Profiles of Polyphenol Intake and Type 2 Diabetes Risk in 60,586 Women Followed for 20 Years: Results from the E3N Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Follow-Up
2.2. Polyphenol Intake
2.3. Ascertainment of Type 2 Diabetes
2.4. Covariables
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Baseline Characteristics
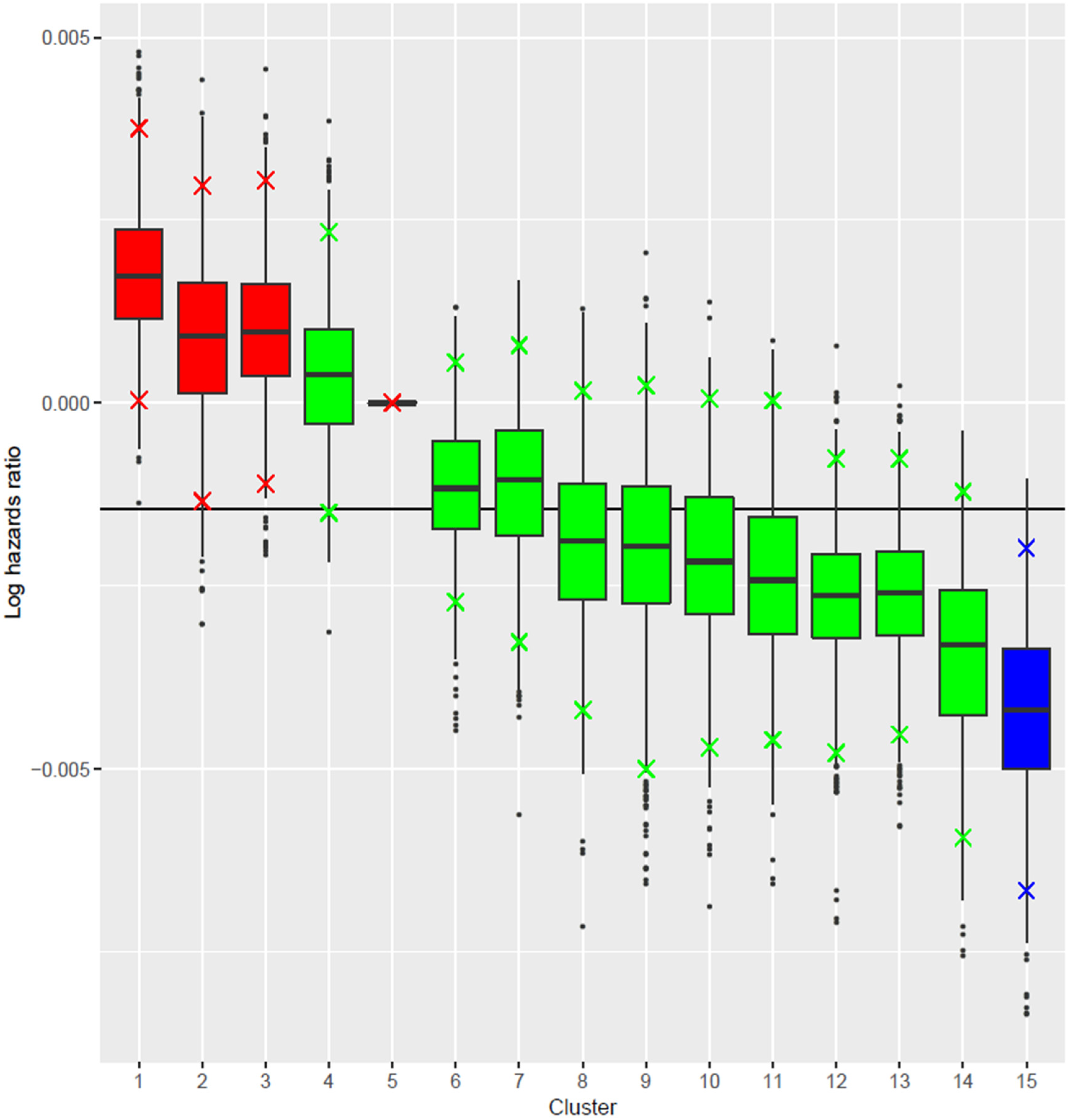
3.2. Clusters of Polyphenol Subclasses Intake and Type 2 Diabetes Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Meigs, J.B.; Li, T.Y.; Rifai, N.; Manson, J.E. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004, 53, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chung, S.J.; Floegel, A.; Song, W.O.; Koo, S.I.; Chun, O.K. Dietary antioxidant capacity is associated with improved serum antioxidant status and decreased serum C-reactive protein and plasma homocysteine concentrations. Eur. J. Nutr. 2013, 52, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Smidowicz, A.; Regula, J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv. Nutr. 2015, 6, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S5–S78. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Goyal, M.R.; Ayeleso, A.O. Bioactive Compounds of Medicinal Plants: Properties and Potential for Human Health; CRC Press: Boca Raton, FL, USA, 2018; 334p. [Google Scholar]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar]
- Clavel-Chapelon, F.; E3N Study Group. Cohort profile: The French E3N cohort study. Int. J. Epidemiol. 2014, 44, 801–809. [Google Scholar] [CrossRef]
- van Liere, M.J.; Lucas, F.; Clavel, F.; Slimani, N.; Villeminot, S. Relative validity and reproducibility of a French dietary history questionnaire. Int. J. Epidemiol. 1997, 26, S128–S136. [Google Scholar] [CrossRef] [PubMed]
- de Securite Sanitaire (ANSES) AN. Table de Composition Nutritionnelle des Aliments Ciqual. Available online: www.ansespro.fr/TableCIQUAL/ (accessed on 12 December 2016).
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Mancini, F.R.; Dow, C.; Affret, A.; Rajaobelina, K.; Dartois, L.; Balkau, B.; Bonnet, F.; Boutron-Ruault, M.-C.; Fagherazzi, G. Micronutrient dietary patterns associated with type 2 diabetes mellitus among women of the E3N-EPIC (Etude Epidémiologique auprès de femmes de l’Education Nationale) cohort study. J. Diabetes 2018, 10, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Laouali, N.; Mancini, F.R.; Hajji-Louati, M.; El Fatouhi, D.; Balkau, B.; Boutron-Ruault, M.-C.; Bonnet, F.; Fagherazzi, G. Dietary inflammatory index and type 2 diabetes risk in a prospective cohort of 70,991 women followed for 20 years: The mediating role of BMI. Diabetologia 2019, 62, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Papathomas, M.; Molitor, J.; Richardson, S.; Riboli, E.; Vineis, P. Examining the joint effect of multiple risk factors using exposure risk profiles: Lung cancer in nonsmokers. Environ. Health Perspect. 2011, 119, 84–91. [Google Scholar] [CrossRef]
- Neal, R.M. Markov chain sampling methods for dirichlet process mixture models. J. Comput. Graph. Stat. 2000, 9, 249–265. [Google Scholar]
- Liverani, S.; Hastie, D.I.; Azizi, L.; Papathomas, M.; Richardson, S. PReMiuM: An R package for profile regression mixture models using dirichlet processes. J. Stat. Softw. 2015, 64, 1–30. [Google Scholar] [CrossRef]
- Coker, E.; Liverani, S.; Ghosh, J.K.; Jerrett, M.; Beckerman, B.; Li, A.; Ritz, B.; Molitor, J. Multi-pollutant exposure profiles associated with term low birth weight in Los Angeles County. Environ. Int. 2016, 91, 1–13. [Google Scholar] [CrossRef]
- Quero, J.; Mármol, I.; Cerrada, E.; Rodríguez-Yoldi, M.J. Insight into the potential application of polyphenol-rich dietary intervention in degenerative disease management. Food Funct. 2020, 11, 2805–2825. [Google Scholar] [CrossRef]
- Romaguera, D.; Guevara, M.; Norat, T.; Langenberg, C.; Forouhi, N.G.; Sharp, S.; Slimani, N.; Schulze, M.B.; Buijsse, B. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: The InterAct project. Diabetes Care 2011, 34, 1913–1918. [Google Scholar] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I.; Suhan, T.O.; de Luca, C.; Korkina, L.G. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur. J. Pharmacol. 2011, 658, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Park, J.O.; Phyu, H.; Dong, Z.; Yang, C.S. Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (-)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate 1. FASEB J. 2001, 15, 2022–2024. [Google Scholar] [CrossRef]
- Muraoka, K.; Shimizu, K.; Sun, X.; Tani, T.; Izumi, R.; Miwa, K.; Yamamoto, K. Flavonoids exert diverse inhibitory effects on the activation of nf-κb. Transpl. Proc. 2002, 34, 1335–1340. [Google Scholar] [CrossRef]
| Type 2 Diabetes Diagnosed during the Follow-Up | |||
|---|---|---|---|
| Overall (N = 60,586) | No (N = 57,846) | Yes (N = 2740) | |
| Age at baseline (years) | 52.69 (6.62) | 52.62 (6.62) | 54.11 (6.62) |
| Educational level (%) | |||
| Undergraduate or less | 6044 (9.98) | 5675 (9.81) | 369 (13.47) |
| Graduate | 31,512 (52.01) | 29,971 (51.81) | 1541 (56.24) |
| Postgraduate or more | 23,030 (38.01) | 22,200 (38.38) | 830 (30.29) |
| Physical activity (MET-h/week) | 49.24 (49.88) | 49.26 (49.83) | 48.81 (51.04) |
| Smoking status (%) | |||
| Current | 8295 (13.69) | 7867 (13.60) | 428 (15.62) |
| Former | 20,256 (33.43) | 19,391 (33.52) | 865 (31.57) |
| Never | 32,035 (52.88) | 30,588 (52.88) | 1447 (52.81) |
| BMI (kg/m2) | 22.88 (3.23) | 22.70 (3.04) | 26.55 (4.58) |
| Hypertension (%) | 30,974 (51.12) | 28,937 (50.02) | 2037 (74.34) |
| Hypercholesterolemia (%) | 4273 (7.05) | 3870 (6.69) | 403 (14.71) |
| Family history of diabetes (%) | 6821 (11.26) | 6194 (10.71) | 627 (22.88) |
| Alcohol consumption (g/day) | 11.64 (13.85) | 11.61 (13.76) | 12.16 (15.53) |
| Coffee intake (ml/day) | 200.81 (148.26) | 200.89 (148.01) | 199.12 (153.51) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laouali, N.; Berrandou, T.; A. Rothwell, J.; Shah, S.; El Fatouhi, D.; Romana Mancini, F.; Boutron-Ruault, M.-C.; Fagherazzi, G. Profiles of Polyphenol Intake and Type 2 Diabetes Risk in 60,586 Women Followed for 20 Years: Results from the E3N Cohort Study. Nutrients 2020, 12, 1934. https://doi.org/10.3390/nu12071934
Laouali N, Berrandou T, A. Rothwell J, Shah S, El Fatouhi D, Romana Mancini F, Boutron-Ruault M-C, Fagherazzi G. Profiles of Polyphenol Intake and Type 2 Diabetes Risk in 60,586 Women Followed for 20 Years: Results from the E3N Cohort Study. Nutrients. 2020; 12(7):1934. https://doi.org/10.3390/nu12071934
Chicago/Turabian StyleLaouali, Nasser, Takiy Berrandou, Joseph A. Rothwell, Sanam Shah, Douae El Fatouhi, Francesca Romana Mancini, Marie-Christine Boutron-Ruault, and Guy Fagherazzi. 2020. "Profiles of Polyphenol Intake and Type 2 Diabetes Risk in 60,586 Women Followed for 20 Years: Results from the E3N Cohort Study" Nutrients 12, no. 7: 1934. https://doi.org/10.3390/nu12071934
APA StyleLaouali, N., Berrandou, T., A. Rothwell, J., Shah, S., El Fatouhi, D., Romana Mancini, F., Boutron-Ruault, M.-C., & Fagherazzi, G. (2020). Profiles of Polyphenol Intake and Type 2 Diabetes Risk in 60,586 Women Followed for 20 Years: Results from the E3N Cohort Study. Nutrients, 12(7), 1934. https://doi.org/10.3390/nu12071934





