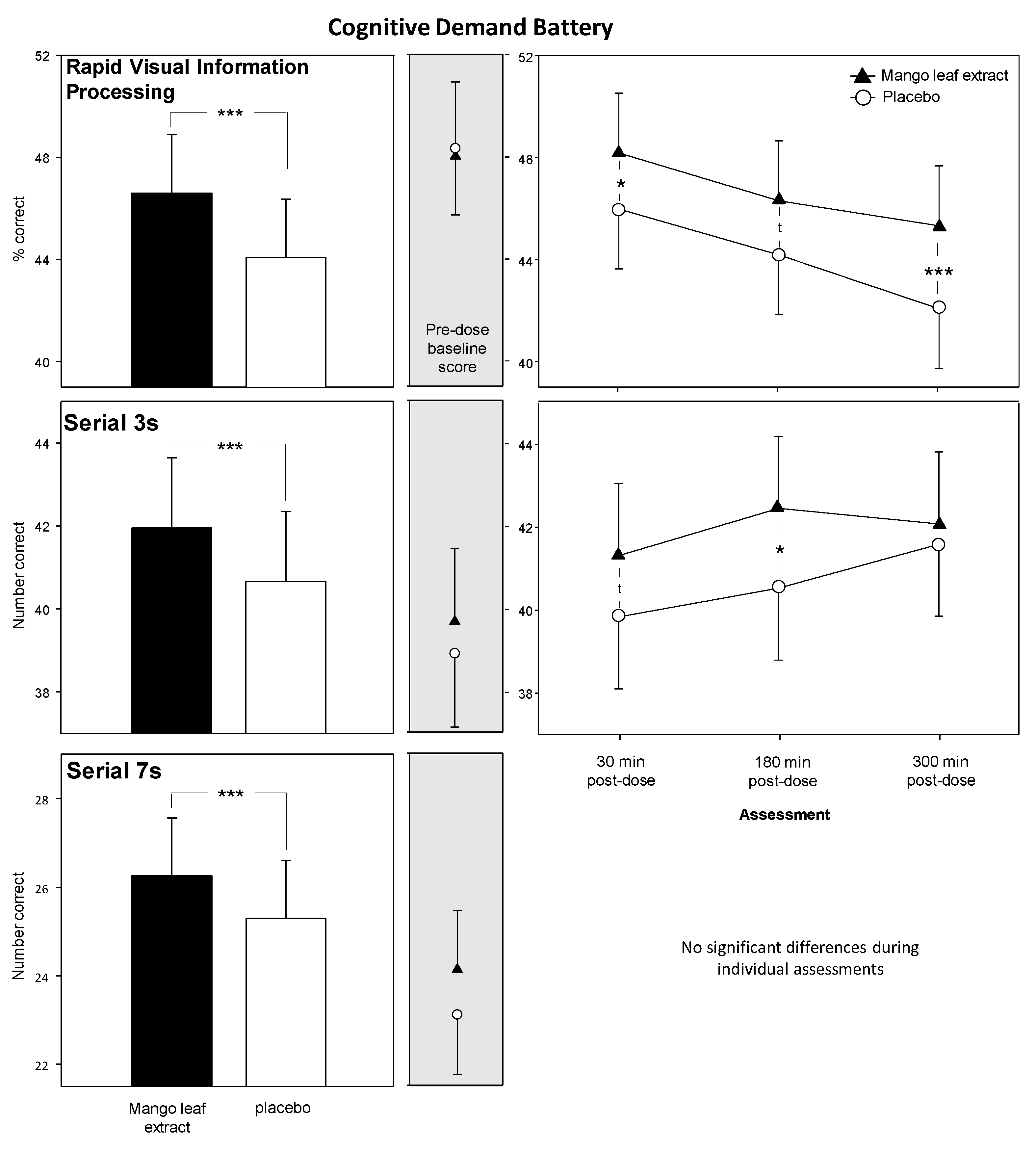
3.2. Cognitive Demand Battery (CDB)
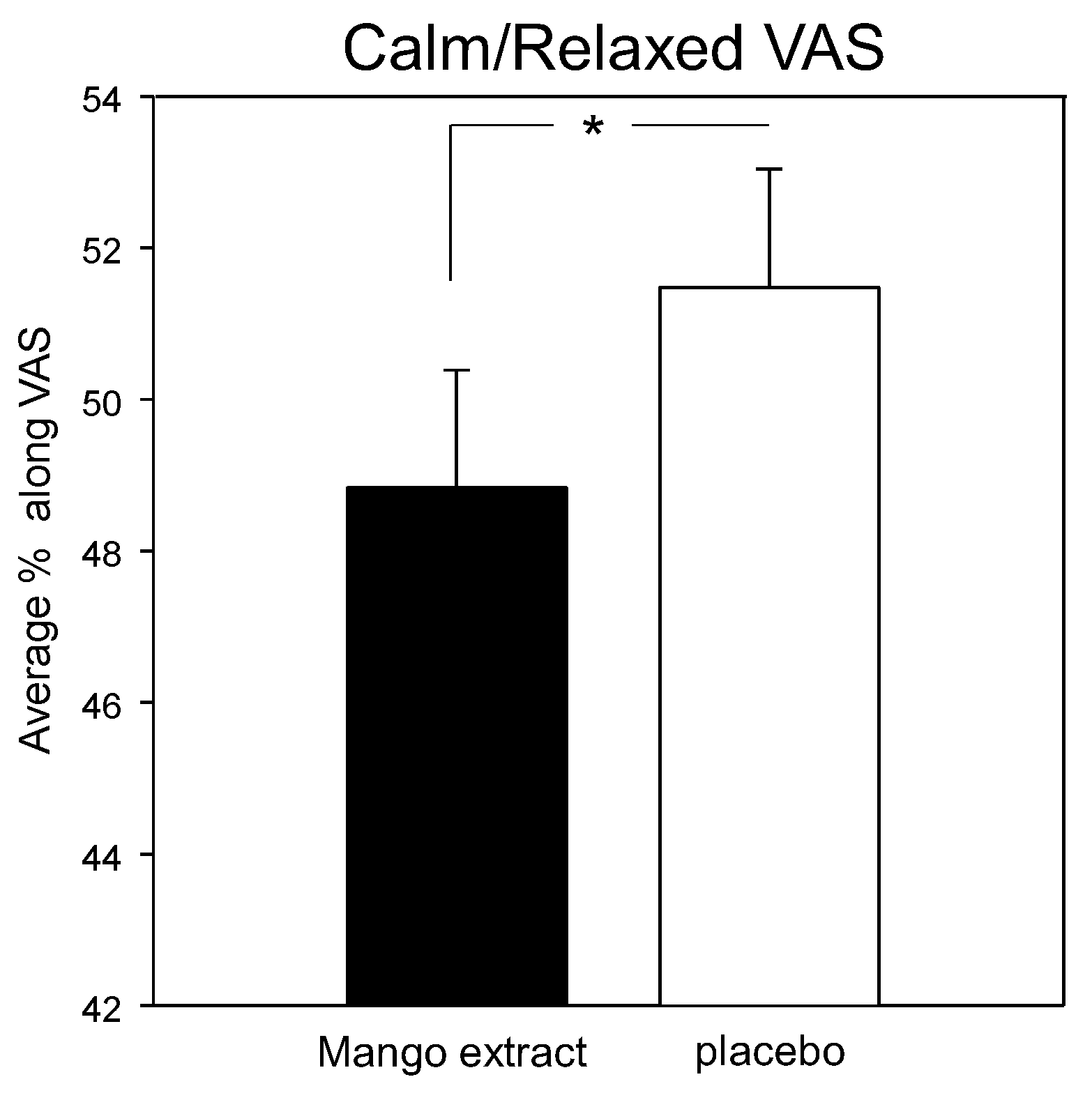
3.3. Mood and Psychological State
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (mango). Evid. Based Complement. Alternat. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Tayana, N.; Inthakusol, W.; Duangdee, N.; Chewchinda, S.; Pandith, H.; Kongkiatpaiboon, S. Mangiferin content in different parts of mango tree (Mangifera indica L.) in Thailand. Songklanakarin J. Sci. Technol. 2019, 41. [Google Scholar] [CrossRef]
- Vieira, L.; Kijjoa, A. Naturally-occurring xanthones: Recent developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef] [PubMed]
- Kitanov, G.M.; Nedialkov, P.T. Mangiferin and isomangiferin in some Hypericum species. Biochem. Syst. Ecol. 1998, 26, 647–653. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Riedl, K.M.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J. Nutr. 2012, 142, 675–680. [Google Scholar] [CrossRef]
- Khare, P.; Shanker, K. Mangiferin: A review of sources and interventions for biological activities. Biofactors 2016, 42, 504–514. [Google Scholar]
- Sekar, V.; Chakraborty, S.; Mani, S.; Sali, V.; Vasanthi, H. Mangiferin from Mangifera indica fruits reduces post-prandial glucose level by inhibiting α-glucosidase and α-amylase activity. S. Afr. J. Bot. 2019, 120, 129–134. [Google Scholar] [CrossRef]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and cancer: Mechanisms of action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef]
- Kasbe, P.; Jangra, A.; Lahkar, M. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J. Trace Elem. Med. Biol. 2015, 31, 107–112. [Google Scholar] [CrossRef]
- Li, H.-W.; Lan, T.-J.; Yun, C.-X.; Du, Z.-C.; Luo, X.-f.; Hao, E.-W.; Deng, J.-G. Mangiferin exerts neuroprotective activity against lead-induced toxicity and oxidative stress via Nrf2 pathway. Chin. Herb. Med. 2020, 12, 36–46. [Google Scholar] [CrossRef]
- Yang, S.; Kuang, G.; Zhang, L.; Wu, S.; Zhao, Z.; Wang, B.; Yin, X.; Gong, X.; Wan, J. Mangiferin Attenuates LPS/D-GalN-Induced Acute Liver Injury by Promoting HO-1 in Kupffer Cells. Front. Immunol. 2020, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.K.; Verma, V.K.; Mutneja, E.; Malik, S.; Nag, T.C.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Mol. Cell. Biochem. 2019, 452, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Kim, Y.-S. PINK1-PRKN mitophagy suppression by Mangiferin promotes a brown-fat-phenotype via PKA-p38 MAPK signalling in murine C3H10T1/2 mesenchymal stem cells. Metabolism 2020, 154228. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Kumari, S.; Ojha, S.; Arya, D.S. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci. Rep. 2017, 7, 42027. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. Polyphenols and the human brain: Plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 2014, 5, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef]
- Vauzour, D. Effect of flavonoids on learning, memory and neurocognitive performance: Relevance and potential implications for Alzheimer’s disease pathophysiology. J. Sci. Food Agric. 2014, 94, 1042–1056. [Google Scholar] [CrossRef]
- Baptista, F.I.; Henriques, A.G.; Silva, A.M.; Wiltfang, J.; da Cruz e Silva, O.A. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’ s disease. ACS Chem. Neurosci. 2014. [Google Scholar] [CrossRef]
- Kennedy, D.O. Plants and the Human Brain; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Shrime, M.G.; Bauer, S.R.; McDonald, A.C.; Chowdhury, N.H.; Coltart, C.E.; Ding, E.L. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J. Nutr. 2011, 141, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, I.; Li, A.; Manson, J.E.; Sesso, H.D.; Wang, L.; Liu, S. Cocoa flavanol intake and biomarkers for cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 2016, 146, 2325. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; French, S.J.; Morris, P.J.; Kennedy, D.O.; Milne, A.L.; Haskell, C.F. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 2010, 24, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Haskell-Ramsay, C.; Stuart, R.; Okello, E.; Watson, A. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Bouaziz, B.; Müller, P.; M Glenn, J.; Bott, N.T.; Müller, N.; Chtourou, H.; Driss, T. Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1598. [Google Scholar] [CrossRef]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C. Benefits in Cognitive Function, Blood Pressure, and Insulin Resistance Through Cocoa Flavanol Consumption in Elderly Subjects With Mild Cognitive ImpairmentNovelty and Significance The Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A randomized controlled trial. Am. J. Clin. Nutr. 2014, 101, 538–548. [Google Scholar] [CrossRef]
- Andreu, G.L.P.; Maurmann, N.; Reolon, G.K.; de Farias, C.B.; Schwartsmann, G.; Delgado, R.; Roesler, R. Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur. J. Pharmacol. 2010, 635, 124–128. [Google Scholar] [CrossRef]
- Jung, K.; Lee, B.; Han, S.J.; Ryu, J.H.; Kim, D.-H. Mangiferin ameliorates scopolamine-induced learning deficits in mice. Biol. Pharm. Bull. 2009, 32, 242–246. [Google Scholar] [CrossRef]
- Feng, X.; Xue, J.H.; Xie, K.X.; Liu, S.P.; Zhong, H.P.; Wang, C.C.; Feng, X.Q. Beneficial effect of Mangiferin against sleep deprivation-induced neurodegeneration and memory impairment in mice. Biomed. Res. 2017, 28, 769–777. [Google Scholar]
- Jangra, A.; Lukhi, M.M.; Sulakhiya, K.; Baruah, C.C.; Lahkar, M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur. J. Pharmacol. 2014, 740, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Gericke, N.; Perez-Valera, M.; Curtelin, D.; Galvan-Alvarez, V.; Lopez-Rios, L.; Morales-Alamo, D.; Calbet, J.A. Mangifera indica l. Leaf extract in combination with luteolin or quercetin enhances vo2peak and peak power output, and preserves skeletal muscle function during ischemia-reperfusion in humans. Front. Physiol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Pérez-López, A.; Vega, T.; Morales-Alamo, D. Enhancement of Exercise Performance by 48 Hours, and 15-Day Supplementation with Mangiferin and Luteolin in Men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-Rebato, M.; Martin-Rincon, M.; Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; Vega-Morales, T.; Wiebe, J.C.; Fernandez-del Castillo, C.; Castilla-Hernandez, E.; Diaz-Tiberio, O. A Single Dose of The Mango Leaf Extract Zynamite® in Combination with Quercetin Enhances Peak Power Output During Repeated Sprint Exercise in Men and Women. Nutrients 2019, 11, 2592. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Gelabert-Rebato, M.; Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; Lopez-Rios, L.; Wiebe, J.C.; Martin-Rodriguez, S.; Arteaga-Ortiz, R.; Dorado, C. Supplementation with a Mango Leaf Extract (Zynamite®) in Combination with Quercetin Attenuates Muscle Damage and Pain and Accelerates Recovery after Strenuous Damaging Exercise. Nutrients 2020, 12, 614. [Google Scholar] [CrossRef]
- Martín Rincón, M.; Galvan Alvarez, V.; Gelabert Rebato, M.; Gallego Selles, Á.; Martínez Cantón, M.; Martín Rodríguez, S.; Pérez Valera, M.; Morales Álamo, D.; López Calbet, J.A. Exercise-induced muscle pain is reduced and recovery accelerated by supplementation with mango leaf extract Zynamite® in combination with quercetin in men and women. In Proceedings of the International Sport Forum on Strength, Conditioning and Nutrition, Madrid, España, 15–16 November 2019. [Google Scholar]
- Dimpfel, W.; Wiebe, J.; Gericke, N.; Schombert, L. Zynamite®(Mangifera indica Leaf Extract) and Caffeine Act in a Synergistic Manner on Electrophysiological Parameters of Rat Central Nervous System. Food Nutr. Sci. 2018, 9, 502. [Google Scholar]
- López-Ríos, L.; Wiebe, J.; Vega-Morales, T.; Gericke, N. Central nervous system activities of extract Mangifera indica L. J. Ethnopharmacol. 2020, 112996. [Google Scholar] [CrossRef]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Jackson, P.A.; Forster, J.; Khan, J.; Grothe, T.; Perrinjaquet-Moccetti, T.; Haskell-Ramsay, C.F. Acute effects of a wild green-oat (Avena sativa) extract on cognitive function in middle-aged adults: A double-blind, placebo-controlled, within-subjects trial. Nutr. Neurosci. 2017, 20, 135–151. [Google Scholar] [CrossRef]
- Kennedy, D.; Wightman, E.; Khan, J.; Grothe, T.; Jackson, P. The Acute and Chronic Cognitive and Cerebral Blood-Flow Effects of Nepalese Pepper (Zanthoxylum armatum DC.) Extract—A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Humans. Nutrients 2019, 11, 3022. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Forster, J.; Khan, J.; Haskell-Ramsay, C.F.; Jackson, P.A. Cognitive and mood effects of a nutrient enriched breakfast bar in healthy adults: A randomised, double-blind, placebo-controlled, parallel groups study. Nutrients 2017, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Okello, E.; Chazot, P.; Howes, M.-J.; Ohiomokhare, S.; Jackson, P.; Haskell-Ramsay, C.; Khan, J.; Forster, J.; Wightman, E. Volatile terpenes and brain function: Investigation of the cognitive and mood effects of Mentha× piperita l. essential oil with in vitro properties relevant to central nervous system function. Nutrients 2018, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Scholey, A.B. A glucose-caffeine ‘energy drink’ ameliorates subjective and performance deficits during prolonged cognitive demand. Appetite 2004, 42, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Reay, J.L.; Kennedy, D.O.; Scholey, A.B. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J. Psychopharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Heuchert, J.P.; McNair, D.M. POMS 2 Manual: Profile of Mood States; Multi-Health Systems Inc.: Toronto, ON, Canada, 2012. [Google Scholar]
- Kennedy, D.O.; Bonnländer, B.; Lang, S.C.; Pischel, I.; Forster, J.; Khan, J.; Jackson, P.A.; Wightman, E.L. Acute and Chronic Effects of Green Oat (Avena sativa) Extract on Cognitive Function and Mood during a Laboratory Stressor in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled Study in Healthy Humans. Nutrients 2020, 12, 1598. [Google Scholar] [CrossRef]
- Long, N.M.; Kuhl, B.A.; Chun, M.M. Memory and attention. Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience 2018, 1, 1–37. [Google Scholar]
- Philip, P.; Sagaspe, P.; Taillard, J.; Mandon, C.; Constans, J.; Pourtau, L.; Pouchieu, C.; Angelino, D.; Mena, P.; Martini, D. Acute Intake of a Grape and Blueberry Polyphenol-Rich Extract Ameliorates Cognitive Performance in Healthy Young Adults During a Sustained Cognitive Effort. Antioxidants 2019, 8, 650. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Nehlig, A. Is caffeine a cognitive enhancer? J. Alzheimers Dis. 2010, 20, 85–94. [Google Scholar] [CrossRef]
- Childs, E.; de Wit, H. Subjective, behavioral, and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacology 2006, 185, 514–523. [Google Scholar] [CrossRef]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R. Nutrition, brain function and cognitive performance. Appetite 2003, 40, 245–254. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.Y.; Lambert, J.D.; Ai, N.; Welsh, W.J.; Yang, C.S. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: Structure–activity relationship and molecular-modeling studies. Biochem. Pharmacol. 2005, 69, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Cuyàs, E.; Verdura, S.; Lozano-Sánchez, J.; Viciano, I.; Llorach-Parés, L.; Nonell-Canals, A.; Bosch-Barrera, J.; Brunet, J.; Segura-Carretero, A.; Sanchez-Martinez, M. The extra virgin olive oil phenolic oleacein is a dual substrate-inhibitor of catechol-O-methyltransferase. Food Chem. Toxicol. 2019, 128, 35–45. [Google Scholar] [CrossRef]
- Apud, J.A.; Mattay, V.; Chen, J.; Kolachana, B.S.; Callicott, J.H.; Rasetti, R.; Alce, G.; Iudicello, J.E.; Akbar, N.; Egan, M.F. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 2007, 32, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Schacht, J.P. COMT val158met moderation of dopaminergic drug effects on cognitive function: A critical review. Pharm. J. 2016, 16, 430–438. [Google Scholar] [CrossRef]
- Valomon, A.; Holst, S.C.; Borrello, A.; Weigend, S.; Müller, T.; Berger, W.; Sommerauer, M.; Baumann, C.R.; Landolt, H.-P. Effects of COMT genotype and tolcapone on lapses of sustained attention after sleep deprivation in healthy young men. Neuropsychopharmacology 2018, 43, 1599–1607. [Google Scholar] [CrossRef]
- Cameron, I.G.; Wallace, D.L.; Al-Zughoul, A.; Kayser, A.S.; D’Esposito, M. Effects of tolcapone and bromocriptine on cognitive stability and flexibility. Psychopharmacology 2018, 235, 1295–1305. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Mishra, S. Investigation of mangiferin, as a promising natural polyphenol xanthone on multiple targets of Alzheimer’s disease. J. Biol. Act. Prod. Nat. 2014, 4, 111–119. [Google Scholar] [CrossRef]
- Morais, T.C.; Lopes, S.C.; Carvalho, K.M.; Arruda, B.R.; de Souza, F.T.C.; Trevisan, M.T.S.; Rao, V.S.; Santos, F.A. Mangiferin, a natural xanthone, accelerates gastrointestinal transit in mice involving cholinergic mechanism. World J. Gastroenterol. WJG 2012, 18, 3207. [Google Scholar]
- Decker, A.L.; Duncan, K. Acetylcholine and the complex interdependence of memory and attention. Curr. Opin. Behav. Sci. 2020, 32, 21–28. [Google Scholar] [CrossRef]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.; Butler, L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacology 2015, 232, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Tagougui, S.; Heyman, E.; Meeusen, R. Acute cocoa flavanol improves cerebral oxygenation without enhancing executive function at rest or after exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. 2), S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wightman, E.L.; Jackson, P.A.; Forster, J.; Khan, J.; Wiebe, J.C.; Gericke, N.; Kennedy, D.O. Acute Effects of a Polyphenol-Rich Leaf Extract of Mangifera indica L. (Zynamite) on Cognitive Function in Healthy Adults: A Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2020, 12, 2194. https://doi.org/10.3390/nu12082194
Wightman EL, Jackson PA, Forster J, Khan J, Wiebe JC, Gericke N, Kennedy DO. Acute Effects of a Polyphenol-Rich Leaf Extract of Mangifera indica L. (Zynamite) on Cognitive Function in Healthy Adults: A Double-Blind, Placebo-Controlled Crossover Study. Nutrients. 2020; 12(8):2194. https://doi.org/10.3390/nu12082194
Chicago/Turabian StyleWightman, Emma L., Philippa A. Jackson, Joanne Forster, Julie Khan, Julia C. Wiebe, Nigel Gericke, and David O. Kennedy. 2020. "Acute Effects of a Polyphenol-Rich Leaf Extract of Mangifera indica L. (Zynamite) on Cognitive Function in Healthy Adults: A Double-Blind, Placebo-Controlled Crossover Study" Nutrients 12, no. 8: 2194. https://doi.org/10.3390/nu12082194
APA StyleWightman, E. L., Jackson, P. A., Forster, J., Khan, J., Wiebe, J. C., Gericke, N., & Kennedy, D. O. (2020). Acute Effects of a Polyphenol-Rich Leaf Extract of Mangifera indica L. (Zynamite) on Cognitive Function in Healthy Adults: A Double-Blind, Placebo-Controlled Crossover Study. Nutrients, 12(8), 2194. https://doi.org/10.3390/nu12082194




