Leptin and Nutrition in Gestational Diabetes
Abstract
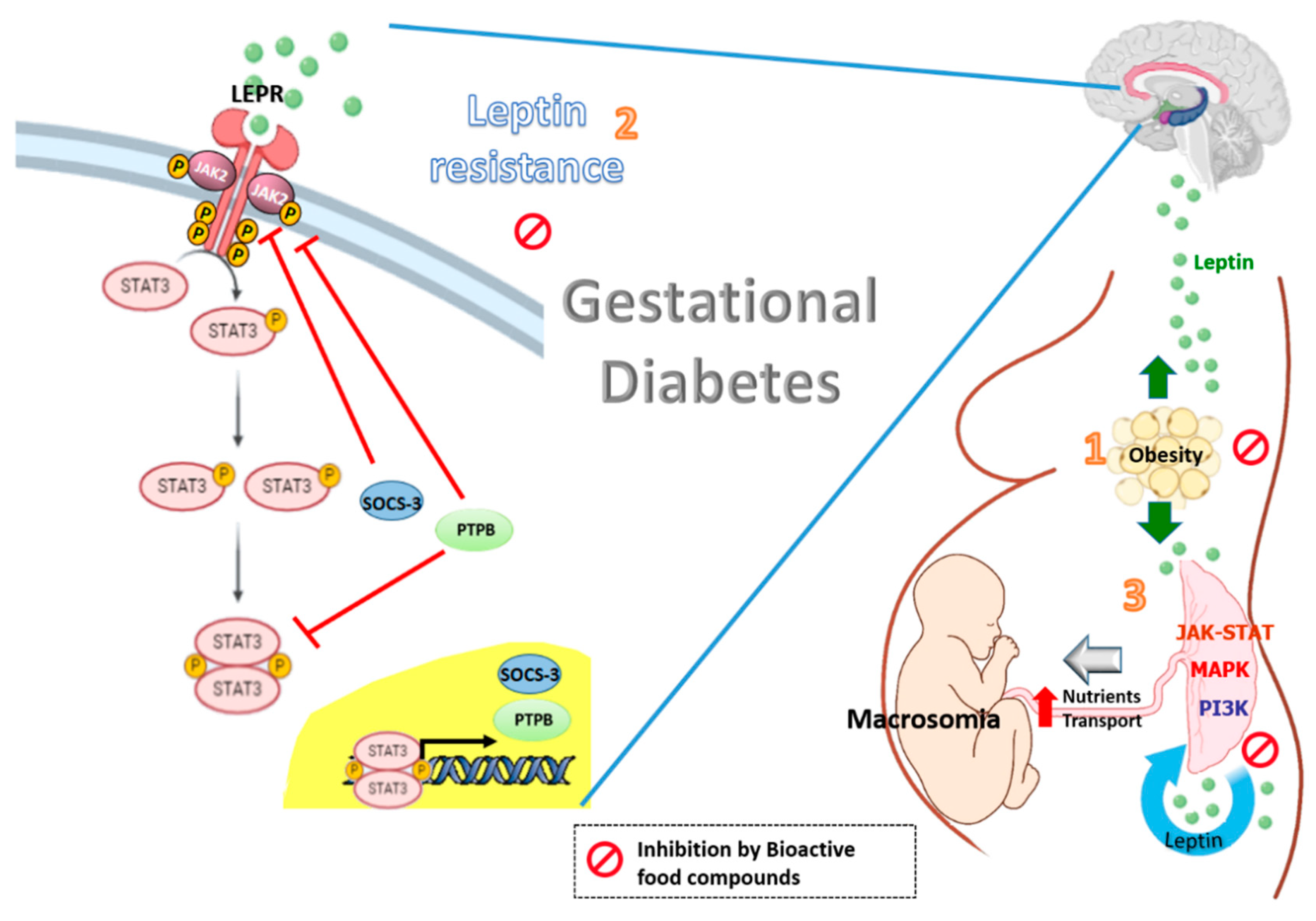
1. Introduction
2. Leptin
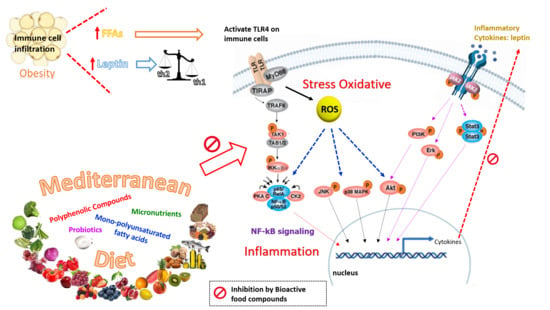
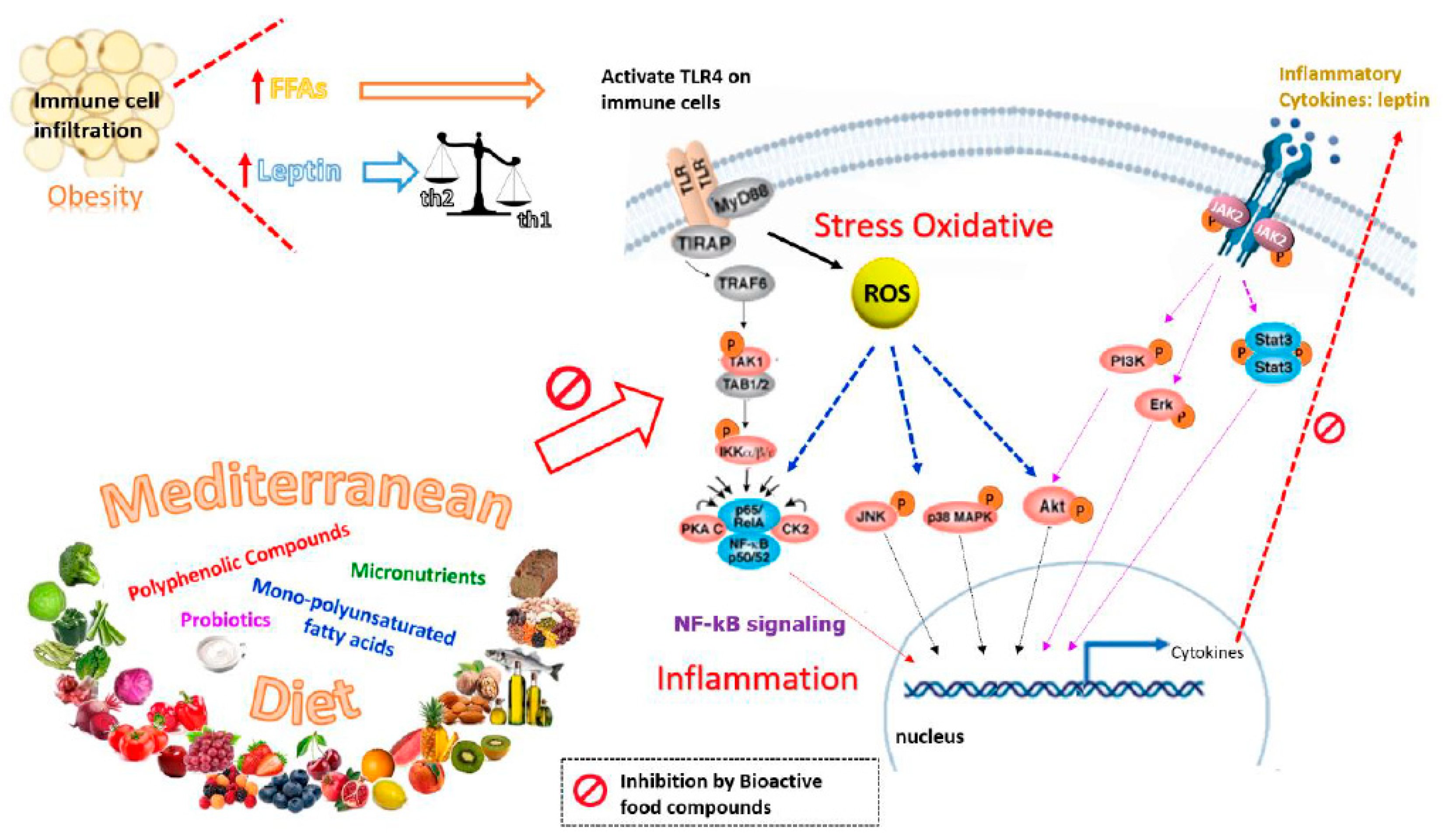
3. Nutrients and Bioactive Food Components Useful for Counteracting Hyperleptinemia and Leptin Resistance in GDM
3.1. Polyphenolic Compounds
3.2. Polyunsaturated Fatty Acids (PUFAs)
3.3. Terpenes
3.4. Probiotics
3.5. Others Bioactive Compounds
3.6. Micronutrients
4. Mediterranean Diet
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, B.; Yu, Y.; Lin, M.; Li, Z.; Wang, L.; Huang, P.; Song, H.; Shi, X.; Yang, S.; Li, X.; et al. High, but stable, trend in the prevalence of gestational diabetes mellitus: A population-based study in Xiamen, China. J. Diabetes Investig. 2019, 10, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, P.S.; Li, X.; Lin, J.; Neese, L.D.; Lin, L.; Nakajima, S.T.; Bohler, H.; Lei, Z. Dysregulation of leptin and testosterone production and their receptor expression in the human placenta with gestational diabetes mellitus. Placenta 2010, 31, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007, 30, S141–S146. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Allard, C.; Battista, M.-C.; Doyon, M.; Bouchard, L.; Ecker, J.L.; Perron, P.; Florez, J.C.; Thadhani, R.; Hivert, M.-F. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 2016, 39, 1052–1055. [Google Scholar] [CrossRef]
- Kim, S.Y.; England, L.; Wilson, H.G.; Bish, C.; Satten, G.A.; Dietz, P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am. J. Public Health 2010, 100, 1047–1052. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diab. Rep. 2015, 15, 9. [Google Scholar] [CrossRef]
- Lee, A.J.; Hiscock, R.J.; Wein, P.; Walker, S.P.; Permezel, M. Gestational diabetes mellitus: Clinical predictors and long-term risk of developing type 2 diabetes: A retrospective cohort study using survival analysis. Diabetes Care 2007, 30, 878–883. [Google Scholar] [CrossRef]
- Thadhani, R.; Powe, C.E.; Tjoa, M.L.; Khankin, E.; Ye, J.; Ecker, J.; Schneyer, A.; Karumanchi, S.A. First-trimester follistatin-like-3 levels in pregnancies complicated by subsequent gestational diabetes mellitus. Diabetes Care 2010, 33, 664–669. [Google Scholar] [CrossRef]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chua, S. Leptin function and regulation. Compr. Physiol. 2018, 8, 351–369. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef]
- Harris, R.B.S.; Apolzan, J.W. Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1327–R1339. [Google Scholar] [CrossRef][Green Version]
- van den Heuvel, J.K.; Eggels, L.; van Rozen, A.J.; Luijendijk, M.C.M.; Fliers, E.; Kalsbeek, A.; Adan, R.A.H.; la Fleur, S.E. Neuropeptide Y and leptin sensitivity is dependent on diet composition. J. Neuroendocrinol. 2014, 26, 377–385. [Google Scholar] [CrossRef]
- Håkansson, M.L.; Brown, H.; Ghilardi, N.; Skoda, R.C.; Meister, B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 1998, 18, 559–572. [Google Scholar] [CrossRef]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000Prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef]
- Matarazzo, V.; Schaller, F.; Nédélec, E.; Benani, A.; Pénicaud, L.; Muscatelli, F.; Moyse, E.; Bauer, S. Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J. Neurosci. 2012, 32, 17097–17107. [Google Scholar] [CrossRef]
- Cheng, A.; Uetani, N.; Simoncic, P.D.; Chaubey, V.P.; Lee-Loy, A.; McGlade, C.J.; Kennedy, B.P.; Tremblay, M.L. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2002, 2, 497–503. [Google Scholar] [CrossRef]
- Reed, A.S.; Unger, E.K.; Olofsson, L.E.; Piper, M.L.; Myers, M.G.; Xu, A.W. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes 2010, 59, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Bence, K.K.; Delibegovic, M.; Xue, B.; Gorgun, C.Z.; Hotamisligil, G.S.; Neel, B.G.; Kahn, B.B. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 2006, 12, 917–924. [Google Scholar] [CrossRef]
- Dodt, C.; Lönnroth, P.; Fehm, H.L.; Elam, M. The subcutaneous lipolytic response to regional neural stimulation is reduced in obese women. Diabetes 2000, 49, 1875–1879. [Google Scholar] [CrossRef][Green Version]
- Coppack, S.W.; Horowitz, J.F.; Paramore, D.S.; Cryer, P.E.; Royal, H.D.; Klein, S. Whole body, adipose tissue, and forearm norepinephrine kinetics in lean and obese women. Am. J. Physiol. Endocrinol. Metab. 1998, 275. [Google Scholar] [CrossRef]
- Morris, D.L.; Rui, L. Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1247–E1259. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Carter-Su, C.; Myers, M.G.; Rui, L. SH2B1 enhances leptin signaling by both janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol. Endocrinol. 2007, 21, 2270–2281. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Li, M.; Rui, L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J. Biol. Chem. 2004, 279, 43684–43691. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Toro, A.; Vilariño-García, T.; Maymó, J.; Guadix, P.; Dueñas, J.L.; Fernández-Sánchez, M.; Varone, C.; Sánchez-Margalet, V. Leptin action in normal and pathological pregnancies. J. Cell. Mol. Med. 2018, 22, 716–727. [Google Scholar] [CrossRef]
- Jiang, L.; Su, H.; Wu, X.; Shen, H.; Kim, M.H.; Li, Y.; Myers, M.G.; Owyang, C.; Rui, L. Leptin receptor-expressing neuron Sh2b1 supports sympathetic nervous system and protects against obesity and metabolic disease. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Farooqi, I.S.; O’Rahilly, S. 20 years of leptin: Human disorders of leptin action. J. Endocrinol. 2014, 223, T63–T70. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Gumbiner, B.; Stevens, C.; Musser, B.; Mallick, M.; Suryawanshi, S.; Maganti, L.; Zhu, H.; Han, T.H.; Scherer, L.; et al. Potent and selective agonism of the melanocortin receptor 4 with MK-0493 does not induce weight loss in obese human subjects: Energy intake predicts lack of weight loss efficacy. Clin. Pharmacol. Ther. 2009, 86, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Houseknecht, K.L.; Portocarrero, C.P. Leptin and its receptors: Regulators of whole-body energy homeostasis. Domest. Anim. Endocrinol. 1998, 15, 457–475. [Google Scholar] [CrossRef]
- Lin, K.C. Increase of maternal plasma leptin concentrations during pregnancy: Comparison with nonpregnant women. Kaohsiung J. Med. Sci. 1999, 15, 640–645. [Google Scholar] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Stewart, B.W.; et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Higgins, M.; Felle, P.; Mooney, E.E.; Bannigan, J.; McAuliffe, F.M. Stereology of the placenta in type 1 and type 2 diabetes. Placenta 2011, 32, 564–569. [Google Scholar] [CrossRef]
- Jirkovská, M.; Kučera, T.; Kaláb, J.; Jadrníček, M.; Niedobová, V.; Janáček, J.; Kubínová, L.; Moravcová, M.; Zižka, Z.; Krejčí, V. The branching pattern of villous capillaries and structural changes of placental terminal villi in type 1 diabetes mellitus. Placenta 2012, 33, 343–351. [Google Scholar] [CrossRef]
- Roberts, K.A.; Riley, S.C.; Reynolds, R.M.; Barr, S.; Evans, M.; Statham, A.; Hor, K.; Jabbour, H.N.; Norman, J.E.; Denison, F.C. Placental structure and inflammation in pregnancies associated with obesity. Placenta 2011, 32, 247–254. [Google Scholar] [CrossRef]
- Vilariño-García, T.; Pérez-Pérez, A.; Dietrich, V.; Fernández-Sánchez, M.; Guadix, P.; Dueñas, J.L.; Varone, C.L.; Damiano, A.E.; Sánchez-Margalet, V. Increased expression of aquaporin 9 in trophoblast from gestational diabetic patients. Horm. Metab. Res. 2016, 48, 535–539. [Google Scholar] [CrossRef]
- Yura, S.; Itoh, H.; Sagawa, N.; Yamamoto, H.; Masuzaki, H.; Nakao, K.; Kawamura, M.; Takemura, M.; Kakui, K.; Ogawa, Y.; et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005, 1, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Lago, F.; Dieguez, C.; Gómez-Reino, J.; Gualillo, O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007, 18, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Margalet, V.; Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Najib, S.; Gonzalez-Yanes, C. Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin. Exp. Immunol. 2003, 133, 11–19. [Google Scholar] [CrossRef]
- Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell. Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef]
- Qiu, C.; Williams, M.A.; Vadachkoria, S.; Frederick, I.O.; Luthy, D.A. Increased maternal plasma leptin in early pregnancy and risk of gestational diabetes mellitus. Obstet. Gynecol. 2004, 103, 519–525. [Google Scholar] [CrossRef]
- Cai, D.; Liu, T. Hypothalamic inflammation: A double-edged sword to nutritional diseases. Ann. N. Y. Acad. Sci. 2011, 1243, E1–E39. [Google Scholar] [CrossRef]
- SMFM Statement: Pharmacological treatment of gestational diabetes SMFM Publications Committee. Am. J. Obstet. Gynecol. 2018, 218, B2–B4. [CrossRef]
- Ge, L.; Wikby, K.; Rask, M. Is gestational diabetes a severe illness? Exploring beliefs and self-care behaviour among women with gestational diabetes living in a rural area of the south east of China. Aust. J. Rural Health 2016, 24, 378–384. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157–170. [Google Scholar] [CrossRef]
- Münzberg, H.; Flier, J.S.; Bjørbæk, C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004, 145, 4880–4889. [Google Scholar] [CrossRef]
- Scarpace, P.J.; Matheny, M.; Tümer, N.; Cheng, K.Y.; Zhang, Y. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia 2005, 48, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Cohen, S.; Danzaki, K.; MacIver, N.J. Nutritional effects on T-cell immunometabolism. Eur. J. Immunol. 2017, 47, 225–235. [Google Scholar] [CrossRef]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in nutritional status impact immune cell metabolism and function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, R.; Feingold, K.R.; Grunfeld, C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001, 15, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Hou, D.X.; Kumamoto, T. Flavonoids as protein kinase inhibitors for cancer chemoprevention: Direct binding and molecular modeling. Antioxid. Redox Signal. 2010, 13, 691–719. [Google Scholar] [CrossRef]
- Yokoyama, T.; Kosaka, Y.; Mizuguchi, M. Structural insight into the interactions between death-associated protein kinase 1 and natural flavonoids. J. Med. Chem. 2015, 58, 7400–7408. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bai, L.; Chen, W.; Xu, S. The NF-κB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin. Ther. Targets 2010, 14, 45–55. [Google Scholar] [CrossRef]
- Schanton, M.; Pérez-Pérez, A.; Gambino, Y.; Maskin, B.; Sánchez-Margalet, V.; Varone, C. Placental leptin expression is mediated by NFκB signaling. Placenta 2018, 62, 79. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Keating, E.; Martel, F. Impact of gestational diabetes mellitus in the maternal-to-fetal transport of nutrients. Curr. Diab. Rep. 2015, 15, 569. [Google Scholar] [CrossRef]
- Desoye, G.; Hauguel-De Mouzon, S. The human placenta in gestational diabetes mellitus: The insulin and cytokine network. Diabetes Care 2007, 30. [Google Scholar] [CrossRef] [PubMed]
- Maymó, J.L.; Pérez Pérez, A.; Dueñas, J.L.; Calvo, J.C.; Sánchez-Margalet, V.; Varone, C.L. Regulation of placental leptin expression by cyclic adenosine 5′-monophosphate involves cross talk between protein kinase A and mitogen-activated protein kinase signaling pathways. Endocrinology 2010, 151, 3738–3751. [Google Scholar] [CrossRef] [PubMed]
- Wahlang, B.; McClain, C.; Barve, S.; Gobejishvili, L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell. Signal. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Masilamani, M.; Wei, J.; Bhatt, S.; Paul, M.; Yakir, S.; Sampson, H.A. Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J. Allergy Clin. Immunol. 2011, 128, 1242–1250.e1. [Google Scholar] [CrossRef]
- Lin, W.; Wang, W.; Wang, D.; Ling, W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- D’Ambrosio, D.; Panina-Bordignon, P.; Sinigaglia, F. Chemokine receptors in inflammation: An overview. J. Immunol. Methods 2003, 273, 3–13. [Google Scholar] [CrossRef]
- Shen, Y.C.; Chiou, W.F.; Chou, Y.C.; Chen, C.F. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur. J. Pharmacol. 2003, 465, 171–181. [Google Scholar] [CrossRef]
- Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2002, 2, 364–371. [Google Scholar] [CrossRef]
- Andreakos, E.T.; Foxwell, B.M.; Brennan, F.M.; Maini, R.N.; Feldmann, M. Cytokines and anti-cytokine biologicals in autoimmunity: Present and future. Cytokine Growth Factor Rev. 2000, 13, 299–313. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Middleton, E. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Natural inhibitors of tumour necrosis factor-alpha production, secretion and function. Planta Med. 2000, 66, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Kotanidou, A.; Xagorari, A.; Bagli, E.; Kitsanta, P.; Fotsis, T.; Papapetropoulos, A.; Roussos, C. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am. J. Respir. Crit. Care Med. 2002, 165, 818–823. [Google Scholar] [CrossRef]
- Xagorari, A.; Papapetropoulos, A.; Mauromatis, A.; Economou, M.; Fotsis, T.; Roussos, C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 2001, 296, 181–187. [Google Scholar]
- Franco, J.G.; Dias-Rocha, C.P.; Fernandes, T.P.; Albuquerque Maia, L.; Lisboa, P.C.; Moura, E.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Resveratrol treatment rescues hyperleptinemia and improves hypothalamic leptin signaling programmed by maternal high-fat diet in rats. Eur. J. Nutr. 2016, 55, 601–610. [Google Scholar] [CrossRef]
- Ardid-Ruiz, A.; Ibars, M.; Mena, P.; Del Rio, D.; Muguerza, B.; Bladé, C.; Arola, L.; Aragonès, G.; Suárez, M. Potential involvement of peripheral leptin/STAT3 signaling in the effects of resveratrol and its metabolites on reducing body fat accumulation. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Ibars, M.; Ardid-Ruiz, A.; Suárez, M.; Muguerza, B.; Bladé, C.; Aragonès, G. Proanthocyanidins potentiate hypothalamic leptin/STAT3 signalling and Pomc gene expression in rats with diet-induced obesity. Int. J. Obes. 2017, 41, 129–136. [Google Scholar] [CrossRef]
- Zhu, F. Proanthocyanidins in cereals and pseudocereals. Crit. Rev. Food Sci. Nutr. 2019, 59, 1521–1533. [Google Scholar] [CrossRef]
- Neilson, A.P.; O’Keefe, S.F.; Bolling, B.W. High-molecular-weight proanthocyanidins in foods: Overcoming analytical challenges in pursuit of novel dietary bioactive components. Annu. Rev. Food Sci. Technol. 2016, 7, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Stürzenbaum, S.R. Proanthocyanidins of natural origin: Molecular mechanisms and implications for lipid disorder and aging-associated diseases. Adv. Nutr. 2019, 10, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Hopmans, T.E.P.; van Houten, C.; Kasius, A.; Kouznetsova, O.I.; Nguyen, L.A.; Rooijmans, S.V.; Voormolen, D.N.; van Vliet, E.O.G.; Franx, A.; Koster, M.P.H.W. Increased risk of type II diabetes mellitus and cardiovascular disease after gestational diabetes mellitus: A systematic review. Ned. Tijdschr. Geneeskd. 2015, 159, A8043. [Google Scholar] [PubMed]
- Thiruchenduran, M.; Vijayan, N.A.; Sawaminathan, J.K.; Devaraj, S.N. Protective effect of grape seed proanthocyanidins against cholesterol cholic acid diet-induced hypercholesterolemia in rats. Cardiovasc. Pathol. 2011, 20, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Weseler, A.R.; Bast, A. Masquelier’s grape seed extract: From basic flavonoid research to a well-characterized food supplement with health benefits. Nutr. J. 2017, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, H.; Zhao, J.; Yan, J.; Meng, H.; Zhan, H.; Chen, L.; Yuan, L. Grape seed proanthocyanidin inhibits monocrotaline-induced pulmonary arterial hypertension via attenuating inflammation: In vivo and in vitro studies. J. Nutr. Biochem. 2019, 67, 72–77. [Google Scholar] [CrossRef]
- Pascual-Serrano, A.; Arola-Arnal, A.; Suárez-García, S.; Bravo, F.I.; Suárez, M.; Arola, L.; Bladé, C. Grape seed proanthocyanidin supplementation reduces adipocyte size and increases adipocyte number in obese rats. Int. J. Obes. 2017, 41, 1246–1255. [Google Scholar] [CrossRef]
- Choi, H.N.; Kang, M.J.; Lee, S.J.; Kim, J.I. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-Sucrose diet. Nutr. Res. Pract. 2014, 8, 544–549. [Google Scholar] [CrossRef]
- Washio, K.; Shimamoto, Y.; Kitamura, H. Brazilian propolis extract increases leptin expression in mouse adipocytes. Biomed. Res. 2015, 36, 343–346. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 1996, 271, 665–670. [Google Scholar] [CrossRef]
- Bachiega, T.F.; Orsatti, C.L.; Pagliarone, A.C.; Sforcin, J.M. The effects of propolis and its isolated compounds on cytokine production by murine macrophages. Phyther. Res. 2012, 26, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kwon, C.S.; Son, K.H. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Arimatsu, K.; Minagawa, T.; Matsuda, Y.; Sato, K.; Takahashi, N.; Nakajima, T.; Yamazaki, K. Brazilian propolis mitigates impaired glucose and lipid metabolism in experimental periodontitis in mice. BMC Complement. Altern. Med. 2016, 16, 329. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Charisiadis, P.; Margianni, E.; Lamari, F.N.; Gerothanassis, I.P.; Tzakos, A.G. Olive leaf extracts are a natural source of advanced glycation end product inhibitors. J. Med. Food 2013, 16, 817–822. [Google Scholar] [CrossRef]
- Khalatbary, A.R.; Zarrinjoei, G.R. Anti-inflammatory effect of oleuropein in experimental Rat Spinal cord trauma. Iran. Red Crescent Med. J. 2012, 14, 229–234. [Google Scholar]
- Santiago-Mora, R.; Casado-Díaz, A.; De Castro, M.D.; Quesada-Gómez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef]
- Al-Azzawie, H.F.; Alhamdani, M.S.S. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006, 78, 1371–1377. [Google Scholar] [CrossRef]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Hoek-van den Hil, E.F.; van Schothorst, E.M.; van der Stelt, I.; Swarts, H.J.M.; van Vliet, M.; Amolo, T.; Vervoort, J.J.M.; Venema, D.; Hollman, P.C.H.; Rietjens, I.M.C.M.; et al. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.K.; Valentine, C.J.; Keim, S.A. DHA supplementation: Current implications in pregnancy and childhood. Pharmacol. Res. 2013, 70, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Importance of the omega-6/omega-3 balance in health and disease: Evolutionary aspects of diet. World Rev. Nutr. Diet. 2011, 102, 10–21. [Google Scholar] [CrossRef]
- Haggarty, P. Effect of placental function in fatty acid requirements during pregnancy. Eur. J. Clin. Nutr. 2004, 58, 1559–1570. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Magnusson, A.L.; Waterman, I.J.; Wennergren, M.; Jansson, T.; Powell, T.L. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 4607–4614. [Google Scholar] [CrossRef]
- Prescott, S.L.; Barden, A.E.; Mori, T.A.; Dunstan, J.A. Maternal fish oil supplementation in pregnancy modifies neonatal leukotriene production by cord-blood-derived neutrophils. Clin. Sci. (Lond.) 2007, 113, 409–416. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668 (Suppl. 1), S50–S58. [Google Scholar] [CrossRef]
- Jawerbaum, A.; Capobianco, E. Review: Effects of PPAR activation in the placenta and the fetus: Implications in maternal diabetes. Placenta 2011, 32 (Suppl. 2), S212–S217. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Ning, Y.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Olsen, S.F.; Gillman, M.W. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann. Epidemiol. 2007, 17, 663–668. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Mori, T.A.; Keelan, J.A.; Waddell, B.J. Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol. Reprod. 2013, 88. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Mohamed-Ali, V.; Horakova, O.; Rossmeisl, M.; Hosseinzadeh-Attar, M.J.; Hensler, M.; Ruzickova, J.; Kopecky, J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 2006, 49, 394–397. [Google Scholar] [CrossRef]
- El Akoum, S.; Lamontagne, V.; Cloutier, I.; Tanguay, J.F. Nature of fatty acids in high fat diets differentially delineates obesity-linked metabolic syndrome components in male and female C57BL/6J mice. Diabetol. Metab. Syndr. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: Mechanistic insights. Adv. Nutr. 2011, 2, 304–316. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Scarpace, P.J. Transcriptional regulation of uncoupling protein-2 gene expression in L6 myotubes. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1619–1624. [Google Scholar] [CrossRef][Green Version]
- Al-Gubory, K.H. Mitochondria: Omega-3 in the route of mitochondrial reactive oxygen species. Int. J. Biochem. Cell Biol. 2012, 44, 1569–1573. [Google Scholar] [CrossRef]
- Shang, F.; Li, X.; Jiang, X. Coffee consumption and risk of the metabolic syndrome: A meta-analysis. Diabetes Metab. 2016, 42, 80–87. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Shen, T.; Park, Y.C.; Kim, S.H.; Lee, J.; Cho, J.Y. Nuclear factor-κB/signal Transducers and activators of transcription-1- mediated inflammatory responses in Lipopolysaccharide-activated macrophages are a major inhibitory target of kahweol, a coffee diterpene. Biol. Pharm. Bull. 2010, 33, 1159–1164. [Google Scholar] [CrossRef]
- Hosoi, T.; Toyoda, K.; Nakatsu, K.; Ozawa, K. Caffeine attenuated ER stress-induced leptin resistance in neurons. Neurosci. Lett. 2014, 569, 23–26. [Google Scholar] [CrossRef]
- Kim, H.G.; Hwang, Y.P.; Jeong, H.G. Kahweol blocks STAT3 phosphorylation and induces apoptosis in human lung adenocarcinoma A549 cells. Toxicol. Lett. 2009, 187, 28–34. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Szabo, A.; Wu, Z.; Wang, H.; Li, D.; Huang, X.F. Teasaponin reduces inflammation and central leptin resistance in diet-induced obese male mice. Endocrinology 2013, 154, 3130–3140. [Google Scholar] [CrossRef]
- Zhao, L. The gut microbiota and obesity: From correlation to causality. Nat. Rev. Microbiol. 2013, 11, 639–647. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Barrett, H.L.; Gomez-Arango, L.F.; Wilkinson, S.A.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Marítn-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Kling Bäckhed, H.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: A randomized, double-blind, placebo-controlled trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef]
- Rautava, S.; Kainonen, E.; Salminen, S.; Isolauri, E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J. Allergy Clin. Immunol. 2012, 130, 1355–1360. [Google Scholar] [CrossRef]
- Isolauri, E.; Rautava, S.; Salminen, S. Probiotics in the development and treatment of allergic disease. Gastroenterol. Clin. North. Am. 2012, 41, 747–762. [Google Scholar] [CrossRef]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar] [CrossRef]
- Mangels, A.R.; Holden, J.M.; Beecher, G.R.; Forman, M.R.; Lanza, E. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J. Am. Diet. Assoc. 1993, 93, 284–296. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Romier, B.; Gouranton, E.; Riollet, C.; Gleize, B.; Malezet-Desmoulins, C.; Landrier, J.F. Lycopene attenuates LPS-induced TNF-α secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage-conditioned media. Mol. Nutr. Food Res. 2012, 56, 725–732. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Luvizotto, R.D.A.M.; Nascimento, A.F.; Imaizumi, E.; Pierine, D.T.; Conde, S.J.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L.A. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br. J. Nutr. 2013, 110, 1803–1809. [Google Scholar] [CrossRef]
- Saravanan, S.; Pari, L. Role of thymol on hyperglycemia and hyperlipidemia in high fat diet-induced type 2 diabetic C57BL/6J mice. Eur. J. Pharmacol. 2015, 761, 279–287. [Google Scholar] [CrossRef]
- Shibata, T.; Kimura, Y.; Mukai, A.; Mori, H.; Ito, S.; Asaka, Y.; Oe, S.; Tanaka, H.; Takahashi, T.; Uchida, K. Transthiocarbamoylation of proteins by thiolated isothiocyanates. J. Biol. Chem. 2011, 286, 42150–42161. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Dyba, M.A.; Holland, R.J.; Liang, Y.H.; Singh, S.V.; Ji, X. Irreversible inhibition of glutathione S-transferase by phenethyl isothiocyanate (PEITC), a dietary cancer chemopreventive phytochemical. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Cross, J.V.; Foss, F.W.; Rady, J.M.; Macdonald, T.L.; Templeton, D.J. The isothiocyanate class of bioactive nutrients covalently inhibit the MEKK1 protein kinase. BMC Cancer 2007, 7. [Google Scholar] [CrossRef]
- Chiarugi, P.; Cirri, P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem. Sci. 2003, 28, 509–514. [Google Scholar] [CrossRef]
- Lewis, S.M.; Li, Y.; Catalano, M.J.; Laciak, A.R.; Singh, H.; Seiner, D.R.; Reilly, T.J.; Tanner, J.J.; Gates, K.S. Inactivation of protein tyrosine phosphatases by dietary isothiocyanates. Bioorganic Med. Chem. Lett. 2015, 25, 4549–4552. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Nakatsuji, Y.; Maeda, A.; Ota, H.; Kamikubo, R.; Miyoshi, N.; Nakamura, Y.; Akagawa, M. Phenethyl isothiocyanate activates leptin signaling and decreases food intake. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Scholl, T.O.; Hediger, M.L.; Bendich, A.; Schall, J.I.; Smith, W.K.; Krueger, P.M. Use of multivitamin/mineral prenatal supplements: Influence on the outcome of pregnancy. Am. J. Epidemiol. 1997, 146, 134–141. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.V.; Vanderlelie, J.J. Multiple micronutrient supplementation and birth outcomes: The potential importance of selenium. Placenta 2016, 48, S61–S65. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.M.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef]
- Kawai, K.; Spiegelman, D.; Shankar, A.H.; Fawzi, W.W. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: Meta-analysis and meta-regression. Bull. World Health Organ. 2011, 89, 402B–411B. [Google Scholar] [CrossRef]
- The International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: Meta-analysis of individual participant data from randomised trials. BMJ 2017, 358, j3119. [Google Scholar] [CrossRef]
- Rogozińska, E.; Marlin, N.; Jackson, L.; Rayanagoudar, G.; Ruifrok, A.E.; Dodds, J.; Molyneaux, E.; van Poppel, M.N.; Poston, L.; Vinter, C.A.; et al. Effects of antenatal diet and physical activity on maternal and fetal outcomes: Individual patient data meta-analysis and health economic evaluation. Health Technol. Assess. 2017, 21, 1–158. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Al Wattar, B.H.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Pérez, A.; Vilariño-García, T.; Guadix, P.; Dueñas, J.L.; Sánchez-Margalet, V. Leptin and Nutrition in Gestational Diabetes. Nutrients 2020, 12, 1970. https://doi.org/10.3390/nu12071970
Pérez-Pérez A, Vilariño-García T, Guadix P, Dueñas JL, Sánchez-Margalet V. Leptin and Nutrition in Gestational Diabetes. Nutrients. 2020; 12(7):1970. https://doi.org/10.3390/nu12071970
Chicago/Turabian StylePérez-Pérez, Antonio, Teresa Vilariño-García, Pilar Guadix, José L. Dueñas, and Víctor Sánchez-Margalet. 2020. "Leptin and Nutrition in Gestational Diabetes" Nutrients 12, no. 7: 1970. https://doi.org/10.3390/nu12071970
APA StylePérez-Pérez, A., Vilariño-García, T., Guadix, P., Dueñas, J. L., & Sánchez-Margalet, V. (2020). Leptin and Nutrition in Gestational Diabetes. Nutrients, 12(7), 1970. https://doi.org/10.3390/nu12071970