Impact of Glutathione and Vitamin B-6 in Cirrhosis Patients: A Randomized Controlled Trial and Follow-Up Study
Abstract
1. Introduction
2. Subjects and Methods
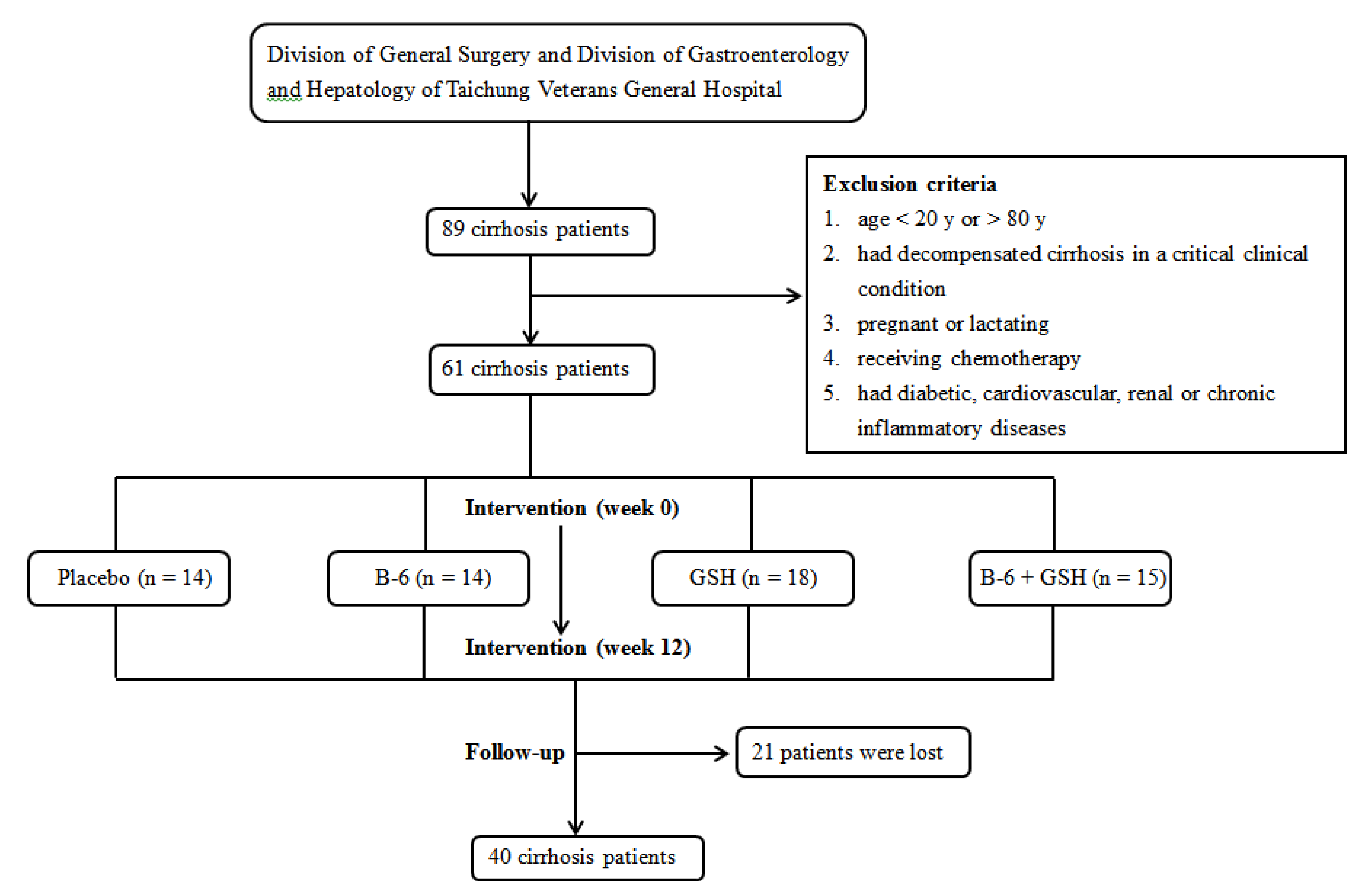
2.1. Study Design and Sample Size Calculation
2.2. Subjects
2.3. Intervention and Follow-Up Procedure
2.4. Data Collection and Biochemical Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| GSH | glutathione |
| GSH-Px | glutathione peroxidase |
| GSH-Rd | glutathione reductase |
| GSSG | glutathione disulfide |
| GSH-St | glutathione S-Transferase |
| INR | international normalized ratio |
| MDA | malondialdehyde |
| PLP | pyridoxal 5′-phosphate |
| SOD | superoxide dismutase |
| TEAC | trolox equivalent antioxidant capacity |
References
- Czeczot, H.; Scibior, D.; Skrzycki, M.; Podsiad, M. Glutathione and GSH-dependent enzymes in patients with liver cirrhosis and hepatocellular carcinoma. Acta Biochim. Pol. 2006, 53, 237–241. [Google Scholar] [CrossRef]
- Geetha, A.; Priya, M.D.L.; Jeyachristy, S.A.; Surendran, R. Level of oxidative stress in the red blood cells of patients with liver cirrhosis. Indian J. Med. Res. 2007, 126, 204–210. [Google Scholar]
- Bhandari, S.; Agarwal, M.P.; Dwivedi, S.; Banerjee, B.D. Monitoring oxidative stress across worsening child pugh class of cirrhosis. Indian J. Med. Sci. 2008, 62, 444. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Rosique-Oramas, D.; Medina-Avila, Z.; Álvarez-Torres, T.; Falcón, D.; La Tijera, F.H.-D.; Béjar, Y.L.; Cordero-Perez, P.; Munoz, L.; Pérez-Hernández, J.L.; et al. Behavior of Oxidative Stress Markers in Alcoholic Liver Cirrhosis Patients. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Sangeetha, K.; Krishnasamy, N.; Padma, K.; Rajendran, K. Evaluation of Oxidative Stress in Liver Cirrhosis Patients to Early Diagnosis of Minimal Hepatic Encephalopathy. Int. Neuropsychiatr. Dis. J. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Lauterburg, B.H.; Adams, J.D.; Mitchell, J.R. Hepatic Glutathione Homeostasis in the Rat: Efflux Accounts for Glutathione Turnover. Hepatology 1984, 4, 586–590. [Google Scholar] [CrossRef]
- Bianchi, G.; Bugianesi, E.; Ronchi, M.; Fabbri, A.; Zoli, M.; Marchesini, G. Glutathione kinetics in normal man and in patients with liver cirrhosis. J. Hepatol. 1997, 26, 606–613. [Google Scholar] [CrossRef]
- Leach, N.V.; Dronca, E.; Vesa Ştefan, C.; Sâmpelean, D.P.; Craciun, E.C.; Lupsor-Platon, M.; Crisan, D.; Tarau, R.; Rusu, R.; Para, I.; et al. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur. J. Intern. Med. 2014, 25, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Bradley, R. Effects of Oral Glutathione Supplementation on Systemic Oxidative Stress Biomarkers in Human Volunteers. J. Altern. Complement. Med. 2011, 17, 827–833. [Google Scholar] [CrossRef]
- Richie, J.P., Jr.; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015, 54, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Matte, J.J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S. Oxidant/antioxidant status in liver tissue of vitamin B6 deficient rats. Clin. Nutr. 2005, 24, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Lin, P.T.; Liu, H.T.; Peng, Y.S.; Huang, S.C.; Huang, Y.C. Vitamin B-6 Supplementation Could Mediate Antioxidant Capacity by Reducing Plasma Homocysteine Concentration in Patients with Hepatocellular Carcinoma after Tumor Resection. BioMed Res. Int. 2016, 2016, 7658981. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.K.; Lewis, F.W.; Kutner, M.H.; Bate, D.M.; Roy, R.G.; Rudman, D. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology 1984, 87, 770–776. [Google Scholar] [CrossRef]
- Zaman, S.N.; Tredger, J.M.; Johnson, P.J.; Williams, R. Vitamin B6 concentrations in patients with chronic liver disease and hepatocellular carcinoma. Br. Med. J. 1986, 293, 175. [Google Scholar] [CrossRef]
- Henderson, J.M.; Scott, S.S.; Merrill, A.H.; Hollins, B.; Kutner, M.H. Vitamin B6 repletion in cirrhosis with oral pyridoxine: Failure to improve amino acid metabolism. Hepatology 1989, 9, 582–588. [Google Scholar] [CrossRef]
- Bianchi, G.; Brizi, M.; Rossi, B.; Ronchi, M.; Grossi, G.; Marchesini, G. Synthesis of glutathione in response to methionine load in control subjects and in patients with cirrhosis. Metabolism 2000, 49, 1434–1439. [Google Scholar] [CrossRef]
- Loguercio, C.; De Girolamo, V.; Federico, A.; Feng, S.L.; Crafa, E.; Cataldi, V.; Gialanella, G.; Moro, R.; Blanco, C.D.V. Relationship of Blood Trace Elements to Liver Damage, Nutritional Status, and Oxidative Stress in Chronic Nonalcoholic Liver Disease. Boil. Trace Elem. Res. 2001, 81, 245–254. [Google Scholar] [CrossRef]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Lapenna, D.; Ciofani, G.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxidesin human plasma. Free Radic. Boil. Med. 2001, 31, 331–335. [Google Scholar] [CrossRef]
- Araki, A.; Sako, Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B: Biomed. Sci. Appl. 1987, 422, 43–52. [Google Scholar] [CrossRef]
- Talwar, D.; Quasim, T.; McMillan, D.C.; Kinsella, J.; Williamson, C.; O’Reilly, D.S. Pyridoxal phosphate decreases in plasma but not erythrocytes during systemic Inflammatory response. Chin. Chem. 2003, 49, 515–518. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H 2 O 2 -treated U937 monocytes. Free Radic. Boil. Med. 2004, 36, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.; Al, B.; Gümüştekin, K.; Demircan, B.; Özbey, I.; Akyüz, M.; Yilmaz, A.; Demir, E.; Uyanik, A.; Ziypak, T.; et al. Antioxidative status and lipid peroxidation in kidney tissue of rats fed with vitamin B6-deficient diet. Ren. Fail. 2010, 32, 618–622. [Google Scholar] [CrossRef]
- Henderson, J.M.; Codner, M.A.; Hollins, B.; Kutner, M.H.; Merrill, A.H. The fasting B6 vitamer profile and response to a pyridoxine load in normal and cirrhotic subjects. Hepatology 1986, 6, 464–471. [Google Scholar] [CrossRef]
- Anand, S.S. Protective effect of vitamin B6 in chromium-induced oxidative stress in liver. J. Appl. Toxicol. 2005, 25, 440–443. [Google Scholar] [CrossRef]
- Giustina, A.D.; Danielski, L.G.; Novochadlo, M.M.; Goldim, M.P.; Joaquim, L.; Metzker, K.L.; De Carli, R.J.; Denicol, T.; Cidreira, T.; Vieira, T.; et al. Vitamin B6 reduces oxidative stress in lungs and liver in experimental sepsis. An. Acad. Bras. Ciênc. 2019, 91, e20190434. [Google Scholar] [CrossRef]
- Horowitz, J.H.; Rypins, E.B.; Henderson, J.M.; Heymsfield, S.B.; Moffitt, S.D.; Bain, R.P.; Chawla, R.K.; Bleier, J.C.; Daniel, R. Evidence for impairment of transsulfuration pathway in cirrhosis. Gastroenterology 1981, 81, 668–675. [Google Scholar] [CrossRef]
- Mari, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernándezcheca, J.C. Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef]
- Chai, Y.C.; Ashraf, S.S.; Rokutan, K.; Johnston, R.B., Jr.; Thomas, J.A. S-thiolation of individual human neutrophil proteins including action by stimulation of the respiratory burst: Evidence against a role for glutathione disulfide. Arch. Biochem. Biophys. 1994, 310, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.; Kaminsky, M.; Kaminsky, A.; Valencia, M.; Lee, L.; Hermenegildo, C.; Felipo, V. Superoxide Production and Antioxidant Enzymes in Ammonia Intoxication in Rats. Free Radic. Res. 1997, 27, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.; Hudson, V.M.; Hilton, S.C.; Wilde, C. A Pilot Study of the Effect of Inhaled Buffered Reduced Glutathione on the Clinical Status of Patients with Cystic Fibrosis. Chest 2005, 127, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Arosio, E.; De Marchi, S.; Zannoni, M.; Prior, M.; Lechi, A. Effect of Glutathione Infusion on Leg Arterial Circulation, Cutaneous Microcirculation, and Pain-Free Walking Distance in Patients with Peripheral Obstructive Arterial Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Mayo Clin. Proc. 2002, 77, 754–759. [Google Scholar] [CrossRef]
- Hauser, R.A.; Lyons, K.E.; McClain, T.; Carter, S.; Perlmutter, D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson’s disease. Mov. Disord. 2009, 24, 979–983. [Google Scholar] [CrossRef]
- Kern, J.K.; Geier, D.A.; Adams, J.B.; Garver, C.R.; Audhya, T.; Geier, M.R. A clinical trial of glutathione supplementation in autism spectrum disorders. Med Sci. Monit. 2011, 17, CR677–CR682. [Google Scholar] [CrossRef]
- Viña, J.; Perez, C.; Furukawa, T.; Palacin, M.; Viña, J.R. Effect of oral glutathione on hepatic glutathione levels in rats and mice. Br. J. Nutr. 1989, 62, 683–691. [Google Scholar] [CrossRef]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017, 17, 96. [Google Scholar] [CrossRef]
- Duce, A.M.; Ortiz, P.; Cabrero, C.; Mato, J.M. S-adenosyl-L-methionine synthetase and phospholipid methyltransferase are inhibited in human cirrhosis. Hepatology 1988, 8, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Skrinska, V.A.; Lucas, F.V. The influence of glutathione and other thiols on human platelet aggregation. Thromb. Res. 1986, 44, 859–866. [Google Scholar] [CrossRef]
- Bayele, H.K.; Murdock, P.J.; Perry, D.J.; Pasi, K.J. Simple shifts in redox/thiol balance that perturb blood coagulation. FEBS Lett. 2001, 510, 67–70. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Hesketh, J.; Huang, D.; Gan, F.; Hao, S.; Tang, S.; Guo, Y.; Huang, K. Protective effects of selenium-glutathione-enriched probiotics on CCl4-induced liver fibrosis. J. Nutr. Biochem. 2018, 58, 138–149. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Ye, G.; Khan, A.; Liu, J.; Gan, F.; Zhang, X.; Kumbhar, S.; Huang, K. Protective Effects of Selenium-Enriched Probiotics on Carbon Tetrachloride-Induced Liver Fibrosis in Rats. J. Agric. Food Chem. 2014, 63, 242–249. [Google Scholar] [CrossRef]
- Lai, C.Y.; Cheng, S.B.; Lee, T.Y.; Liu, H.T.; Huang, S.C.; Huang, Y.C. Possible Synergistic Effects of Glutathione and C-Reactive Protein in the Progression of Liver Cirrhosis. Nutrients 2018, 10, 678. [Google Scholar] [CrossRef]
| Parameters | Placebo (n = 14) | B-6 (n = 14) | GSH (n = 18) | B-6 + GSH (n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | Week 0 | Week 12 | Week 0 | Week 12 | |
| Age (y) | 55.0 ± 3.41 | 62.86 ± 2.09 | 62.39 ± 2.35 | 56.40 ± 1.84 | ||||
| Sex (male/female) | 10/4 | 9/5 | 14/4 | 14/1 | ||||
| BMI (kg/m2) | 25.70 ± 1.21 | 25.43 ± 1.30 | 25.96 ± 0.91 | 25.87 ± 0.79 | 23.71 ± 0.89 | 23.38 ± 0.88 | 25.40 ± 0.67 | 25.82 ± 0.60 |
| Blood pressure (mmHg) | ||||||||
| Systolic | 136.71 ± 6.02 | 126.86 ± 5.39 | 137.0 ± 6.04 | 134.29 ± 7.03 | 127.61 ± 4.65 | 124.61 ± 2.38 | 128.53 ± 3.29 | 129.33 ± 2.97 |
| Diastolic | 83.14 ± 4.44 | 78.43 ± 3.41 | 78.64 ± 3.61 | 78.14 ± 4.26 | 79.44 ± 3.10 | 75.50 ± 1.73 | 79.33 ± 2.29 | 78.20 ± 2.70 |
| Serum ALT (U/L) | 48.21 ± 11.67 | 43.36 ± 10.48 | 56.86 ± 17.40 | 42.43 ± 6.38 | 54.22 ± 12.75 | 44.78 ± 8.50 | 73.60 ± 19.86 | 59.13 ± 9.81 |
| Serum AST (U/L) | 42.07 ± 8.07 | 42.21 ± 8.80 | 60.93 ± 13.92 | 57.21 ± 11.20 | 82.33 ± 42.31 | 38.61 ± 2.86 | 59.47 ± 13.97 | 54.67 ± 8.80 |
| Serum creatinine (mg/dL) | 0.96 ± 0.10 | 0.99 ± 0.12 | 0.78 ± 0.04 | 0.94 ± 0.08 * | 0.88 ± 0.03 | 0.93 ± 0.06 | 1.18 ± 0.21 | 1.23 ± 0.23 |
| Serum albumin (g/dL) | 4.05 ± 0.19 | 3.95 ± 0.18 | 3.94 ± 0.23 | 3.95 ± 0.16 | 4.32 ± 0.13 | 4.22 ± 0.12 | 4.09 ± 0.15 | 4.10 ± 0.15 |
| Serum total bilirubin (mg/dL) | 0.93 ± 0.22 | 1.16 ± 0.18 | 1.29 ± 0.18 | 1.15 ± 0.17 | 1.11 ± 0.17 | 0.99 ± 0.12 | 1.59 ± 0.58 | 1.31 ±0.44 |
| INR | 1.10 ± 0.04 a,b | 1.11 ± 0.03 | 1.14 ± 0.02 a | 1.13 ± 0.03 | 1.04 b ± 0.01 | 1.05 ± 0.02 | 1.12 ± 0.04 a,b | 1.12 ± 0.05 |
| Child–Turcotte–Pugh scores | ||||||||
| A (n, %) | 12, 85.71% | 13, 92.86% | 12, 85.71% | 12, 85.71% | 18, 100% | 18, 100% | 14, 93.33% | 13, 86.67% |
| B (n, %) | 2, 14.29% | 1, 0.07% | 2, 14.29% | 2, 14.29% | 0 | 0 | 1, 6.67% | 2, 13.33% |
| Smoking (n, %) | 3, 21.43% | 3, 21.43% | 6, 33.33% | 6, 40% | ||||
| Drinking (n, %) | 1, 7.14% | 2, 14.29% | 1, 5.56% | 4, 26.67% | ||||
| Parameters | Placebo (n = 14) | B-6 (n = 14) | GSH (n = 18) | B-6 + GSH (n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | Week 0 | Week 12 | Week 0 | Week 12 | |
| PLP (nmol/L) | 67.59 ± 10.58 | 107.63 ± 35.69 b | 55.21 ± 7.37 | 276.56 ± 38.50 *,a | 131.14 ± 33.80 | 63.93 ± 11.33 b | 116.74 ± 37.06 | 319.87 ± 52.63 *,a |
| Cysteine (µmol/L) | 202.53 ± 14.75 | 223.62 ± 11.10 | 217.24 ± 13.57 | 209.73 ± 10.63 | 208.15 ± 9.76 | 225.74 ± 10.21 * | 189.72 ± 9.25 | 192.22 ± 8.07 |
| Oxidative stress indicators | ||||||||
| MDA (µmol/L) | 0.80 ± 0.05 | 0.87 ± 0.08 | 0.77 ± 0.04 | 0.80 ± 0.07 | 0.78 ± 0.06 | 0.75 ± 0.05 | 0.81 ± 0.03 | 0.87 ± 0.06 |
| GSH/GSSG ratio | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.01 * |
| Antioxidant capacities | ||||||||
| TEAC (µmol/L) | 4335.34 ± 264.35 | 4412.06 ± 160.40 | 4448.85 ± 147.24 | 4321.28 ± 113.79 | 4078.22 ± 112.37 | 4324.72 ± 96.95 | 3943.14 ± 143.68 | 4259.35 ± 103.17 |
| GSH (µmol/L) | 72.25 ± 6.96 | 79.59 ± 9.81 | 66.54 ± 10.01 | 68.62 ± 10.63 | 77.43 ± 4.98 | 80.23 ± 5.76 | 78.14 ± 7.81 | 71.52 ± 7.20 |
| GSSG (µmol/L) | 666.06 ± 18.53 | 694.20 ± 13.70 | 667.05 ± 18.33 | 679.30 ± 15.92 | 673.05 ± 19.10 | 691.85 ± 16.17 | 662.61 ± 12.46 | 705.29 ± 17.33 * |
| GSH-St (nmol/mL/min) | 15.97 ± 3.60 | 22.20 ± 4.54 | 16.28 ± 2.68 | 26.46 ± 3.30 | 19.47 ± 3.42 | 27.00 ± 2.84 | 18.98 ± 2.65 | 25.58 ± 3.36 |
| GSH-Px (nmol/mL/min) | 199.39 ± 20.74 | 142.82 ± 10.77 * | 211.94 ± 26.26 | 135.35 ± 8.06 * | 189.89 ± 12.57 | 168.70 ± 13.17 | 175.40 ± 16.43 | 151.29 ± 14.14 |
| GSH-Rd (nmol/mL/min) | 64.91 ± 4.14 | 68.29 ± 5.20 | 70.68 ± 3.98 | 72.73 ± 33.03 | 85.02 ± 12.68 | 69.75 ± 4.03 | 80.28 ± 6.80 | 71.64 ± 6.76 |
| SOD (U/mL) | 8.46 ± 0.93 | 7.81 ± 0.87 | 7.17 ± 1.01 | 8.44 ± 0.74 | 7.07 ± 0.73 | 7.36 ± 0.71 | 8.24 ± 1.05 | 9.08 ± 0.73 |
| Catalase (nmol/mL/min) | 85.68 ± 17.27 | 61.16 ± 10.06 * | 62.91 ± 5.76 | 55.79 ± 8.50 | 85.47 ± 13.50 | 56.16 ± 5.53 * | 79.80 ± 8.67 | 71.45 ± 9.56 |
| Parameters | Child–Turcotte–Pugh Score | ||
|---|---|---|---|
| Week 0 (n = 61) 1 | Week 12 (n = 61) 1 | End of Follow-Up (n = 40) 2 | |
| β (standard error) | |||
| PLP (nmol/L) | −0.001 (0.001) | ||
| at Week 0 | −0.0002 (0.001) | −0.001 (0.001) | |
| at Week 12 | −0.001 (0.001) | −0.001 (0.001) | |
| Cysteine (µmol/L) | 0.001 (0.003) | ||
| at Week 0 | 0.002 (0.003) | 0.002 (0.003) | |
| at Week 12 | 0.001 (0.003) | 0.002 (0.003) | |
| Oxidative stress indicators | 0.573 (0.619) | ||
| MDA (µmol/L) | |||
| at Week 0 | 0.269 (0.644) | −0.159 (0.723) | |
| at Week 12 | 0.830 (0.453) | −0.070 (0.419) | |
| GSH/GSSG ratio | −8.122 (2.514) ** | ||
| at Week 0 | −9.529 (2.523) † | −5.928 (2.469) * | |
| at Week 12 | −6.837 (2.145) ** | −3.153 (2.473) | |
| Antioxidant capacities | −0.0004 (0.0001) * | ||
| TEAC (µmol/L) | |||
| at Week 0 | −0.0004 (0.0002) * | −0.001 (0.0001) | |
| at Week 12 | −0.0002 (0.0002) | −0.0004 (0.0003) | |
| GSH (µmol/L) | −0.011 (0.004) ** | ||
| at Week 0 | −0.013 (0.004) † | −0.009 (0.004) * | |
| at Week 12 | −0.009 (0.003) ** | −0.004 (0.004) | |
| GSSG (µmol/L) | 0.001 (0.002) | ||
| at Week 0 | 0.0004 (0.002) | −0.002 (0.001) | |
| at Week 12 | −0.004 (0.002) * | −0.003 (0.002) | |
| GSH-St (nmol/mL/min) | −0.020 (0.009) * | ||
| at Week 0 | −0.022 (0.009) * | −0.022(0.009) * | |
| at Week 12 | −0.015 (0.008) | −0.010 (0.009) | |
| GSH-Px (nmol/mL/min) | −0.0001 (0.001) | ||
| at Week 0 | 0.001 (0.002) | 0.001 (0.002) | |
| at Week 12 | −0.002 (0.002) | −0.002 (0.002) | |
| GSH-Rd (nmol/mL/min) | −0.001 (0.003) | ||
| at Week 0 | 0.001 (0.003) | −0.003 (0.006) | |
| at Week 12 | 0.016 (0.005) ** | 0.007 (0.005) | |
| SOD (U/mL) | −0.071 (0.030) * | ||
| at Week 0 | −0.030 (0.032) | 0.012 (0.032) | |
| at Week 12 | −0.008 (0.034) | 0.020 (0.036) | |
| Catalase (nmol/mL/min) | −0.006 (0.002) ** | ||
| at Week 0 | −0.006 (0.002) ** | −0.002 (0.002) | |
| at Week 12 | −0.008 (0.003) * | −0.007 (0.004) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-Y.; Cheng, S.-B.; Lee, T.-Y.; Hsiao, Y.-F.; Liu, H.-T.; Huang, Y.-C. Impact of Glutathione and Vitamin B-6 in Cirrhosis Patients: A Randomized Controlled Trial and Follow-Up Study. Nutrients 2020, 12, 1978. https://doi.org/10.3390/nu12071978
Lai C-Y, Cheng S-B, Lee T-Y, Hsiao Y-F, Liu H-T, Huang Y-C. Impact of Glutathione and Vitamin B-6 in Cirrhosis Patients: A Randomized Controlled Trial and Follow-Up Study. Nutrients. 2020; 12(7):1978. https://doi.org/10.3390/nu12071978
Chicago/Turabian StyleLai, Chia-Yu, Shao-Bin Cheng, Teng-Yu Lee, Yung-Fang Hsiao, Hsiao-Tien Liu, and Yi-Chia Huang. 2020. "Impact of Glutathione and Vitamin B-6 in Cirrhosis Patients: A Randomized Controlled Trial and Follow-Up Study" Nutrients 12, no. 7: 1978. https://doi.org/10.3390/nu12071978
APA StyleLai, C.-Y., Cheng, S.-B., Lee, T.-Y., Hsiao, Y.-F., Liu, H.-T., & Huang, Y.-C. (2020). Impact of Glutathione and Vitamin B-6 in Cirrhosis Patients: A Randomized Controlled Trial and Follow-Up Study. Nutrients, 12(7), 1978. https://doi.org/10.3390/nu12071978






