Natural Fermentation of Cowpea (Vigna unguiculata) Flour Improves the Nutritive Utilization of Indispensable Amino Acids and Phosphorus by Growing Rats
Abstract
1. Introduction
2. Materials and Methods
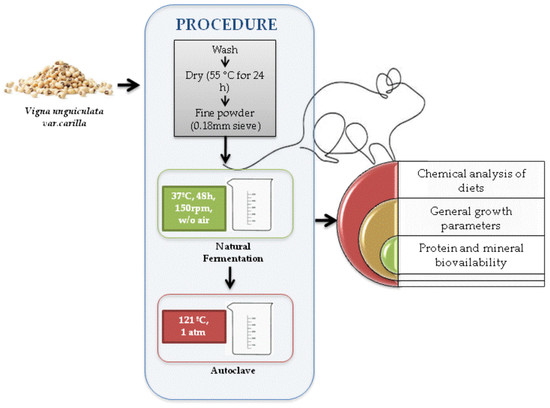
2.1. Plant Material, Fermentation and Thermal Treatment of the Fermented Flours
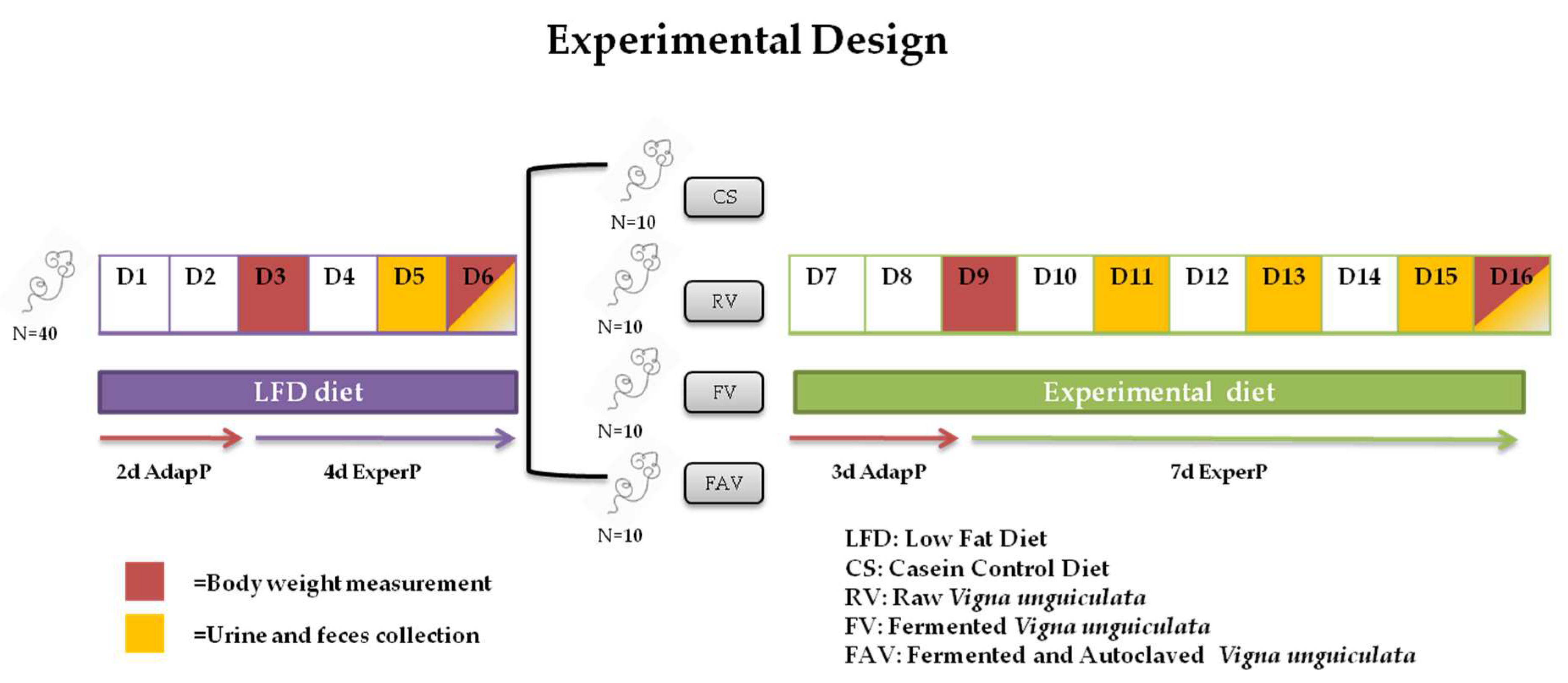
2.2. Animal Trial
2.3. Chemical Analysis
2.4. Biological Indices
2.5. Statistical Analysis
3. Results
3.1. Chemical Analysis
3.2. Biological Analysis
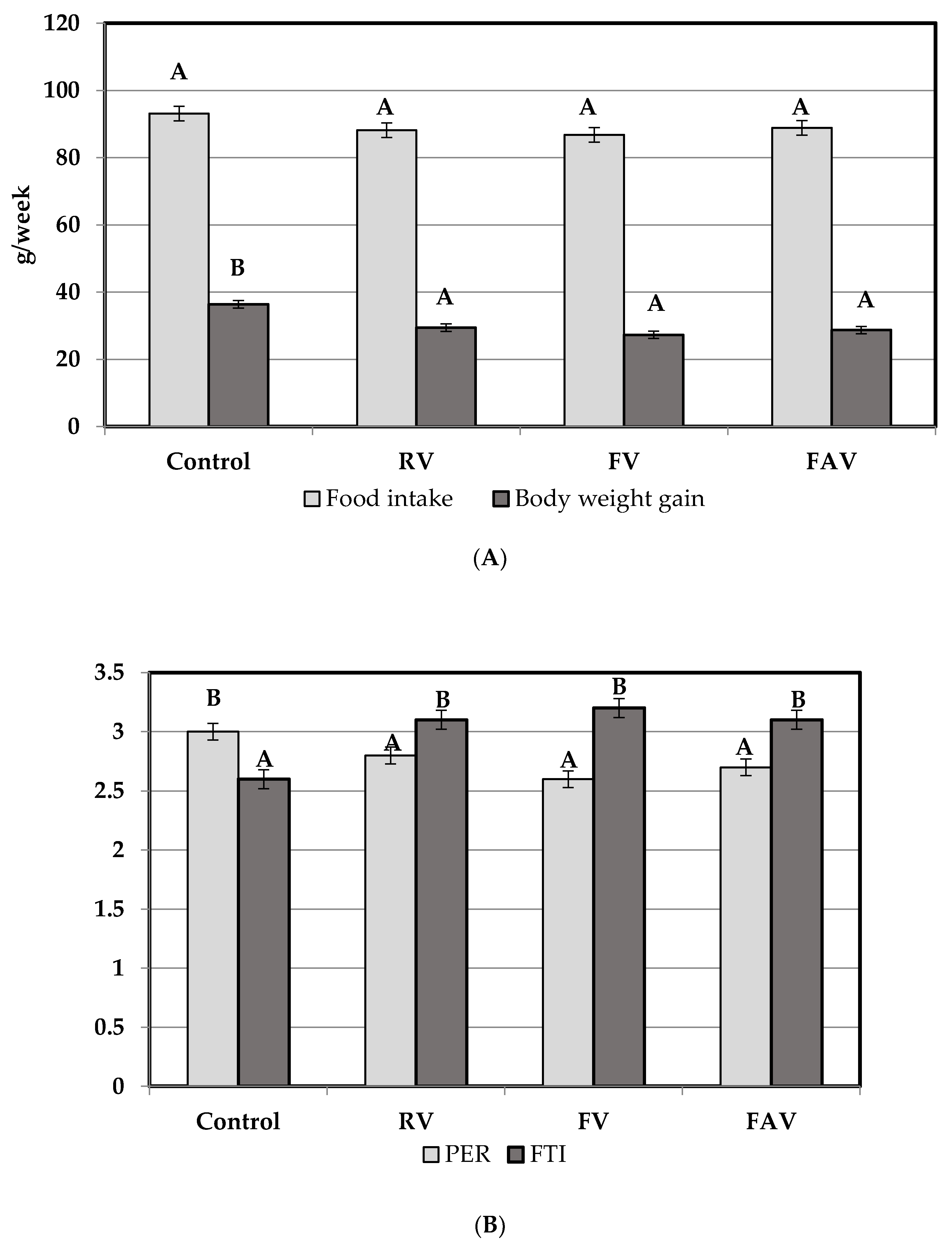
3.2.1. General Growth Parameters and Nutritive Utilization of N
3.2.2. Protein and Amino Acids Digestibility
3.2.3. Protein Quality Indices
3.2.4. Mineral Bioavailability
Phosphorus and Calcium
Magnesium and Potassium
3.2.5. Mineral Content in Tissues and Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Limitations of the Study
Appendix A

References
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Porres, J.M.; Aranda, P.; López-Jurado, M.; Urbano, G. Effect of Natural and controlled fermentation on chemical composition and nutrient dialyzability from beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2003, 51, 5144–5149. [Google Scholar] [CrossRef] [PubMed]
- Urbano, G.; López-Jurado, M.; Aranda, C.; Vilchez, A.; Cabrera, L.; Porres, J.M.; Aranda, P. Evaluation of zinc and magnesium bioavailability from pea (Pisum sativum, L.) sprouts. Effect of illumination and different germination periods. Int. J. Food Sci. Technol. 2006, 41, 618–626. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; López Chaves, C.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cañizares, F.J.; Galisteo, M.; Porres, J.M. Improvement of the antioxidant and hypolipidaemic effects of cowpea flours (Vigna unguiculata) by fermentation: Results of in vitro and in vivo experiments. J. Sci. Food Agric. 2014, 95, 1207–1216. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Nebot, E.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cantarero, S.; Galisteo, M.; Porres, J.M. The combined intervention with germinated Vigna radiata and aerobic interval training protocol is an effective strategy for the treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) and other alterations related to the metabolic syndrome in Zucker rats. Nutrients 2017, 9, 774. [Google Scholar] [CrossRef]
- Nwokolo, E.; Ilechukwu, S.N. Cowpea (Vigna unguiculata (L.) Walp.). In Food and Feed from Legumes and Oilseeds; Nwokolo, E., Smartt, J., Eds.; Chapman and Hall Publishing: London, UK, 1996; pp. 229–242. [Google Scholar]
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-Perles, R.; Trindade, H.; Rosa, E.A.S.; Ferreira, L.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agri-food system: Nutritional advantages and constraints. J. Sci. Food Agric. 2016, 96, 2941–2951. [Google Scholar] [CrossRef]
- Phillips, R.D.; McWatters, K.H.; Chinnan, M.S.; Hung, Y.C.; Beuchat, L.R.; Sefa-Dedeh, S.; Sakyi-Dawson, E.; Ngoddy, P.; Nnanyelugo, D.; Enwere, J.; et al. Utilization of cowpeas for human food. Field Crop. Res. 2003, 82, 193–213. [Google Scholar] [CrossRef]
- Ayogu, R.N.B.; Nnam, N.M.; Mbah, M. Evaluation of two local cowpea species for nutrient, antinutrient, and phytochemical compositions and organoleptic attributes of their wheat-based cookies. Food Nutr. Res. 2016, 60, 29600. [Google Scholar] [CrossRef][Green Version]
- Svanberg, U.; Lorri, W. Fermentation and nutrient availability. Food Control 1997, 8, 319–327. [Google Scholar] [CrossRef]
- Chawla, P.; Bhandari, L.; Sadh, P.K.; Kaushik, R. Impact of solid-state fermentation (Aspergillus oryzae) on functional properties and mineral bioavailability of black-eyed pea (Vigna unguiculata) seed flour. Cereal Chem. 2017, 94, 437–442. [Google Scholar] [CrossRef]
- Doblado, R.; Frias, J.; Muñoz, R.; Vidal-Valverde, C. Fermentation of Vigna sinensis var. carilla flours by natural microflora and Lactobacillus species. J. Food Prot. 2003, 66, 2313–2320. [Google Scholar]
- Valencia, S.; Svanberg, U.; Sandberg, A.S.; Ruales, J. Processing of quinoa (Chenopodium quinoa, Willd): Effects on in vitro iron availability and phytate hydrolysis. Int. J. Food Sci. Nutr. 1999, 50, 203–211. [Google Scholar] [CrossRef]
- Karlund, A.; Gomez-Gallego, C.; Korhonen, J.; Palo-oja, O.M.; El-Nezami, H.; Kolehmainen, M. Harnessing microbes for sustainable development: Food fermentation as a tool for improving the nutritional quality of alternative protein sources. Nutrients 2020, 12, 1020. [Google Scholar] [CrossRef]
- Yadav, N.; Kaur, D.; Malaviya, R.; Singh, M.; Fatima, M.; Singh, L. Effect of thermal and non-thermal processing on antioxidant potential of cowpea seeds. Int. J. Food Prop. 2018, 21, 437–451. [Google Scholar] [CrossRef]
- Torres, J.; Peters, M.; Montoya, C.A. Boiling influences the nutritional value of three seed cowpea (Vigna unguiculata) varieties using in vivo and in vitro methods. Food Chem. 2019, 297, UNSP 124940. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia faba). Nutrients 2018, 10, 671. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Hu, X.Z. Nutritional quality and techno-functional changes in raw, germinated and fermented yellow field pea (Pisum sativum L.) upon pasteurization. LWT. Food Sci. Technol. 2018, 92, 147–154. [Google Scholar] [CrossRef]
- Sarwar, G.; Peace, R.W. The protein-quality of some enteral products is inferior to that of casein as assessed by rat growth methods and digestibility-corrected amino-acid scores. J. Nutr. 1994, 124, 2223–2232. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Urbano, G.; Porres, J.M.; Frias, J.; Vidal-Valverde, C. Improvement in food intake and nutritive utilization of protein from Lupinus albus var. multolupa protein isolates supplemented with ascorbic acid. Food Chem. 2007, 103, 944–951. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey Jr, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- National Research Council (US) Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals, 4th ed.; National Academies Press: Washington, DC, USA, 1995; ISBN 978-0-309-05126-2. [Google Scholar]
- Martín-Cabrejas, M.A.; Sanfiz, B.; Vidal, A.; Mollá, E.; Esteban, R.; López-Andréu, F.J. Effect of fermentation and autoclaving on dietary fiber fractions and antinutritional factors of beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2004, 52, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Porres, J.M.; Urbano, G.; Fernández-Fígares, I.; Prieto, C.; Pérez, L.; Aguilera, J.F. Digestive utilisation of protein and amino acids from raw and heated lentils by growing rats: Effect of heat on digestibility of lentil protein and amino acids. J. Sci. Food Agric. 2002, 82, 1740–1747. [Google Scholar] [CrossRef]
- Chen, P.S.; Toribara, T.Y.; Warner, H. Microdetermination of Phosphorus. Anal. Chem. 1956, 28, 1756–1758. [Google Scholar] [CrossRef]
- Bell, S.J.; Bistrian, B.R.; Ainsley, B.M.; Manji, N.; Lewis, E.J.; Joyce, C.; Blackburn, G.L. A chemical score to evaluate the protein-quality of commercial parenteral and enteral formulas with emphasis on formulas for patients with liver-failure. J. Am. Diet. Assoc. 1991, 91, 586–589. [Google Scholar]
- Muranaka, S.; Shono, M.; Myoda, T.; Takeuchi, J.; Franco, J.; Nakazawa, Y.; Boukar, O.; Takagi, H. Genetic diversity of physical, nutritional and functional properties of cowpea grain and relationships among the traits. Plant. Genet. Resourc. 2016, 14, 67–76. [Google Scholar] [CrossRef]
- Carvalho, A.F.U.; de Sousa, N.M.; Farias, D.F.; da Rocha-Bezerra, L.C.B.; da Silva, R.M.P.; Viana, M.P.; Gouveia, S.T.; Sampaio, S.S.; de Sousa, M.B.; de Lima, G.P.G. Nutritional ranking of 30 Brazilian genotypes of cowpeas including determination of antioxidant capacity and vitamins. J. Food Comp. Anal. 2012, 26, 81–88. [Google Scholar] [CrossRef]
- Granito, M.; Torres, A.; Frias, J.; Guerra, M.; Vidal-Valverde, C. Influence of fermentation on the nutritional value of two varieties of Vigna sinensis. Eur. Food Res. Technol. 2005, 220, 176–181. [Google Scholar] [CrossRef]
- Akinyele, I.O.; Akinlosotu, A. Effect of soaking, dehulling and fermentation on the oligosaccharides and nutrient content of cowpeas (Vigna unguiculata). Food Chem. 1991, 41, 43–53. [Google Scholar] [CrossRef]
- Jirapa, P.; Normah, H.; Zamaliah, M.M.; Asmah, R.; Mohamad, K. Nutritional quality of germinated cowpea flour (Vigna unguiculata) and its application in home prepared powdered weaning foods. Plant. Foods Hum. Nutr. 2001, 56, 203–216. [Google Scholar] [CrossRef]
- Avanza, M.; Acevedo, B.; Chaves, M.; Añón, M. Nutritional and anti-nutritional components of four cowpea varieties under thermal treatments: Principal component analysis. LWT Food Sci. Technol. 2013, 51, 148–157. [Google Scholar] [CrossRef]
- Tenorio, M.D.; Espinosa-Martos, I.; Préstamo, G.; Rupérez, P. Soybean whey enhance mineral balance and caecal fermentation in rats. Eur. J. Nutr. 2010, 49, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Giami, S.Y. Compositional and nutritional properties of selected newly developed lines of cowpea (Vigna unguiculata L. Walp). J. Food Comp. Anal. 2005, 18, 665–673. [Google Scholar] [CrossRef]
- Urbano, G.; Lopez-Jurado, M.; Hernandez, J.; Fernandez, M.; Moreu, M.C.; Frias, J.; Diaz-Pollan, C.; Prodanov, M.; Vidal-Valverde, C. Nutritional assessment of raw, heated, and germinated lentils. J. Agric. Food Chem. 1995, 43, 1871–1877. [Google Scholar] [CrossRef]
- Urbano, G.; López-Jurado, M.; Ławomir Frejnagel, S.; Gómez-Villalva, E.; Porres, J.M.; Frías, J.; Vidal-Valverde, C.; Aranda, P. Nutritional assessment of raw and germinated pea (Pisum sativum L.) protein and carbohydrate by in vitro and in vivo techniques. Nutrition 2005, 21, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Nestares, T.; López-Frías, M.; Barrionuevo, M.; Urbano, G. Nutritional assessment of raw and processed Chickpea (Cicer arietinum L.) protein in growing rats. J. Agric. Food Chem. 1996, 44, 2760–2765. [Google Scholar] [CrossRef]
- Seabra, M.; Carvalho, S.; Freire, J.; Ferreira, R.; Mourato, M.; Cunha, L.; Cabral, F.; Teixeira, A.; Aumaitre, A. Lupinus luteus, Vicia sativa and Lathyrus cicera as protein sources for piglets: Ileal and total tract apparent digestibility of amino acids and antigenic effects. Anim. Feed Sci. Technol. 2001, 89, 1–16. [Google Scholar] [CrossRef]
- Rangel, A.; Saraiva, K.; Schwengber, P.; Narciso, M.S.; Domont, G.B.; Ferreira, S.T.; Pedrosa, C. Biological evaluation of a protein isolate from cowpea (Vigna unguiculata) seeds. Food Chem. 2004, 87, 491–499. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Jensen, B.B.; Andersen, J.O.; Hansen, I. Gastrointestinal implications in pigs of wheat and oat fractions. 2. Microbial activity in the gastrointestinal tract. Br. J. Nutr. 1991, 65, 233–248. [Google Scholar] [CrossRef]
- Goodlad, J.S.; Mathers, J.C. Digestion of complex carbohydrates and large bowel fermentation in rats fed on raw and cooked peas (Pisum sativum). Br. J. Nutr. 1992, 67, 475–488. [Google Scholar] [CrossRef]
- Rubio, L.A. Carbohydrates digestibility and faecal nitrogen excretion in rats fed raw or germinated faba bean (Vicia faba)-and chickpea (Cicer arietinum)-based diets. Br. J. Nutr. 2003, 90, 301–309. [Google Scholar] [CrossRef]
- Fernández-Fígares, I.; Ruiz, R.; Kapravelou, G.; Porres, J.M.; Rubio, L.A. Total bacteria as part of fecal endogenous losses of N in rats fed with Vigna unguiculata. In Proceedings of the XIV Jornadas Sobre Producción Animal, Zaragoza, España, 17–18 May 2011; pp. 869–871. [Google Scholar]
- Frota, K.M.G.; Lopes, L.A.R.; Silva, I.C.V.; Arêas, J.A.G. Nutritional quality of the protein of Vigna unguiculata L. Walp and its protein isolate. Rev. Ciênc. Agron. 2017, 48, 792–798. [Google Scholar] [CrossRef]
- Tshovhote, N.J.; Nesamvuni, A.E.; Raphulu, T.; Gous, R.M. The chemical composition, energy and amino acid digestibility of cowpeas used in poultry nutrition. S. Afr. J. Anim. Sci. 2003, 33, 65–69. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Habiba, R.A.; Shatta, A.A.; Embaby, H.E. Effect of soaking, germination, cooking and fermentation on antinutritional factors in cowpeas. Nahrung 2002, 46, 92–95. [Google Scholar] [CrossRef]
- Jezierny, D.; Mosenthin, R.; Bauer, E. The use of grain legumes as a protein source in pig nutrition: A review. Anim. Feed Sci. Technol. 2010, 157, 111–128. [Google Scholar] [CrossRef]
- Nielsen, H.K.; De Weck, D.; Finot, P.A.; Liardon, R.; Hurrell, R.F. Stability of tryptophan during food processing and storage. 1. Comparative losses of tryptophan, lysine and methionine in different model systems. Br. J. Nutr. 1985, 53, 281–292. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Carpenter, K.J. The estimation of available lysine foodstuffs after Maillard reactions. Prog. Food Nutr. Sci. 1981, 5, 159–176. [Google Scholar]
- Porres, J.M.; Aranda, P.; López-Jurado, M.; Urbano, G. Nutritional potential of raw and free alpha-galactosides lupin (Lupinus albus Var. multolupa) seed flours. Effect of phytase treatment on nitrogen and mineral dialyzability. J. Agric. Food Chem. 2005, 53, 3088–3094. [Google Scholar] [CrossRef]
| Diet a | LPD4% | Control | RV | FV | FAV |
|---|---|---|---|---|---|
| Ingredients (g·kg−1) | |||||
| Casein | 45.5 | 136.0 | - | - | - |
| Cowpea flour | - | - | 500.0 | 500.0 | 500.0 |
| Methionine | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Olive oil | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 |
| Cellulose | 50.0 | 26.0 | - | - | - |
| Agar | - | 27.0 | - | - | - |
| Oat xylan | - | 4.4 | - | - | - |
| Potato starch | - | 16.0 | - | - | - |
| Lignin | - | 13.0 | - | - | - |
| Citrus pectin | - | 38.5 | - | - | - |
| Sucrose | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Mineral mix | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Vitamin mix | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Calcium Citrate | 24.0 | 24.0 | 19.2 | 19.2 | 19.2 |
| CaHPO4 | - | - | 7.5 | 7.5 | 7.5 |
| Wheat starch | 658.0 | 495.0 | 250.0 | 250.0 | 250.0 |
| Chemical Composition (In Dry Matter) | |||||
| Total N (g·kg−1) | 20.8 | 19.0 | 19.6 | 19.0 | |
| Total amino acids (g·kg−1) | 132 | 107 | 104 | 101 | |
| Ash (g kg−1) | 35.7 | 41.0 | 42.0 | 42.5 | |
| Ca (mg kg−1) | 4806 | 5126 | 5283 | 5597 | |
| P (mg·kg−1) | 3025 | 4006 | 4302 | 4317 | |
| Mg (mg·kg−1) | 711 | 1165 | 1027 | 1014 | |
| K (mg·kg−1) | 4274 | 7345 | 7796 | 7322 | |
| Diet a | Egg | Control | RV | FV | FAV |
|---|---|---|---|---|---|
| Indispensable | |||||
| Arginine | 6.0 | 4.75 (6.26) | 9.09 (9.76) | 9.35 (9.75) | 8.59 (8.68) |
| Histidine | 2.2 | 2.96 (3.90) | 4.21 (4.53) | 4.17 (4.35) | 4.58 (4.62) |
| Isoleucine | 5.4 | 3.89 (5.12) | 4.80 (5.15) | 4.47 (4.66) | 4.33 (4.37) |
| Leucine | 8.6 | 10.60 (14.0) | 7.68 (8.24) | 7.42 (7.74) | 7.42 (7.50) |
| Lysine | 7.2 | 11.47 (15.1) | 7.08 (7.61) | 7.74 (8.07) | 7.90 (7.98) |
| Methionine | 5.7 (Met + Cys) | 3.23 (4.25) | 3.68 (3.95) | 3.67 (3.83) | 3.63 (3.67) |
| Phenylalanine | 9.3 (Phe + Tyr) | 6.26 (8.24) | 6.76 (7.26) | 5.85 (6.10) | 5.76 (5.82) |
| Tyrosine | - | 4.59 (6.04) | 4.21 (4.52) | 4.85 (5.06) | 4.39 (4.44) |
| Threonine | 4.7 | 4.38 (5.77) | 4.80 (5.15) | 4.05 (4.23) | 4.39 (4.44) |
| Valine | 6.6 | 5.62 (7.40) | 6.25 (6.71) | 5.58 (5.82) | 5.31 (5.37) |
| BCAA | 20.6 | 20.1 (26.5) | 18.7 (20.1) | 17.5 (18.2) | 17.1 (17.2) |
| Total indispensable | 55.7 | 57.4 (76.1) | 58.6 (62.9) | 57.2 (59.6) | 56.3 (56.9) |
| Dispensable | |||||
| Alanine | - | 4.45 (5.86) | 4.95 (5.32) | 4.77 (4.98) | 4.02 (4.06) |
| Aspartate | - | 6.40 (8.43) | 7.3 (7.87) | 8.99 (9.38) | 9.71 (9.82) |
| Cysteine | - | 0.61 (0.80) | 0.91 (0.98) | 1.00 (1.04) | 0.92 (0.93) |
| Glutamate | - | 16.73 (22.02) | 14.91 (16.01) | 13.44 (14.01) | 14.23 (14.39) |
| Glycine | - | 2.15 (2.83) | 4.35 (4.68) | 3.83 (4.00) | 4.06 (4.10) |
| Proline | - | 8.13 (10.70) | 5.15 (5.53) | 6.26 (6.53) | 6.20 (6.26) |
| Serine | - | 3.78 (4.98) | 3.85 (4.14) | 4.54 (4.73) | 4.56 (4.61) |
| Diets a | Control | RV | FV | FAV |
|---|---|---|---|---|
| N Intake (mg/d) | 276 (5.6) B | 239 (5.82) A | 244 (5.7) A | 248 (7.5) A |
| Total fecal N (mg/d) | 30 (0.9) A | 57 (1.6) B | 54 (2.2) B | 57 (2.4) B |
| Endogenous fecal N (mg/d) | 6.7 (0.4) AB | 8.0 (0.4) B | 5.8 (0.3) A | 7.8 (0.9) AB |
| Apparent absorbed N (mg/d) | 246 (5.0) B | 182 (4.9) A | 189 (4.4) A | 191 (5.9) A |
| True absorbed N (mg/d) | 252 (5.1) B | 190 (5.1) A | 195 (4.5) A | 198 (6.3) A |
| Total urinary N (mg/d) | 49 (2.5) | 42 (2.0) | 50 (2.7) | 51 (3.8) |
| Endogenous urinary N (mg/d) | 21 (1.8) | 25 (1.7) | 21 (1.3) | 21 (1.2) |
| Apparent retained N (mg/d) | 197 (3.8) A | 140 (4.7) B | 140 (3.7) B | 140 (6.0) B |
| True retained N (mg/d) | 225 (3.8) B | 173 (4.7) A | 164 (3.8) A | 168 (5.2) A |
| Apparent R/A | 0.80 (0.008) B | 0.77 (0.006) AB | 0.74 (0.012) A | 0.74 (0.014) A |
| True R/A | 0.89 (0.01) B | 0.91 (0.01) B | 0.85 (0.01) A | 0.85 (0.02) A |
| Diets a | Control | RV | FV | FAV |
|---|---|---|---|---|
| Indispensable | ||||
| Arginine | 885 (7.2) B | 834 (8.3) A | 860 (6.6) AB | 835 (9.6) A |
| Histidine | 892 (4.2) B | 837 (26.8) A | 847 (8.4) AB | 839 (9.2) AB |
| Isoleucine | 878 (3.9) B | 779 (10.0) A | 785 (9.8) A | 762 (12.6) A |
| Leucine | 939 (1.5) C | 756 (11.5) A | 810 (9.3) B | 791 (10.6) B |
| Lysine | 928 (1.8) C | 692 (14.6) A | 811 (10.6) B | 798 (12.2) B |
| Methionine | 936 (3.0) B | 892 (10.4) A | 965 (1.3) D | 948 (2.4) C |
| Phenylalanine | 938 (2.7) C | 724 (11.9) A | 808 (9.7) B | 790 (10.4) B |
| Tyrosine | 894 (3.8) C | 774 (10.9) A | 834 (8.4) B | 802 (10.8) AB |
| Threonine | 917 (3.8) B | 820 (8.5) A | 797 (12.1) A | 791 (12.0) A |
| Valine | 907 (2.5) C | 751 (10.6) A | 808 (8.8) B | 795 (10.9) B |
| Dispensable | ||||
| Alanine | 892 (2.7) C | 729 (14.1) A | 790 (10.2) B | 728 (18.8) A |
| Aspartate | 903 (4.3) C | 803 (10.5) A | 896 (5.2) B | 897 (8.3) B |
| Cysteine | 759 (12.3) AB | 727 (25.7) A | 792 (8.3) B | 747 (11.8) A |
| Glutamate | 939 (2.1) B | 835 (8.0) A | 845 (6.8) A | 843 (9.5) A |
| Glycine | 805 (8.2) B | 758 (10.8) A | 778 (11.1) AB | 765 (14.0) AB |
| Proline | 951 (2.3) D | 739 (12.9) A | 793 (11.6) B | 839 (6.8) C |
| Serine | 904 (3.4) B | 799 (9.3) A | 816 (9.9) A | 800 (11.4) A |
| Sum of amino acids | 920 (2.4) C | 804 (8.8) A | 843 (7.2) B | 836 (8.9) B |
| Protein | 891 (2.4) B | 761 (4.0) A | 771 (7.9) A | 787 (9.0) A |
| Diets a | Control | RV | FV | FAV |
|---|---|---|---|---|
| Indispensable | ||||
| Arginine | 968 (6.9) B | 922 (8.5) A | 942 (6.8) AB | 922 (10.1) A |
| Histidine | 953 (3.9) C | 868 (26.2) A | 911 (8.4) B | 907 (9.6) B |
| Isoleucine | 921 (4.0) B | 830 (10.0) A | 829 (9.9) A | 808 (12.9) A |
| Leucine | 970 (1.6) C | 788 (11.5) A | 841 (9.4) B | 823 (10.8) B |
| Lysine | 962 (1.9) C | 728 (14.6) A | 845 (10.6) B | 834 (12.4) B |
| Methionine | 1004 (2.7) D | 961 (8.5) A | 990 (1.3) C | 974 (2.6) B |
| Phenylalanine | 973 (2.7) C | 761 (11.8) A | 841 (9.8) B | 826 (10.6) B |
| Tyrosine | 928 (4.1) C | 810 (11.0) A | 868 (8.5) B | 838 (11.0) AB |
| Threonine | 973 (3.7) B | 880 (8.5) A | 853 (12.1) A | 850 (12.4) A |
| Valine | 951 (2.6) C | 808 (10.7) A | 852 (9.0) B | 841 (11.1) AB |
| Dispensable | ||||
| Alanine | 956 (2.6) C | 856 (14.4) B | 855 (10.2) B | 796 (19.2) A |
| Aspartate | 936 (4.3) B | 839 (10.5) A | 931 (5.4) B | 935 (8.7) B |
| Cysteine | 940 (9.5) C | 929 (25.4) C | 844 (8.4) B | 801 (12.0) A |
| Glutamate | 986 (2.0) B | 884 (8.0) A | 891 (7.0) A | 893 (9.9) A |
| Glycine | 873 (8.0) | 829 (10.8) | 845 (11.1) | 835 (14.4) |
| Proline | 958 (2.2) D | 747 (12.9) A | 801 (11.6) B | 847 (6.8) C |
| Serine | 983 (3.3) B | 882 (9.4) A | 895 (10.0) A | 884 (11.9) A |
| Sum of amino acids | 966 (2.4) C | 852 (8.7) A | 889 (7.3) B | 884 (9.2) B |
| Protein | 915 (2.3) B | 794 (5.8) A | 802 (6.9) A | 801 (5.8) A |
| Diets a | Control | RV | FV | FAV |
|---|---|---|---|---|
| Phosphorus | ||||
| P Intake (mg/d) | 40.1 (0.8) A | 50.6 (1.2) B | 52.9 (1.2) B | 54.7 (1.7) B |
| Fecal Excretion (g/d) | 1.30 (0.04) B | 1.01 (0.04) A | 0.98 (0.04) A | 1.09 (0.04) A |
| Fecal P (mg/d) | 5.99 (0.30) A | 23.8 (0.95) C | 19.8 (0.9) B | 20.6 (1.1) B |
| Urinary P (mg/d) | 0.47 (0.05) | 0.94 (0.14) | 0.31 (0.03) | 0.28 (0.04) |
| Absorbed P (mg/d) | 34.1 (0.6) B | 26.7 (0.5) A | 33.1 (0.97) B | 34.2 (0.90) B |
| P AFD | 0.85 (0.01) C | 0.53 (0.01) A | 0.63 (0.01) B | 0.63 (0.01) B |
| Retained P (mg/d) | 33.7 (0.6) B | 25.8 (0.5) A | 32.8 (1.0) B | 33.9 (0.9) B |
| P R/A | 0.99 (0.001) | 0.97 (0.005) | 0.99 (0.001) | 0.99 (0.001) |
| Calcium | ||||
| Ca Intake (mg/d) | 63.8 (1.3) | 64.7 (1.6) | 65.7 (1.5) | 71.0 (2.1) |
| Fecal Ca (mg/d) | 14.4 (0.6) A | 37.7 (1.4) B | 40.3 (2.5) BC | 45.6 (2.3) C |
| Urinary Ca (mg/d) | 9.14 (0.41) B | 8.56 (0.35) B | 5.81 (0.25) A | 4.92 (0.33) A |
| Absorbed Ca (mg/d) | 48.7 (1.0) B | 27.0 (1.23) A | 23.6 (1.8) A | 25.4 (1.3) A |
| Ca AFD | 0.76 (0.01) B | 0.42 (0.02) A | 0.37 (0.03) A | 0.36 (0.02) A |
| Retained Ca (mg/d) | 39.5 (1.0) B | 18.7 (1.0) A | 17.8 (1.7) A | 20.5 (1.2) A |
| Ca R/A | 0.81 (0.01) B | 0.69 (0.01) A | 0.74 (0.02) AB | 0.80 (0.01) B |
| Diets a | Control | RV | FV | FAV |
|---|---|---|---|---|
| Magnesium | ||||
| Mg Intake (mg/d) | 9.4 (0.2) A | 14.7 (0.4) C | 12.8 (0.3) B | 12.9 (0.4) B |
| Fecal Mg (mg/d) | 3.53 (0.13) A | 5.55 (0.19) B | 4.87 (0.21) B | 5.62 (0.23) B |
| Urinary Mg (mg/d) | 2.77 (0.16) A | 5.60 (0.25) C | 4.76 (0.30) BC | 4.33 (0.27) B |
| Absorbed Mg (mg/d) | 5.90 (0.17) A | 9.16 (0.27) C | 7.90 (0.21) B | 7.24 (0.20) B |
| Mg AFD | 0.63 (0.01) B | 0.62 (0.01) B | 0.61 (0.01) B | 0.56 (0.01) A |
| Retained Mg (mg/d) | 3.14 (0.15) | 3.56 (0.08) | 3.14 (0.31) | 2.91 (0.22) |
| Mg R/A | 0.53 (0.02) B | 0.39 (0.01) A | 0.40 (0.03) A | 0.40 (0.03) A |
| Potassium | ||||
| K Intake (mg/d) | 56.7 (1.2) A | 92.8 (2.3) B | 96.9 (2.3) B | 92.9 (2.8) B |
| Fecal K (mg/d) | 5.23 (0.67) | 8.40 (1.05) | 5.64 (0.81) | 6.20 (0.70) |
| Urinary K (mg/d) | 23.6 (0.90) A | 52.5 (3.1) B | 56.4 (4.0) B | 51.9 (3.4) B |
| Absorbed K (mg/d) | 51.5 (1.0) A | 84.4 (3.0) B | 91.3 (2.2) B | 85.7 (2.3) B |
| K AFD | 0.91 (0.01) A | 0.91 (0.01) A | 0.94 (0.08) A | 0.92 (0.01) A |
| Retained K (mg/d) | 27.9 (0.9) | 31.9 (1.1) | 32.9 (2.0) | 33.7 (1.9) |
| K R/A | 0.54 (0.01) B | 0.38 (0.02) A | 0.36 (0.03) A | 0.40 (0.03) A |
| Diets a | Control | RV | FV | FAV |
|---|---|---|---|---|
| Femur Ash (%) | 55.7 (0.7) | 55.5 (0.5) | 55.8 (0.4) | 55.1 (0.4) |
| LD muscle Ash (%) | 4.58 (0.18) AB | 4.29 (0.27) A | 5.33 (0.22) B | 4.52 (0.23) AB |
| P | ||||
| Blood (mg·100 mL−1) | 48.6 (1.4) | 49.0 (0.6) | 46.5 (2.6) | 44.8 (1.5) |
| Femur (mg·g−1 Ash) | 174.8 (1.0) | 174.8 (1.6) | 173.2 (0.7) | 173.0 (0.7) |
| LD muscle (mg·g−1 Ash) | 199.7 (1.5) B | 193.0 (1.3) A | 190.8 (1.3) A | 189.2 (2.2) A |
| Ca | ||||
| Blood (mg·100 mL−1) | 6.56 (0.70) B | 5.00 (0.18) A | 5.45 (0.44) AB | 5.66 (0.36) AB |
| Femur (mg·g−1 Ash) | 310.1 (2.7) B | 320.1 (4.5) B | 291.6 (7.9) A | 271.1 (5.4) A |
| LD muscle (mg·g−1 Ash) | 27.5 (7.8) | 33.2 (7.5) | 26.5 (5.9) | 25.5 (6.3) |
| Mg | ||||
| Blood (mg·100 mL−1) | 4.34 (0.14) | 4.37 (0.07) | 4.27 (0.22) | 4.24 (0.13) |
| Femur (mg·g−1 Ash) | 7.27 (0.11) A | 7.93 (0.10) B | 7.61 (0.04) AB | 7.52 (0.03) A |
| LD muscle (mg·g−1 Ash) | 22.5 (0.5) B | 21.6 (0.4) AB | 20.9 (0.5) AB | 20.0 (0.8) A |
| K | ||||
| Blood (mg·100 mL−1) | 207.8 (12.6) | 197.6 (4.5) | 197.9 (15.9) | 224.2 (10.5) |
| Femur (mg·g−1 Ash) | 9.94 (0.39) | 11.08 (0.58) | 9.66 (0.27) | 11.24 (0.31) |
| LD muscle (mg·g−1 Ash) | 290.7 (9.1) | 287.2 (8.4) | 286.2 (9.9) | 277.0 (7.9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapravelou, G.; Martínez, R.; Martino, J.; Porres, J.M.; Fernández-Fígares, I. Natural Fermentation of Cowpea (Vigna unguiculata) Flour Improves the Nutritive Utilization of Indispensable Amino Acids and Phosphorus by Growing Rats. Nutrients 2020, 12, 2186. https://doi.org/10.3390/nu12082186
Kapravelou G, Martínez R, Martino J, Porres JM, Fernández-Fígares I. Natural Fermentation of Cowpea (Vigna unguiculata) Flour Improves the Nutritive Utilization of Indispensable Amino Acids and Phosphorus by Growing Rats. Nutrients. 2020; 12(8):2186. https://doi.org/10.3390/nu12082186
Chicago/Turabian StyleKapravelou, Garyfallia, Rosario Martínez, Jole Martino, Jesus M. Porres, and Ignacio Fernández-Fígares. 2020. "Natural Fermentation of Cowpea (Vigna unguiculata) Flour Improves the Nutritive Utilization of Indispensable Amino Acids and Phosphorus by Growing Rats" Nutrients 12, no. 8: 2186. https://doi.org/10.3390/nu12082186
APA StyleKapravelou, G., Martínez, R., Martino, J., Porres, J. M., & Fernández-Fígares, I. (2020). Natural Fermentation of Cowpea (Vigna unguiculata) Flour Improves the Nutritive Utilization of Indispensable Amino Acids and Phosphorus by Growing Rats. Nutrients, 12(8), 2186. https://doi.org/10.3390/nu12082186