Nerolidol Mitigates Colonic Inflammation: An Experimental Study Using both In Vivo and In Vitro Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Experimental Design
2.4. Evaluation of the Clinical Score for Colitis and Construction of Disease Activity Index (DAI)
2.5. Quantification of Proinflammatory Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Histopathological Evaluation
2.7. RNA Extraction and Real Time RT-PCR
2.8. Preparation of Cytosolic and Nuclear Fractions
2.9. Measurement of Superoxide Dismutase, Catalase Enzyme Activity, and Tissue Nitrite Concentration
2.10. Western Blot
2.11. HT-29 Cell Culture
2.12. Cell Viability Assay
2.13. Statistical Analysis
3. Results
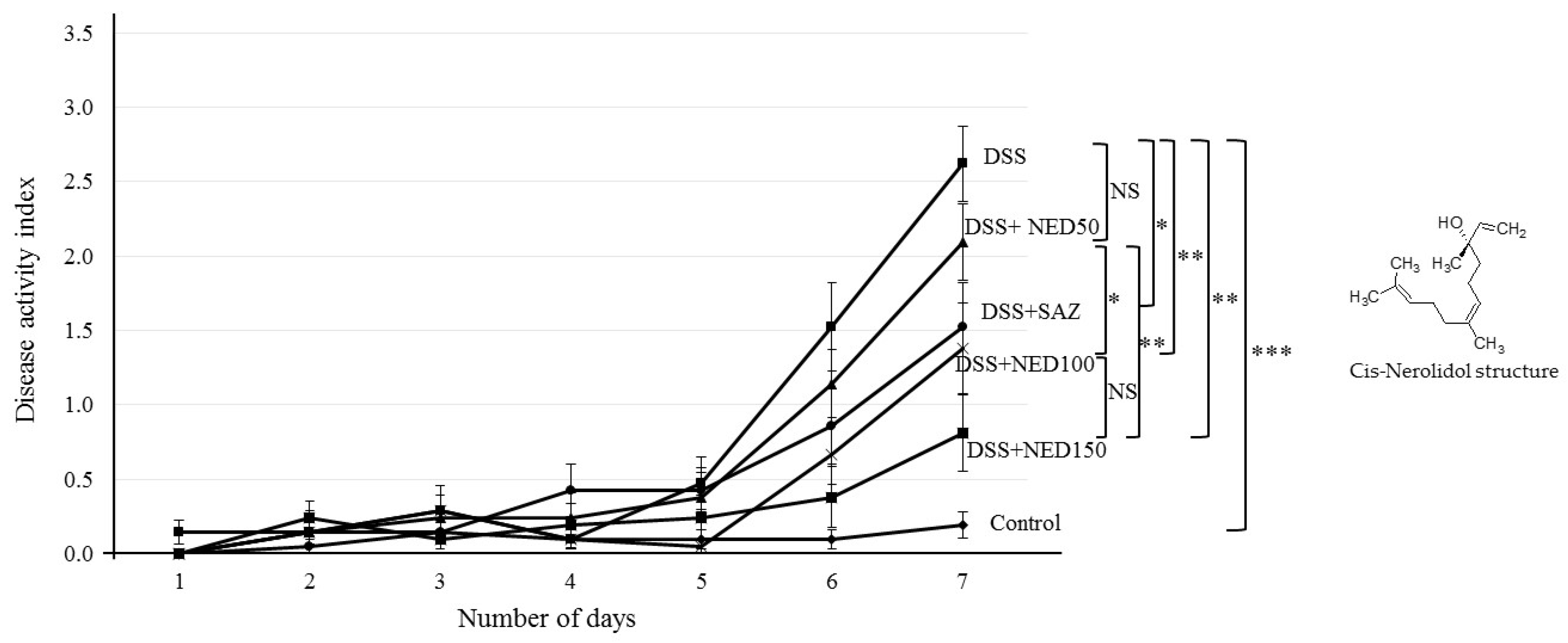
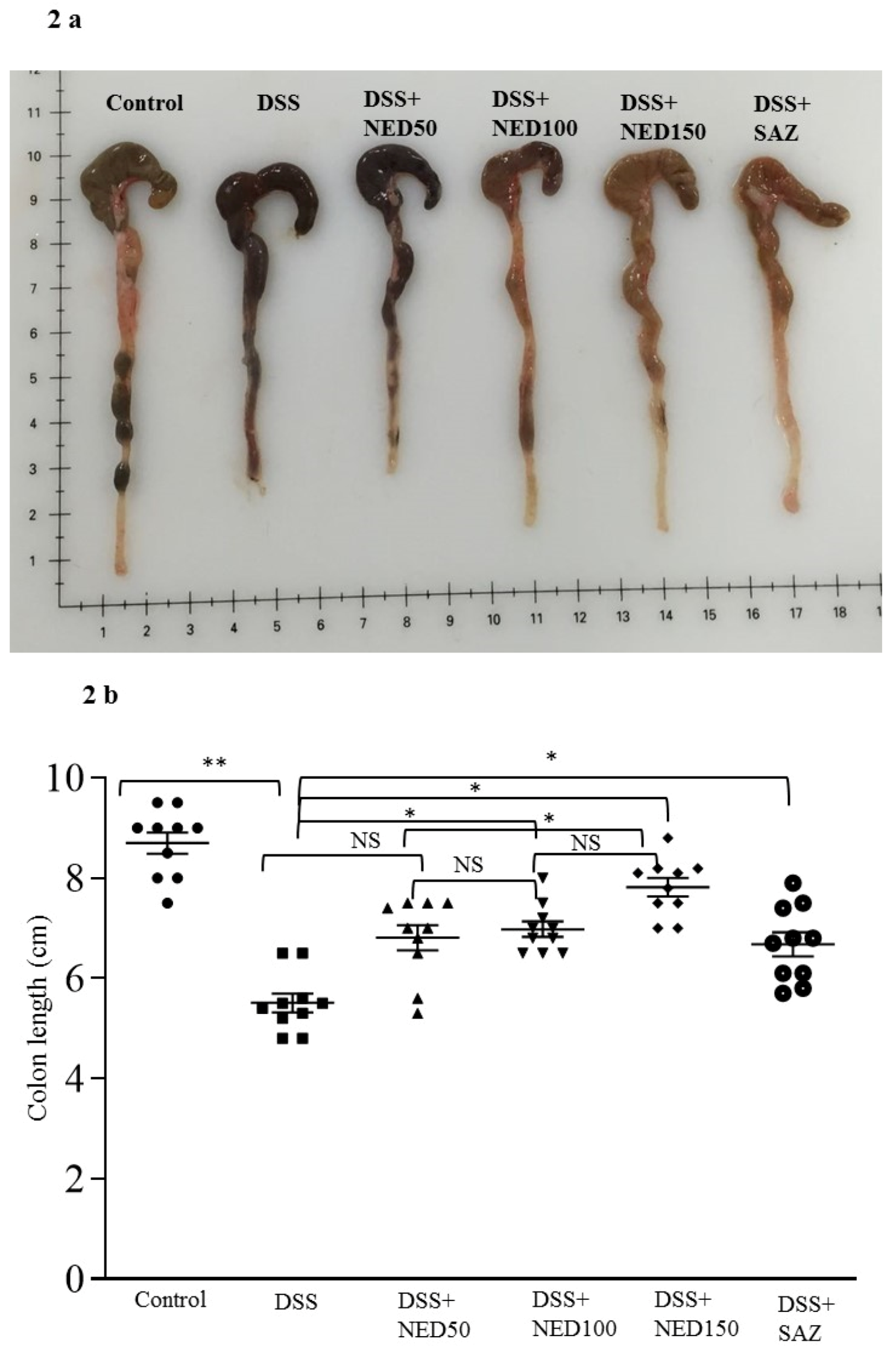
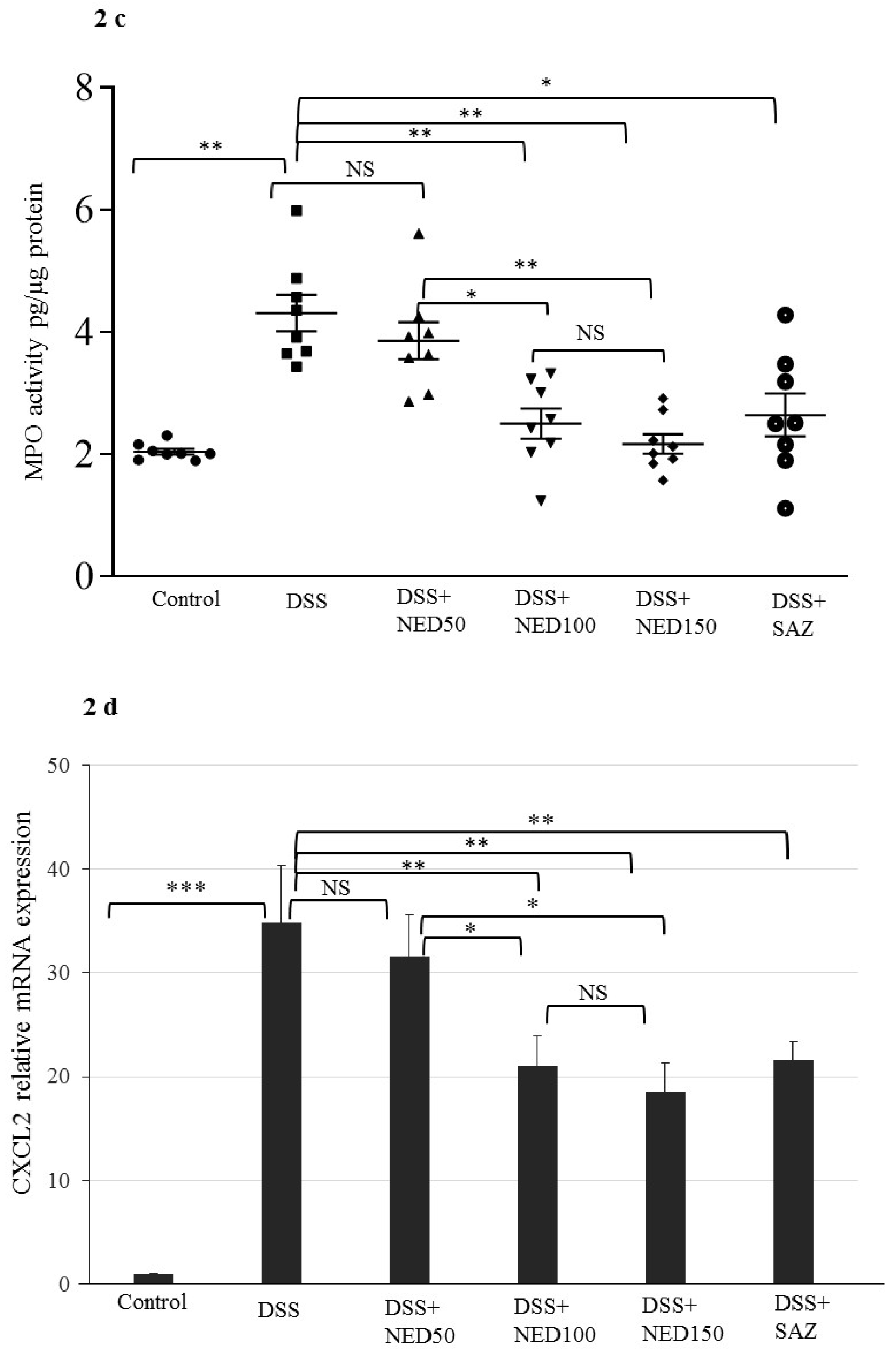
3.1. Effect of NED on Disease Activity Index (DAI), Colon Length, MPO Concentration, and CXCL2 and CCL2 mRNA
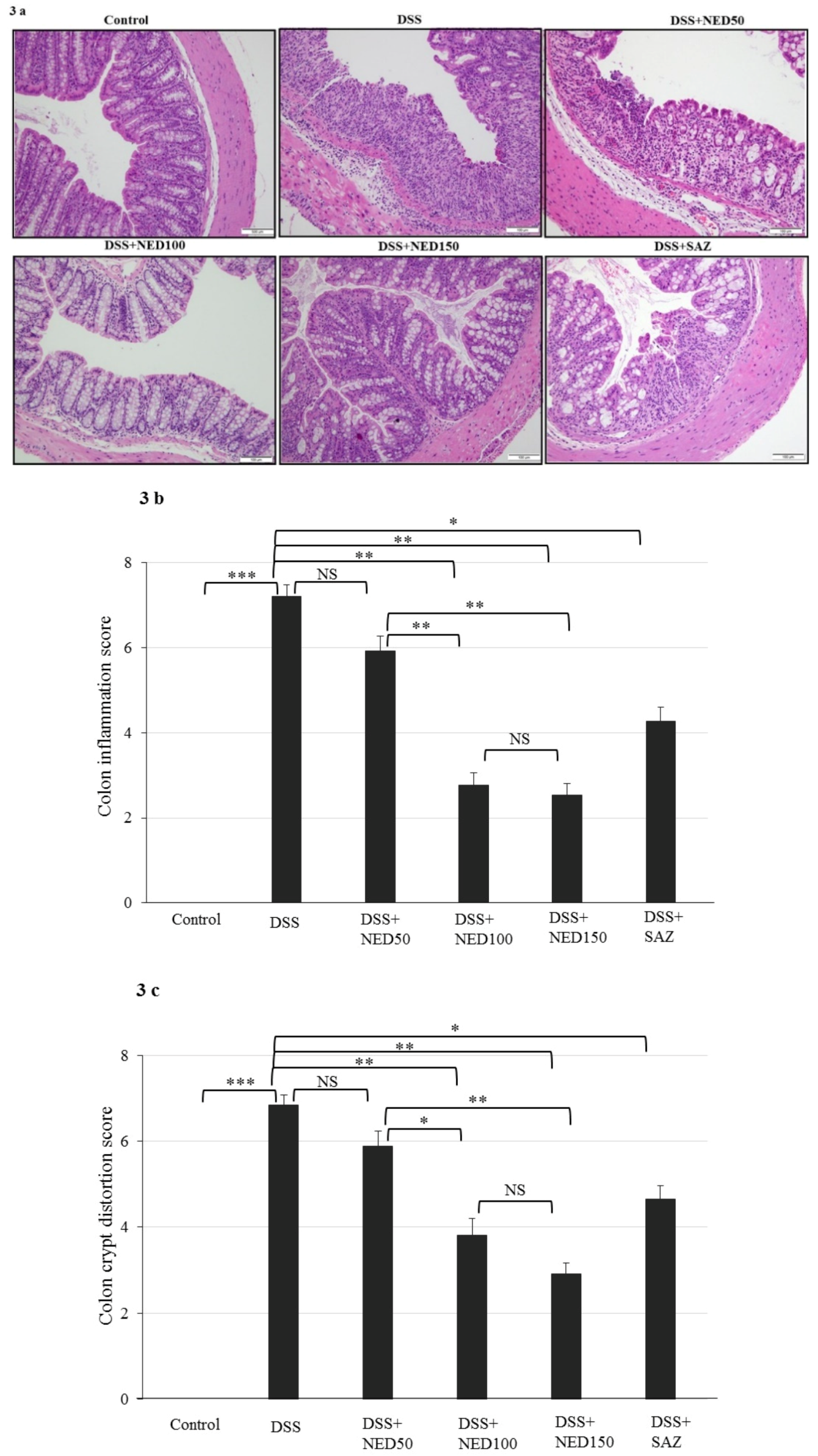
3.2. Effect of NED on Colon Microscopic Architecture
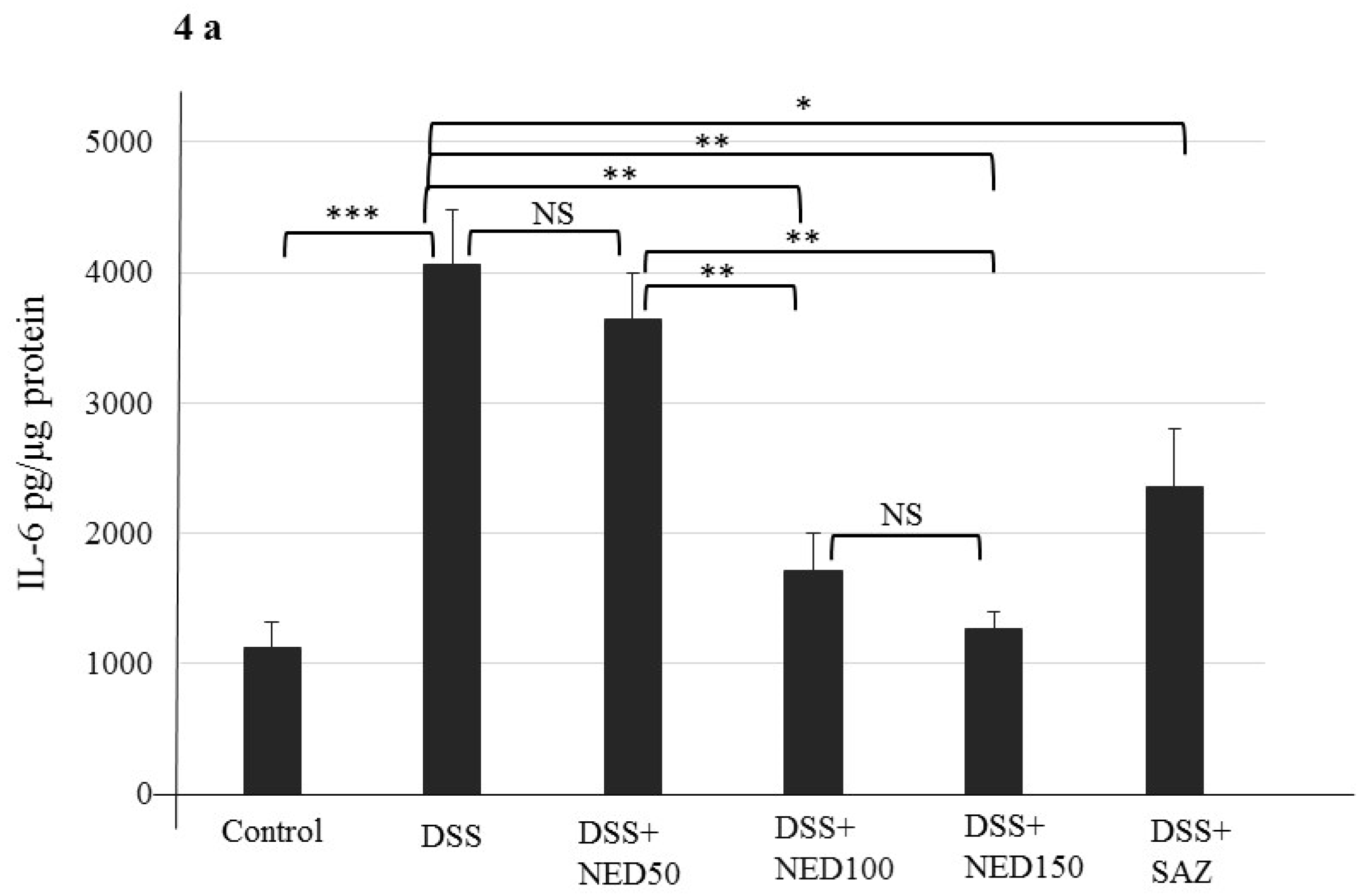
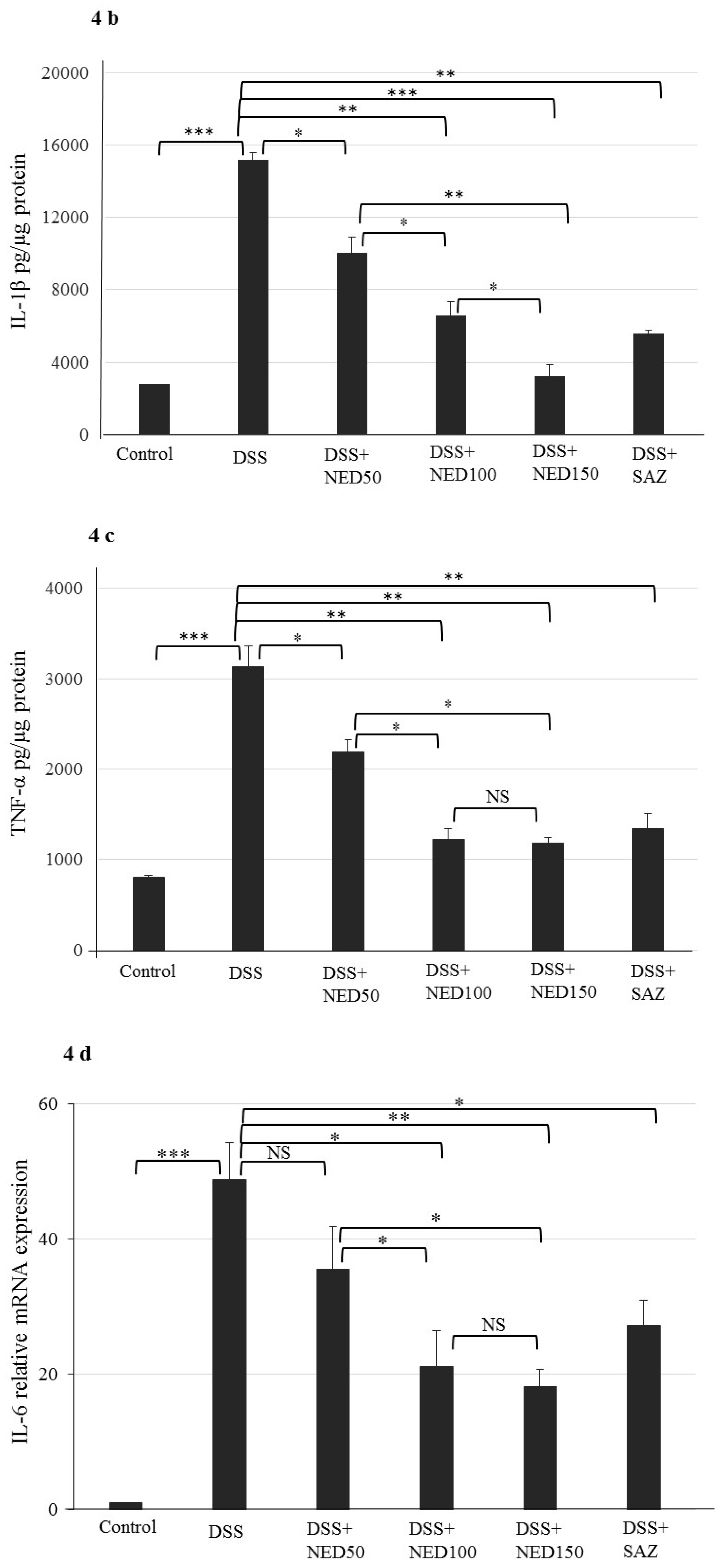
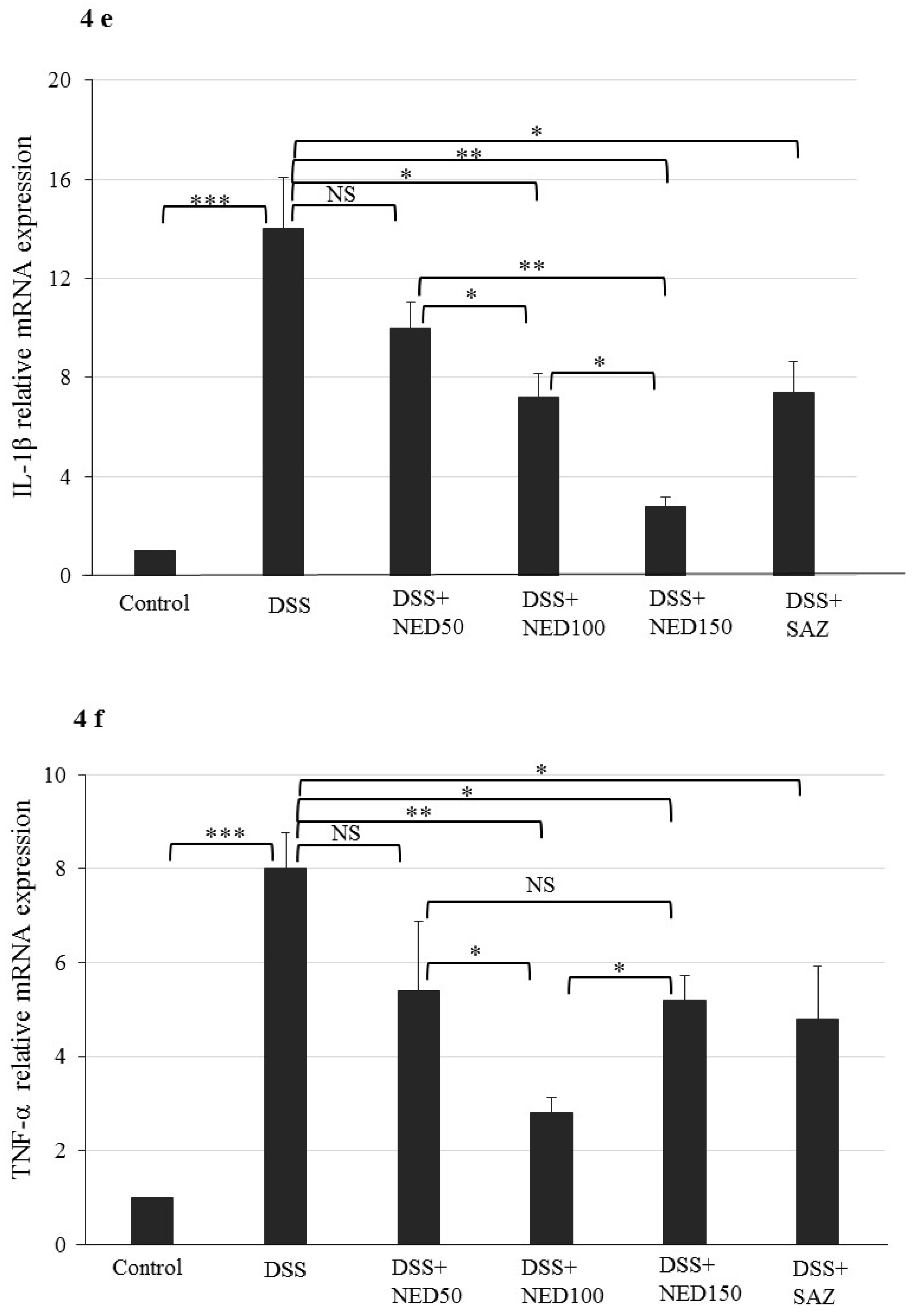
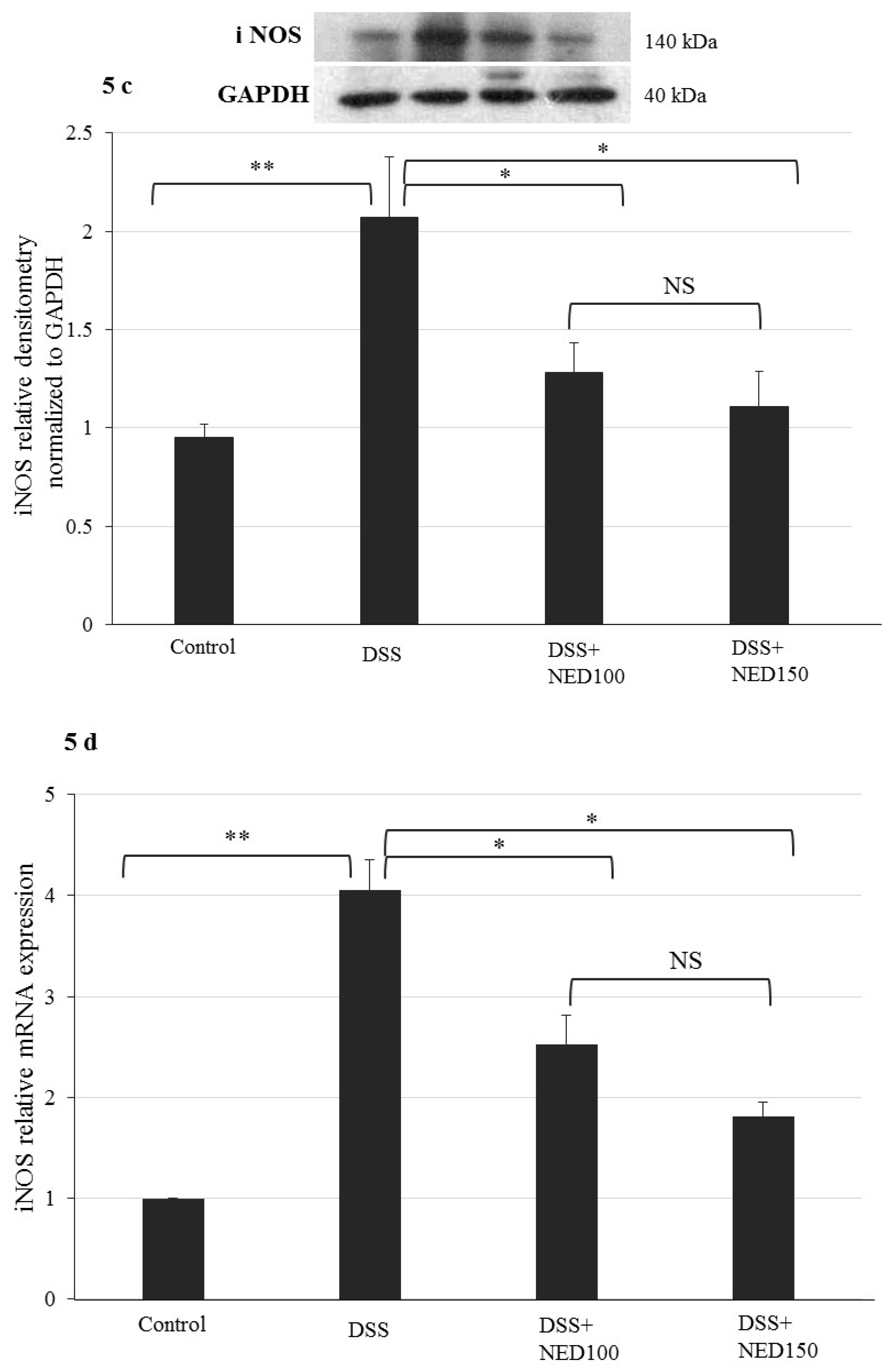
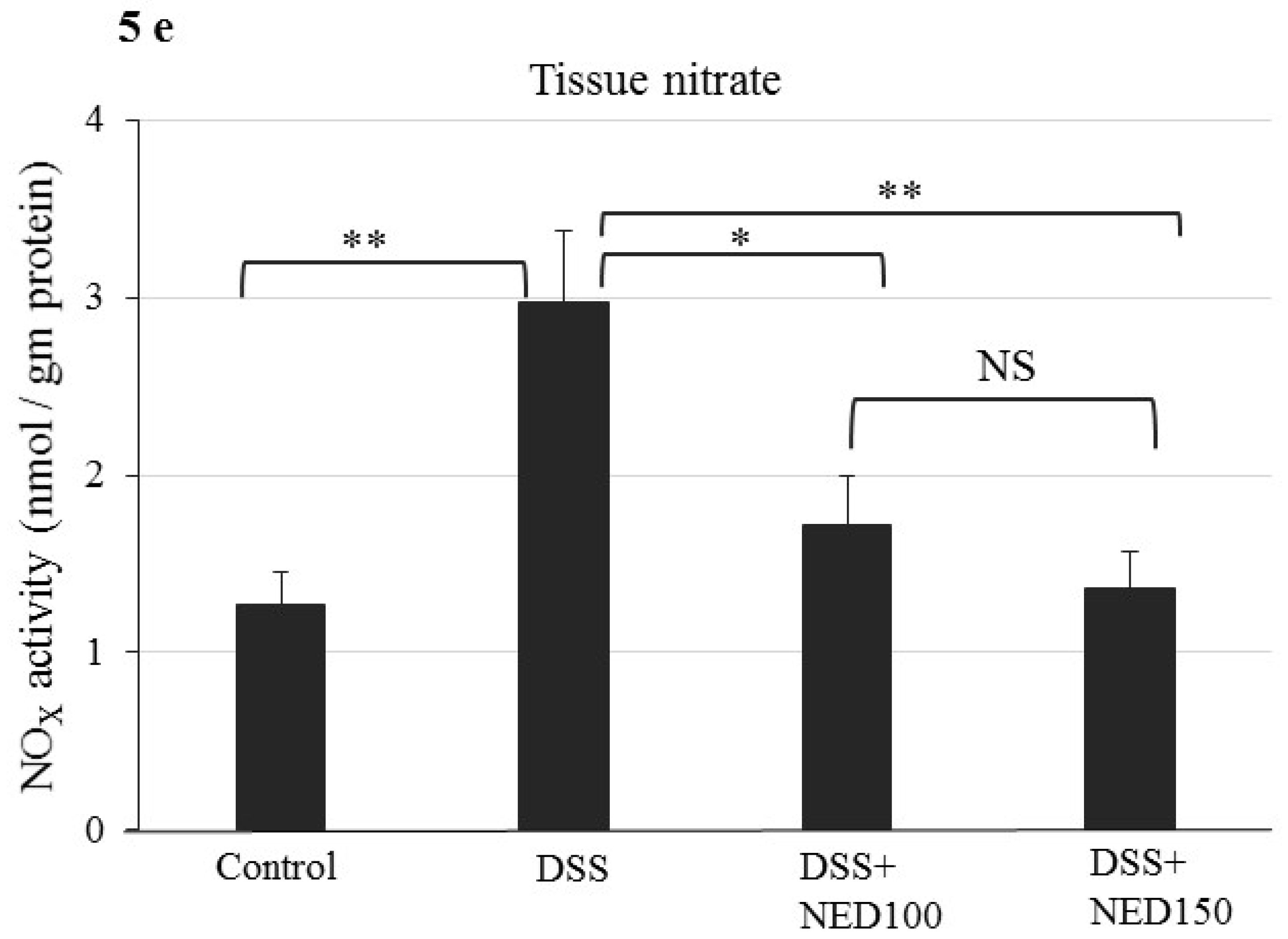
3.3. Effect of NED on Proinflammatory Cytokines and Proinflammatory Mediators
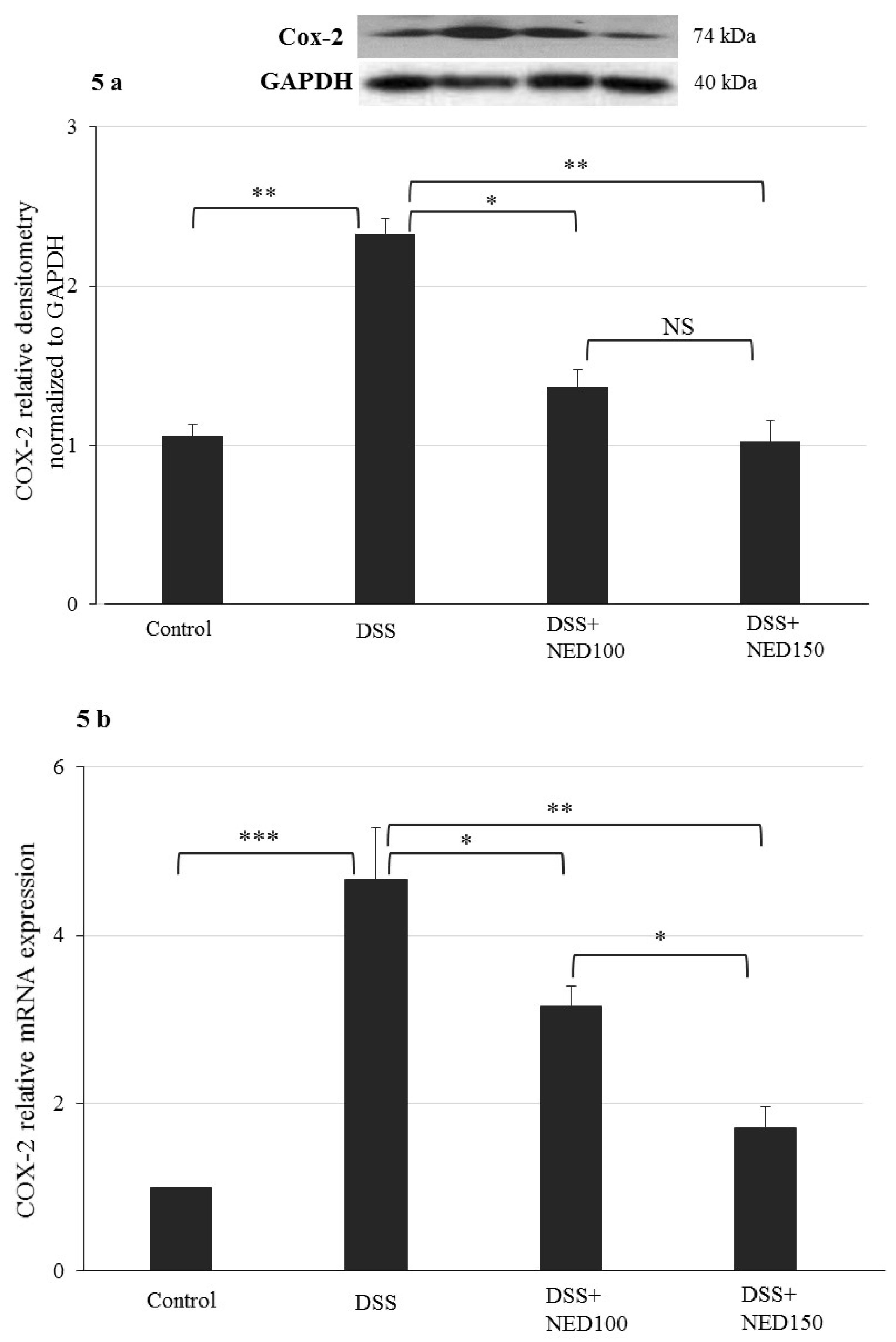
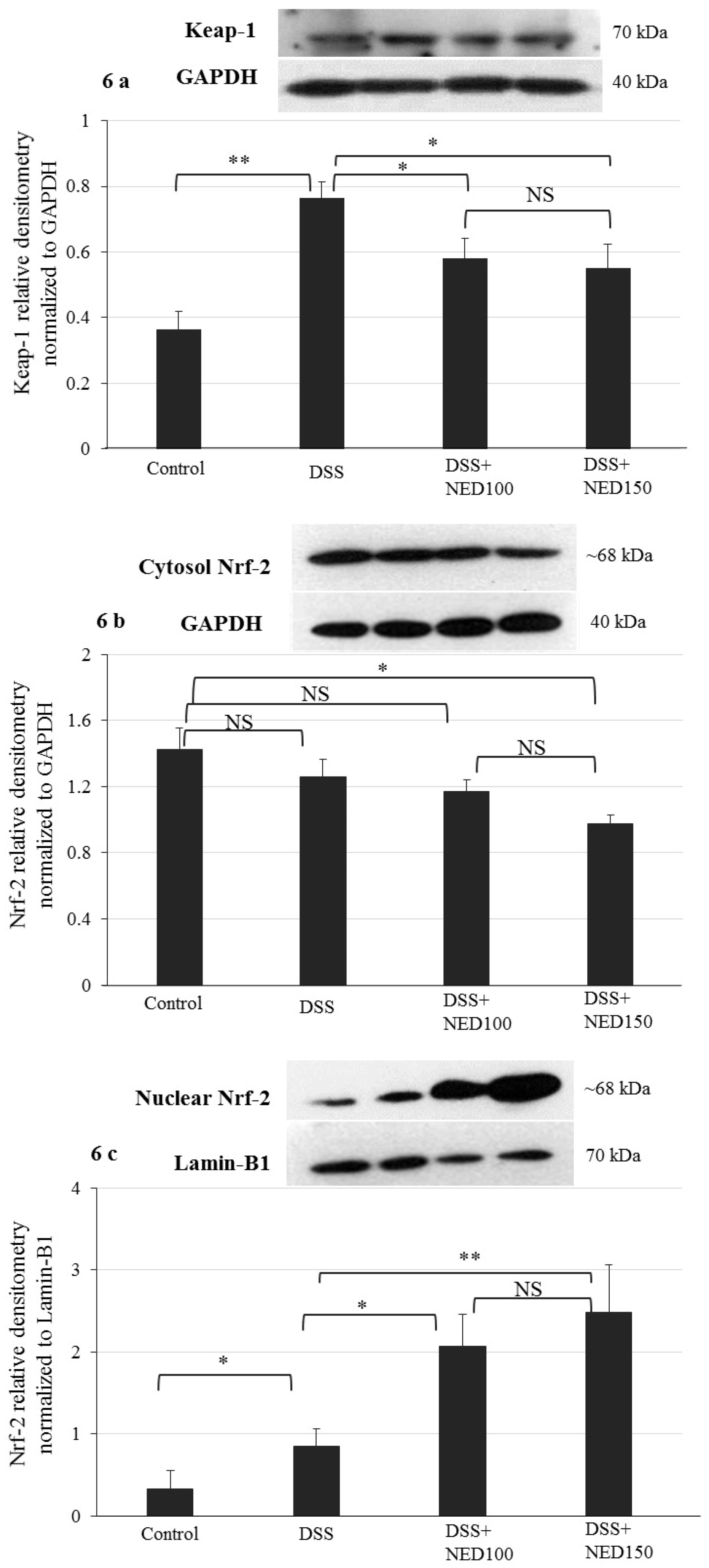
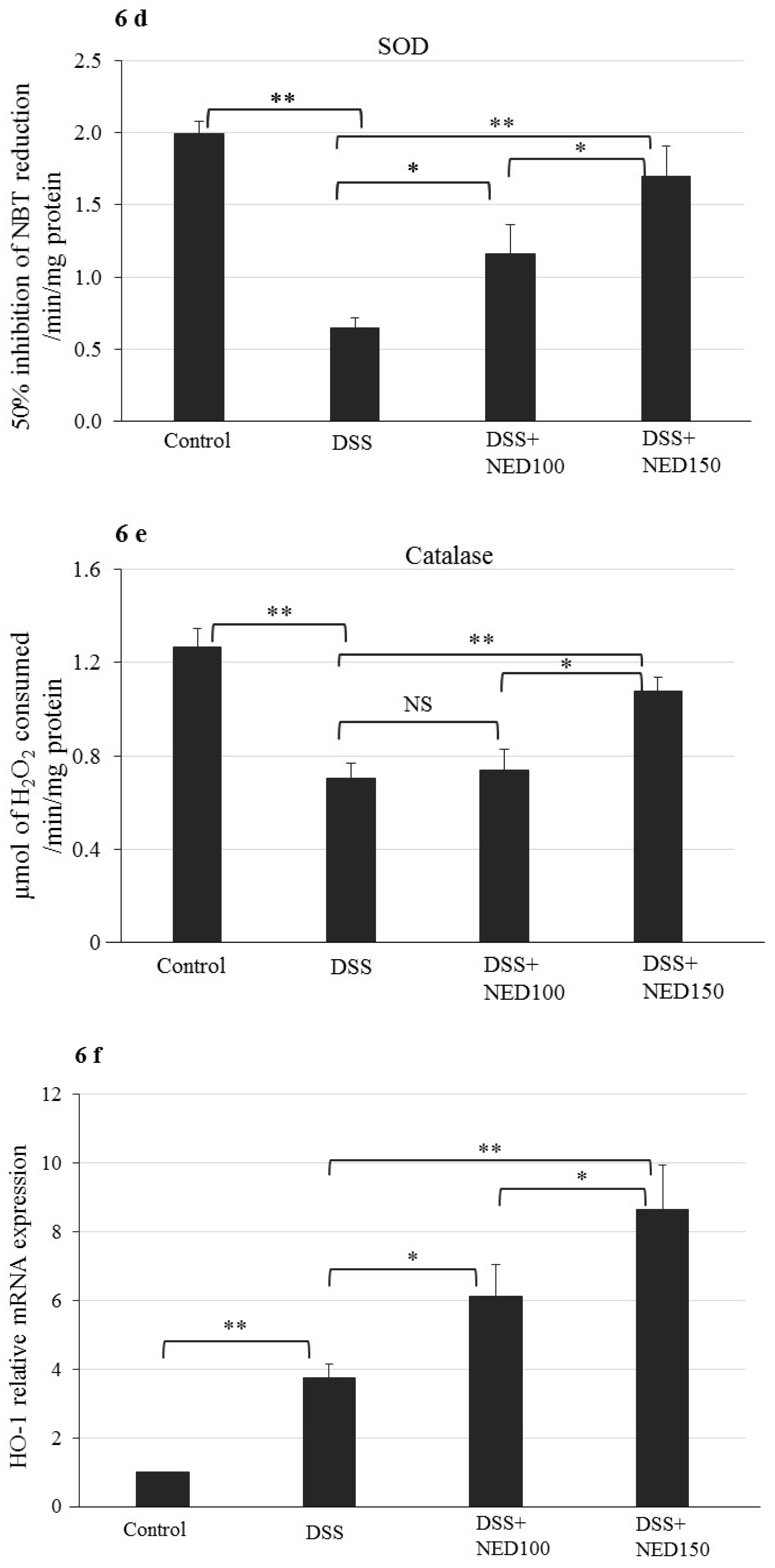
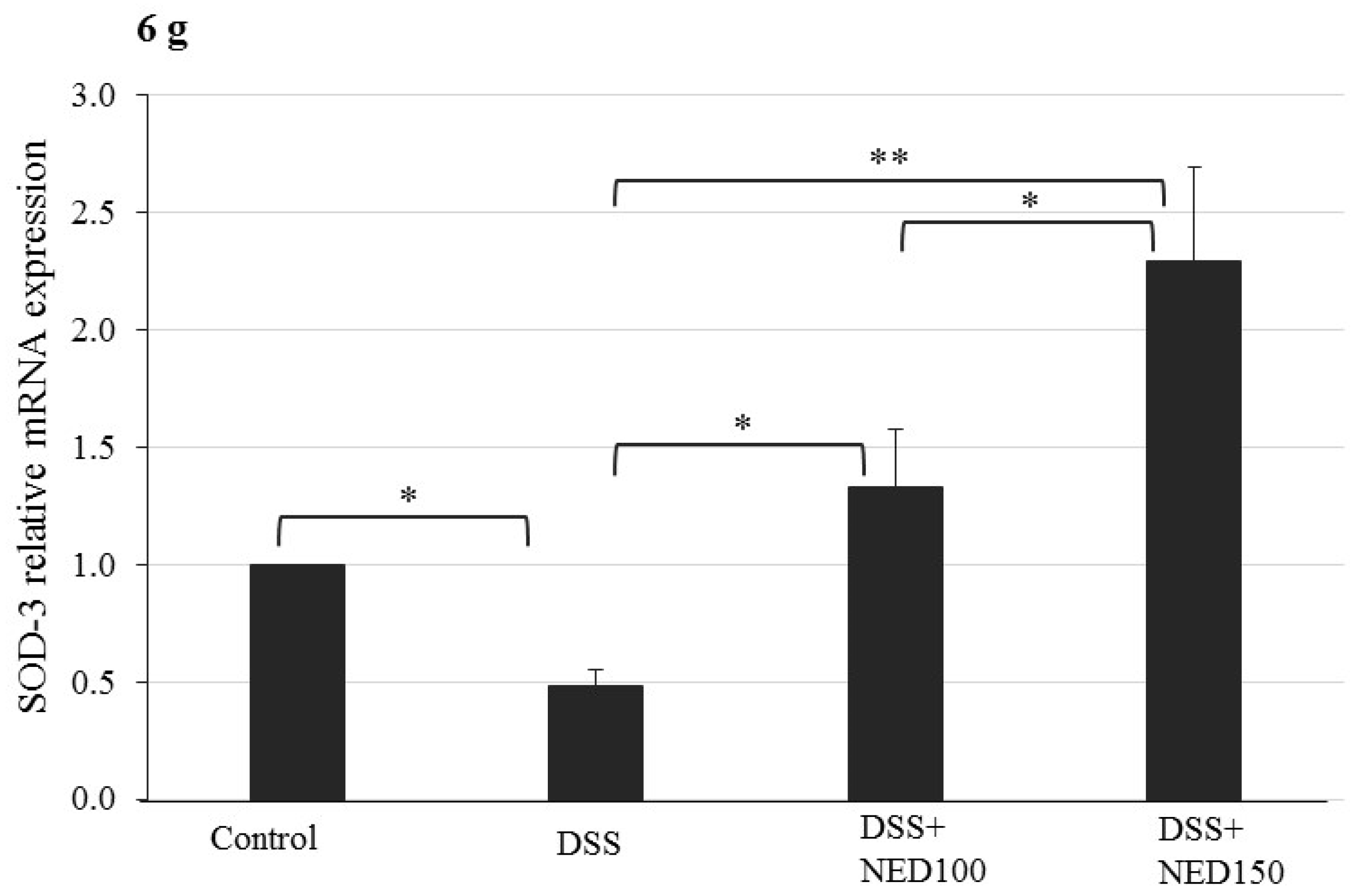
3.4. Effect of NED on Antioxidant Keap-1 and Nrf-2 Transcription Factor Protein, SOD, CAT Activity, and Downstream Target mRNA Expression
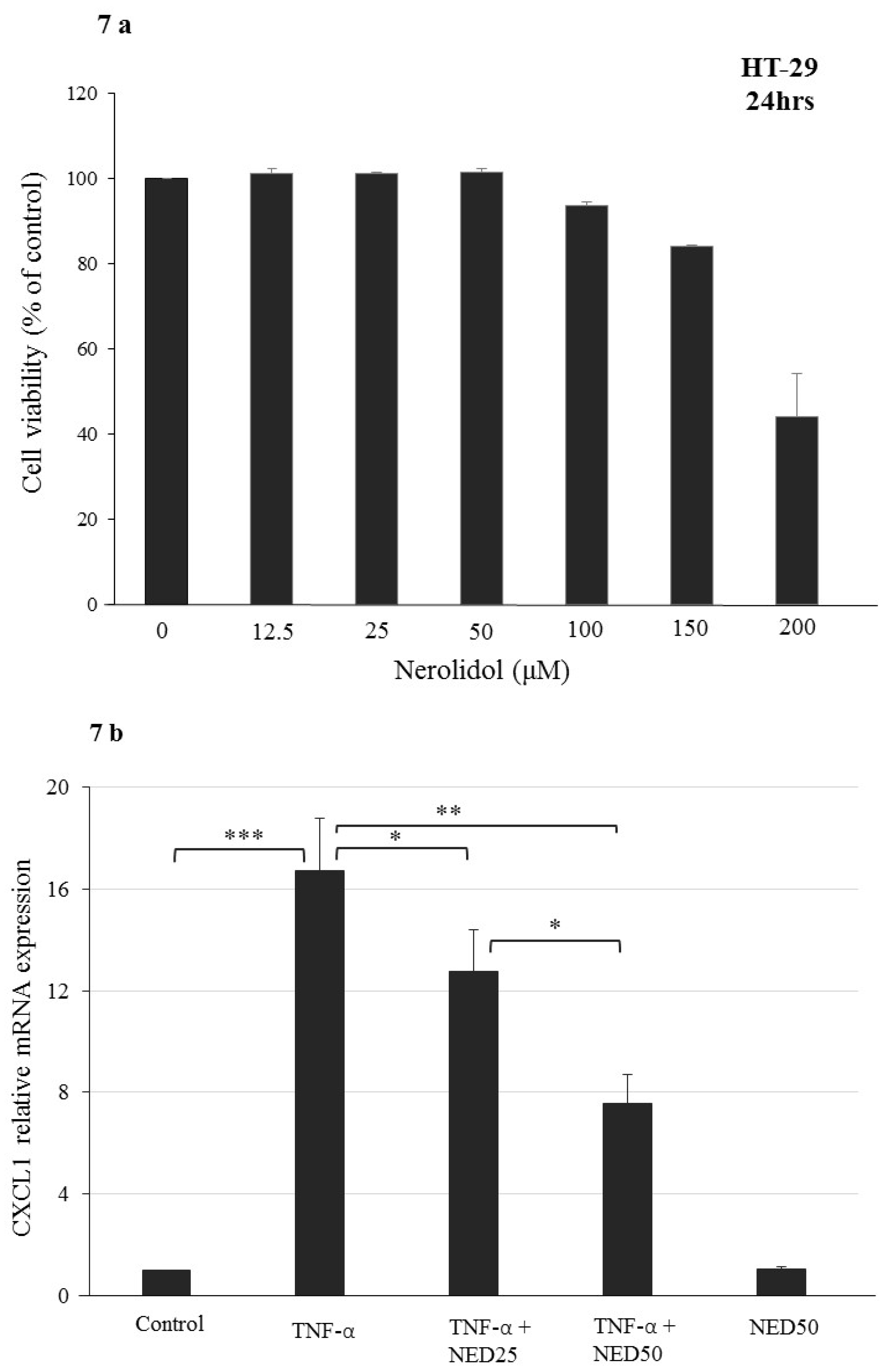
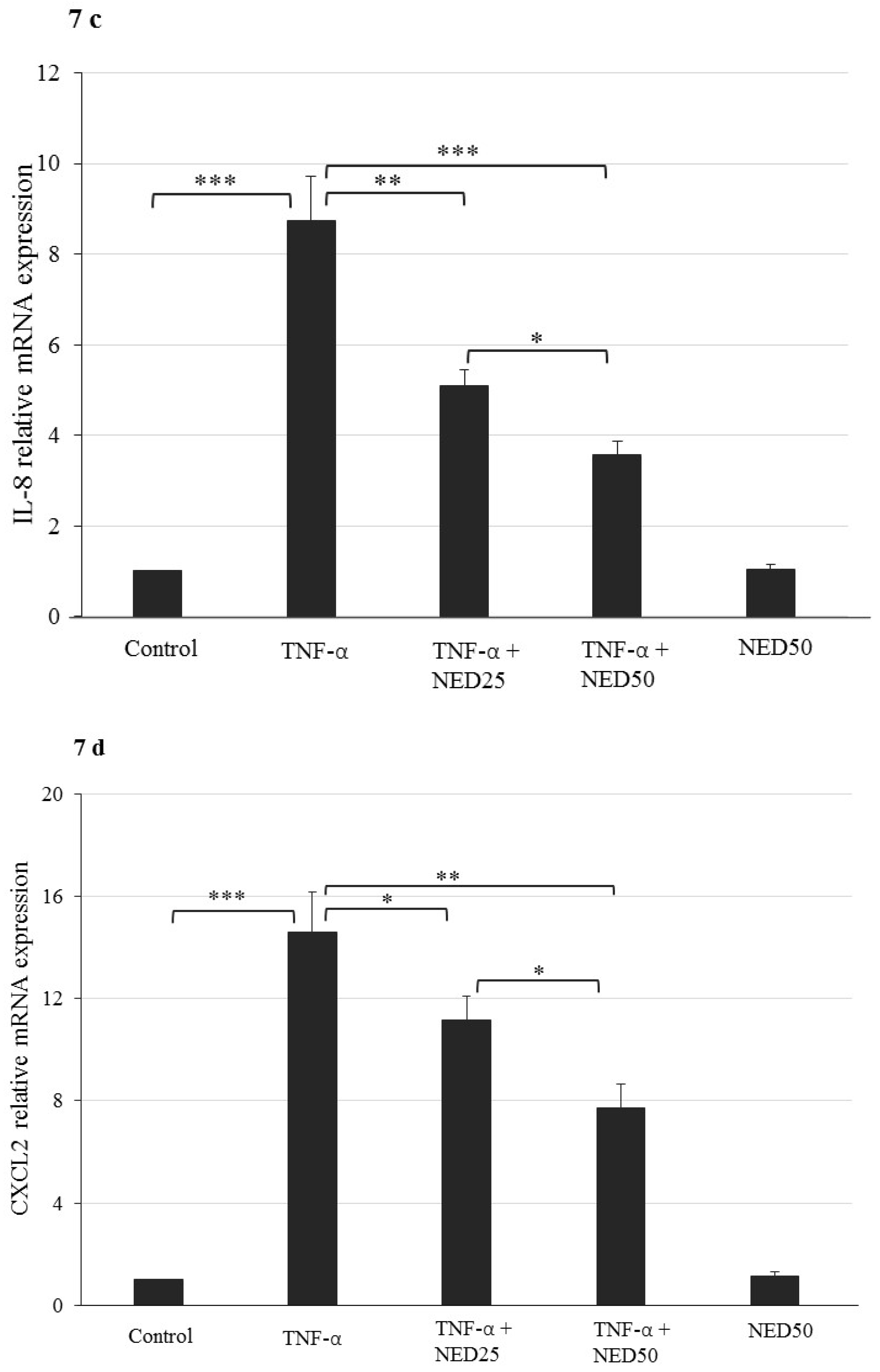
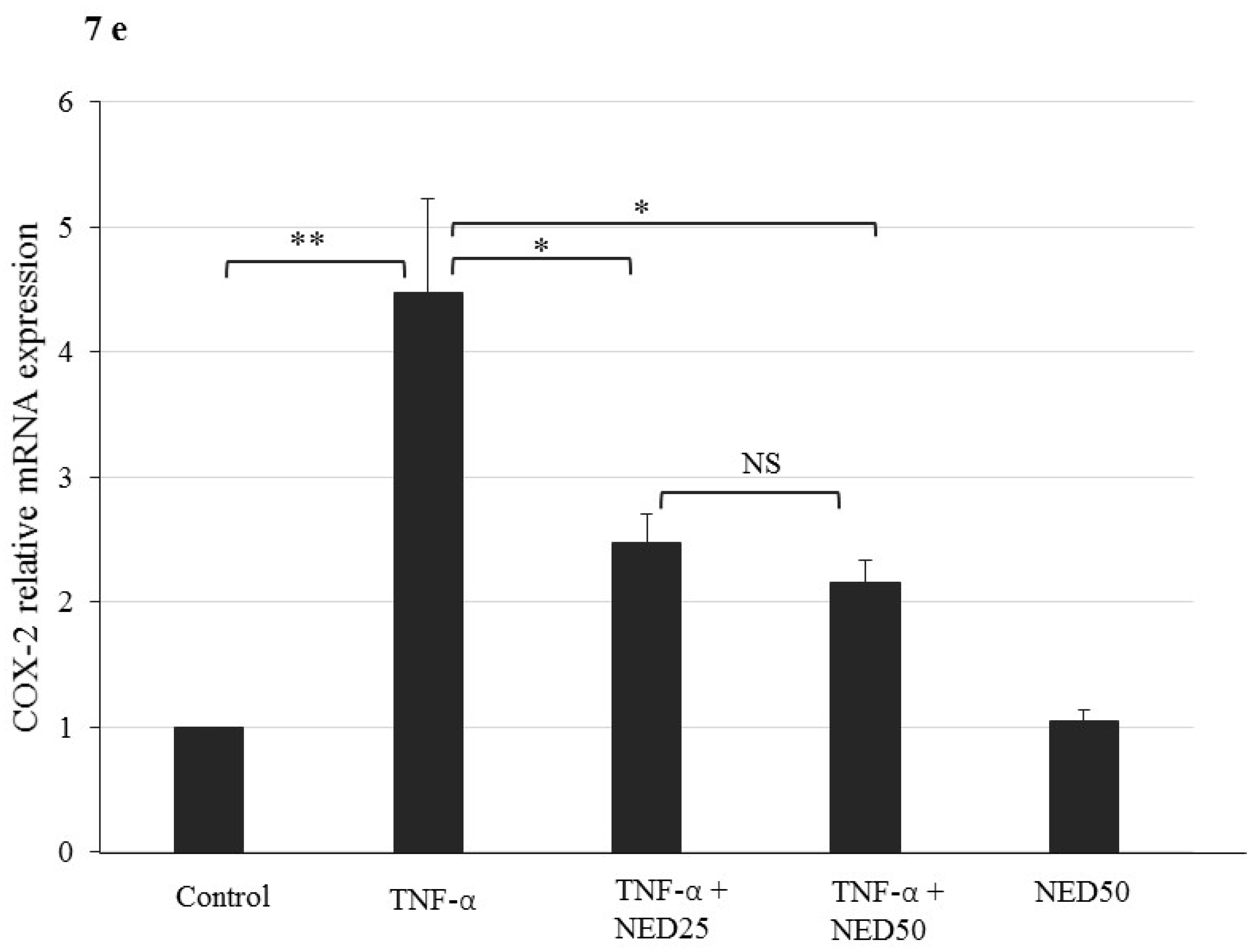
3.5. Effect of NED on TNF-α stimulated HT-29 Colon Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ballester Ferre, M.P.; Bosca-Watts, M.M.; Minguez Perez, M. Crohn’s disease. Med. Clínica 2018, 151, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ghishan, F.K.; Kiela, P.R. Epithelial transport in inflammatory bowel diseases. Inflamm. Bowel Dis. 2014, 20, 1099–1109. [Google Scholar] [CrossRef][Green Version]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Barbosa, D.S.; Cecchini, R.; El Kadri, M.Z.; Rodriguez, M.A.; Burini, R.C.; Dichi, I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition 2003, 19, 837–842. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef]
- Monk, J.M.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Liu, R.; Pauls, K.P.; Wood, G.A.; Tsao, R.; Robinson, L.E.; et al. White and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. J. Nutr. Biochem. 2015, 26, 752–760. [Google Scholar] [CrossRef]
- Binienda, A.; Ziolkowska, S.; Ingvild, H.H.; Salaga, M. The Role of Immune and Epithelial Stem Cells in Inflammatory Bowel Disease Therapy. Curr. Drug Targets 2020. [Google Scholar] [CrossRef]
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, C.; Giacomin, P.; Navarro, S.; Miller, C.; Loukas, A.; Wangchuk, P. A medicinal plant compound, capnoidine, prevents the onset of inflammation in a mouse model of colitis. J. Ethnopharmacol. 2018, 211, 17–28. [Google Scholar] [CrossRef]
- Kwak, M.S.; Cha, J.M.; Ahn, J.H.; Chae, M.K.; Jeong, S.; Lee, H.H. Practical strategy for optimizing the timing of anti-tumor necrosis factor-alpha therapy in Crohn disease: A nationwide population-based study. Medicine 2020, 99, e18925. [Google Scholar] [CrossRef]
- Braus, N.A.; Elliott, D.E. Advances in the pathogenesis and treatment of IBD. Clin. Immunol. 2009, 132, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Head, K.A.; Jurenka, J.S. Inflammatory bowel disease Part 1: Ulcerative colitis--pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2003, 8, 247–283. [Google Scholar]
- Guo, B.J.; Bian, Z.X.; Qiu, H.C.; Wang, Y.T.; Wang, Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1401, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. 2015, 28, 210–220. [Google Scholar]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.A.; Soica, C. Main Isoflavones Found in Dietary Sources as Natural Anti-inflammatory Agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Sang, L.X.; Chang, B. Isoflavones and inflammatory bowel disease. World J. Clin. Cases 2020, 8, 2081–2091. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, L.; Liu, X.; Gui, J.; Mei, X.; Fu, X.; Dong, F.; Tang, J.; Zhang, L.; Yang, Z. Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem. 2017, 231, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.F.; Nascimento, A.M.D.; Nogueira, J.M.F. Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal. Chim. Acta 2005, 546, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Chen, X.; Nieuwenhuizen, N.J.; Matich, A.J.; Wang, M.Y.; Bunn, B.J.; Yauk, Y.K.; Atkinson, R.G. Identification, functional characterization, and regulation of the enzyme responsible for floral (E)-nerolidol biosynthesis in kiwifruit (Actinidia chinensis). J. Exp. Bot. 2012, 63, 1951–1967. [Google Scholar] [CrossRef]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- De Carvalho, R.B.F.; De Almeida, A.A.C.; Campelo, N.B.; Lellis, D.; Nunes, L.C.C. Nerolidol and its Pharmacological Application in Treating Neurodegenerative Diseases: A Review. Recent Pat. Biotechnol. 2018, 12, 158–168. [Google Scholar] [CrossRef]
- Zhou, M.X.; Li, G.H.; Sun, B.; Xu, Y.W.; Li, A.L.; Li, Y.R.; Ren, D.M.; Wang, X.N.; Wen, X.S.; Lou, H.X.; et al. Identification of novel Nrf2 activators from Cinnamomum chartophyllum H.W. Li and their potential application of preventing oxidative insults in human lung epithelial cells. Redox Biol. 2018, 14, 154–163. [Google Scholar] [CrossRef]
- Fonseca, D.V.; Salgado, P.R.; de Carvalho, F.L.; Salvadori, M.G.; Penha, A.R.; Leite, F.C.; Borges, C.J.; Piuvezam, M.R.; Pordeus, L.C.; Sousa, D.P.; et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundam. Clin. Pharmacol. 2016, 30, 14–22. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S.; Haque, M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Subramanya, S.B.; Chandran, S.; Almarzooqi, S.; Raj, V.; Al Zahmi, A.S.; Al Katheeri, R.A.; Al Zadjali, S.A.; Collin, P.D.; Adrian, T.E. Frondanol, a Nutraceutical Extract from Cucumaria frondosa, Attenuates Colonic Inflammation in a DSS-Induced Colitis Model in Mice. Mar. Drugs 2018, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar] [PubMed]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Lu, S.C.; Wu, H.W.; Lin, Y.J.; Chang, S.F. The essential role of Oct-2 in LPS-induced expression of iNOS in RAW 264.7 macrophages and its regulation by trichostatin A. Am. J. Physiol. Cell Physiol. 2009, 296, C1133–C1139. [Google Scholar] [CrossRef]
- Wilson, C.A.; Browning, J.L. Death of HT29 adenocarcinoma cells induced by TNF family receptor activation is caspase-independent and displays features of both apoptosis and necrosis. Cell Death Differ. 2002, 9, 1321–1333. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Gautam, M.; Goel, S.; Ghatule, R.; Singh, A.; Nath, G.; Goel, R. Curative effect of Terminalia chebula extract on acetic acid-induced experimental colitis: Role of antioxidants, free radicals and acute inflammatory marker. Inflammopharmacology 2013, 21, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Al-Alwan, L.A.; Chang, Y.; Mogas, A.; Halayko, A.J.; Baglole, C.J.; Martin, J.G.; Rousseau, S.; Eidelman, D.H.; Hamid, Q. Differential roles of CXCL2 and CXCL3 and their receptors in regulating normal and asthmatic airway smooth muscle cell migration. J. Immunol. 2013, 191, 2731–2741. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Jurjus, A.; Eid, A.; Al Kattar, S.; Zeenny, M.N.; Gerges-Geagea, A.; Haydar, H.; Hilal, A.; Oueidat, D.; Matar, M.; Tawilah, J.; et al. Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: The links. BBA Clin. 2016, 5, 16–24. [Google Scholar] [CrossRef]
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38, 1654–1661. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, D.; Bao, Y.; Shi, Y.; Cui, Y.; Guo, M. Nerolidol Protects Against LPS-induced Acute Kidney Injury via Inhibiting TLR4/NF-kappaB Signaling. Phytother Res. 2017, 31, 459–465. [Google Scholar] [CrossRef]
- Minhas, R.; Bansal, Y.; Bansal, G. Inducible nitric oxide synthase inhibitors: A comprehensive update. Med. Res. Rev. 2019. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef]
- Ko, Y.J.; Ahn, G.; Ham, Y.M.; Song, S.M.; Ko, E.Y.; Cho, S.H.; Yoon, W.J.; Kim, K.N. Anti-inflammatory effect and mechanism of action of Lindera erythrocarpa essential oil in lipopolysaccharide-stimulated RAW264.7 cells. EXCLI J. 2017, 16, 1103–1113. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef]
- Yu, M.; Wei, Z.Y.; Xu, Z.H.; Pan, J.Q.; Chen, J.H. Oxidative Damage and Nrf2 Translocation Induced by Toxicities of Deoxynivalenol on the Placental and Embryo on Gestation Day 12.5 d and 18.5 d. Toxins 2018, 10, 370. [Google Scholar] [CrossRef]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2 by costunolide provides neuroprotective effect in PC12 cells. Food Funct. 2019, 10, 4143–4152. [Google Scholar] [CrossRef]
- Liu, L.; Wu, W.; Li, J.; Jiao, W.H.; Liu, L.Y.; Tang, J.; Sun, F.; Han, B.N.; Lin, H.W. Two sesquiterpene aminoquinones protect against oxidative injury in HaCaT keratinocytes via activation of AMPKalpha/ERK-Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. 2018, 100, 417–425. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, J.W.; Ju, E.J.; Pae, A.N.; Park, K.D. Antioxidant and Anti-Inflammatory Activities of a Natural Compound, Shizukahenriol, through Nrf2 Activation. Molecules 2015, 20, 15989–16003. [Google Scholar] [CrossRef]
- Intagliata, S.; Salerno, L.; Ciaffaglione, V.; Leonardi, C.; Fallica, A.N.; Carota, G.; Amata, E.; Marrazzo, A.; Pittala, V.; Romeo, G. Heme Oxygenase-2 (HO-2) as a therapeutic target: Activators and inhibitors. Eur. J. Med. Chem. 2019, 183, 111703. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Mizushima, K.; Hirai, Y.; Harusato, A.; Okayama, T.; Katada, K.; Kamada, K.; Uchiyama, K.; Handa, O.; et al. Heme oxygenase-1 prevents murine intestinal inflammation. J. Clin. Biochem. Nutr. 2018, 63, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Mizushima, K.; Nukigi, Y.; Okada, H.; Suzuki, T.; Hirata, I.; Omatsu, T.; Okayama, T.; Handa, O.; et al. Increased intestinal expression of heme oxygenase-1 and its localization in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23, S229–S233. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Kuiper, I.; van Duijn, W.; Marklund, S.L.; van Hogezand, R.A.; Lamers, C.B.; Verspaget, H.W. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J. Pathol. 2003, 201, 7–16. [Google Scholar] [CrossRef]
- Laukkanen, M.O. Extracellular Superoxide Dismutase: Growth Promoter or Tumor Suppressor? Oxid. Med. Cell. Longev. 2016, 2016, 3612589. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Lee, J.; Stamm, D.S.; Sanderson, I.R. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 2001, 49, 526–533. [Google Scholar] [CrossRef]
- Fuhr, L.; Rousseau, M.; Plauth, A.; Schroeder, F.C.; Sauer, S. Amorfrutins Are Natural PPARgamma Agonists with Potent Anti-inflammatory Properties. J. Nat. Prod. 2015, 78, 1160–1164. [Google Scholar] [CrossRef]
- Ogata, H.; Sekikawa, A.; Yamagishi, H.; Ichikawa, K.; Tomita, S.; Imura, J.; Ito, Y.; Fujita, M.; Tsubaki, M.; Kato, H.; et al. GROalpha promotes invasion of colorectal cancer cells. Oncol. Rep. 2010, 24, 1479–1486. [Google Scholar] [PubMed]
- Imada, A.; Ina, K.; Shimada, M.; Yokoyama, T.; Yokoyama, Y.; Nishio, Y.; Yamaguchi, T.; Ando, T.; Kusugami, K. Coordinate upregulation of interleukin-8 and growth-related gene product-alpha is present in the colonic mucosa of inflammatory bowel. Scand. J. Gastroenterol. 2001, 36, 854–864. [Google Scholar] [CrossRef]
- Hua, K.F.; Yang, T.J.; Chiu, H.W.; Ho, C.L. Essential oil from leaves of Liquidambar formosana ameliorates inflammatory response in lipopolysaccharide-activated mouse macrophages. Nat. Prod. Commun. 2014, 9, 869–872. [Google Scholar] [CrossRef] [PubMed]

| Weight Loss | Score | Stool Consistency | Score | Rectal Bleeding | Score |
|---|---|---|---|---|---|
| No loss | 0 | Normal | 0 | No Blood | 0 |
| 1–5% | 1 | Loose stool | 2 | Heme occult + ve and visual pellet bleeding | 2 |
| 5–10% | 2 | Diarrhea | 4 | Gross bleeding and blood around anus | 4 |
| 10–20% | 3 | ||||
| >20% | 4 |
| Inflammation Graded | Percentage of Inflammation Involvement of Mucosal Surface Area | Hyperplastic Epithelium—Graded Based on Extent of Involvement | ||
|---|---|---|---|---|
| none | 0 | no inflammation | 0 | none |
| mild | 1 | 1–25% | 1 | 1–25% |
| moderate | 2 | 26–50% | 2 | 26–50% |
| severe | 3 | 51–75% | 3 | 51–75% |
| 4 | 76–100% | 4 | 76–100% | |
| Crypt Grade | Quantification on Percentage of Crypt Change | Crypt Distortion—Graded Based on Extent of Involvement | |||
|---|---|---|---|---|---|
| Grade 0 | Intact crypt | 1 | 1–25% | 0 | no crypt distortion |
| Grade 1 | Shortening and loss of basal 1/3 of crypts | 2 | 26–50% | 1 | 1–25% |
| Grade 2 | Loss of basal 2/3 of crypts | 3 | 51–75% | 2 | 26–50% |
| Grade 3 | Loss of entire crypt with intact surface epithelium | 4 | 76–100% | 3 | 51–75% |
| Grade 4 | Loss of both entire crypt and surface epithelium (erosion) | 4 | 76–100% | ||
| Gene | Forward | Reverse | PMID No | Gene Accession Number |
|---|---|---|---|---|
| CXCL2 (mouse) | 5′-GGATGGCTTTCATGGAAGGAG-3′ | 5′-TTGCTAAGCAAGGCACTGTGC-3′ | 22326488 | NM_009140.2 |
| CCL2 (mouse) | 5′-CAGCCAGATGCAGTTAACGC-3′ | 5′-GCCTACTCATTGGGATCATCTTG-3′ | 10953027 | NM_011333.2 |
| IL-6 (mouse) | 5′-ACAAGTCGGAGGCTTAATTACACAT-3′ | 5′-TTGCCATTGCACAACTCTTTTC-3′ | 21735552 | X06203 |
| TNF-α (mouse) | 5′-AGGCTGCCCCGACTACGT-3′ | 5′-GACTTTCTCCTGGTATGAGATAGCAAA-3′ | 21705622 | NM_013693.2 |
| IL-1β (mouse) | 5′-TCGCTCAGGGTCACAAGAAA-3′ | 5′-CATCAGAGGCAAGGAGGAAAC-3′ | 21735552 | NM_008361.4 |
| COX-2 (mouse) | 5′-AACCGCATTGCCTCTGAAT-3′ | 5′-CATGTTCCAGGAGGATGGAG-3′ | 22158945 | NM_011198.4 |
| iNOS (mouse) | 5′-CGAAACGCTTCACTTCCAA-3′ | 5′-TGAGCCTATATTGCTGTGGCT-3′ | 22158945 | BC062378.1 |
| NRF-2 (mouse) | 5′-GAGCTAGATAGTGCCCCTGG-3′ | 5′-CAGGACTCACGGGAACTTCT-3′ | 29162986 | U20532.1 |
| HO-1 (mouse) | 5′-AAGCCGAGAATGCTGAGTTCA-3′ | 5′-GCCGTGTAGATATGGTACAAGGA-3′ | 25112868 | BC010757.1 |
| SOD3 (mouse) | 5′-TTCTACGGCTTGCTACTGGC-3′ | 5′-GCTAGGTCGAAGCTGGACTC-3′ | 26513461 | NM_011435.3 |
| 18S (mouse) | 5′-CCCCTCGATGACTTTAGCTGAGTGT-3′ | 5′-CGCCGGTCCAAGAATTTCACCTCT-3′ | 22427817 | NR_003278 |
| CXCL1 (human) | 5′-GCGGAAAGCTTGCCTCAATC-3′ | 5′-GGTCAGTTGGATTTGTCACTGT-3′ | 25938459 | BC011976.1 |
| IL-8 (human) | 5′-CTGATTTCTGCAGCTCTGTG-3′ | 5′-GGGTGGAAAGGTTTGGAGTATG-3′ | 20150959 | BC013615.1 |
| CXCL2 (human) | 5′-TTTATTGTGGGCTTCACACG-3′ | 5′-GATTTGCGCACACAGACAAC-3′ | 17591792 | NM_004591.3 |
| COX2 (human) | 5′-ACAGTGTGTGGTCAACATTTCTC-3′ | 5′-TCGAAACCTCTCTGCTCTAACAC-3′ | 25938459 | BC015753.1 |
| 18S (human) | 5′-GTGGAGCGATTTGTCTGGTT-3′ | 5′-AACGCCACTTGTCCCTCTAA-3′ | 25369870 | NR_003286 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, V.; Venkataraman, B.; Almarzooqi, S.; Chandran, S.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; Subramanya, S.B. Nerolidol Mitigates Colonic Inflammation: An Experimental Study Using both In Vivo and In Vitro Models. Nutrients 2020, 12, 2032. https://doi.org/10.3390/nu12072032
Raj V, Venkataraman B, Almarzooqi S, Chandran S, Ojha SK, Attoub S, Adrian TE, Subramanya SB. Nerolidol Mitigates Colonic Inflammation: An Experimental Study Using both In Vivo and In Vitro Models. Nutrients. 2020; 12(7):2032. https://doi.org/10.3390/nu12072032
Chicago/Turabian StyleRaj, Vishnu, Balaji Venkataraman, Saeeda Almarzooqi, Sanjana Chandran, Shreesh K. Ojha, Samir Attoub, Thomas E. Adrian, and Sandeep B. Subramanya. 2020. "Nerolidol Mitigates Colonic Inflammation: An Experimental Study Using both In Vivo and In Vitro Models" Nutrients 12, no. 7: 2032. https://doi.org/10.3390/nu12072032
APA StyleRaj, V., Venkataraman, B., Almarzooqi, S., Chandran, S., Ojha, S. K., Attoub, S., Adrian, T. E., & Subramanya, S. B. (2020). Nerolidol Mitigates Colonic Inflammation: An Experimental Study Using both In Vivo and In Vitro Models. Nutrients, 12(7), 2032. https://doi.org/10.3390/nu12072032






