Intermittent Hypoxic Exposure with High Dose of Arginine Impact on Circulating Mediators of Tissue Regeneration
Abstract
1. Introduction
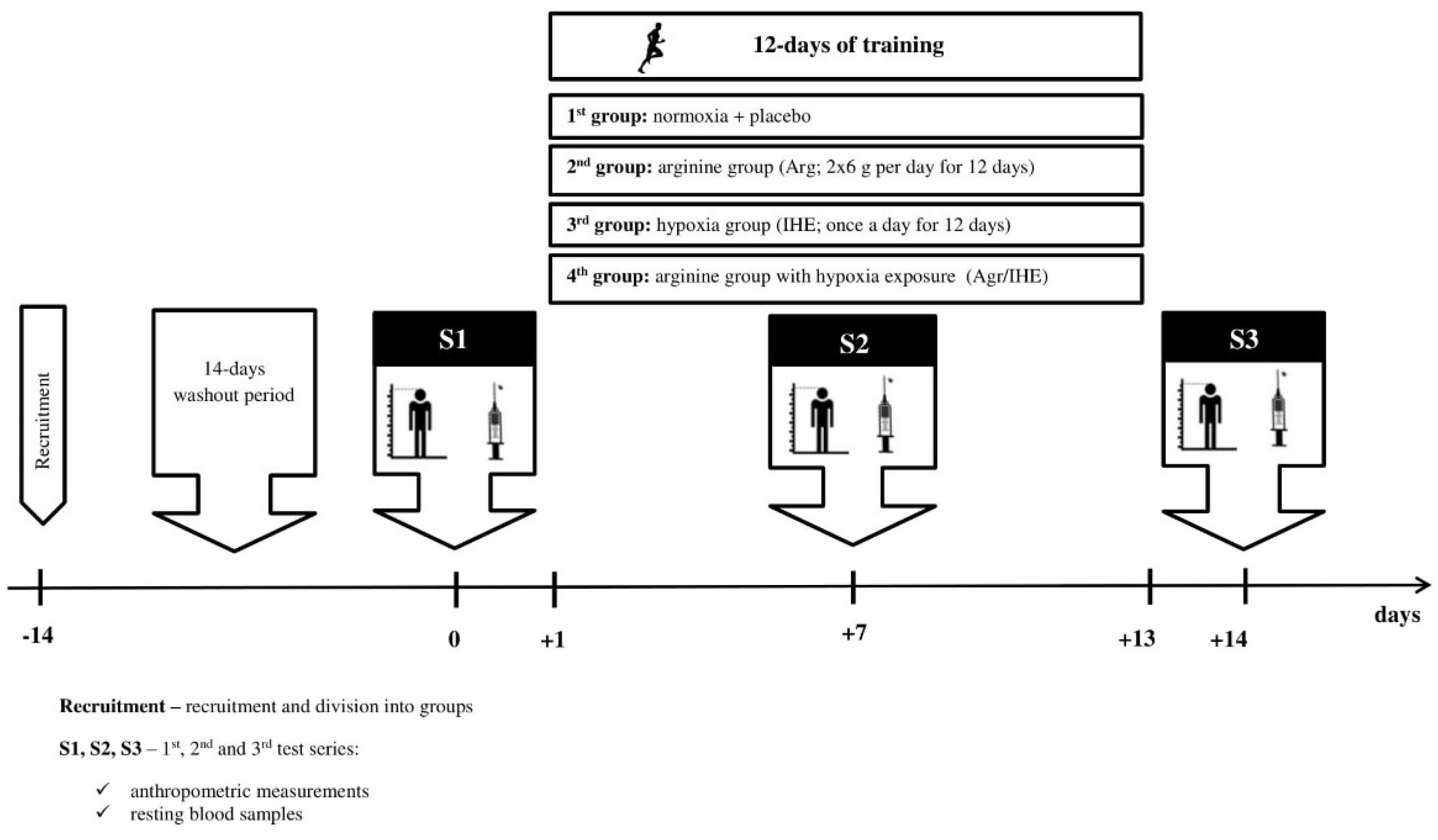
2. Materials and Methods
2.1. Subjects
2.2. Arginine Supplementation
2.3. Intermittent Hypoxic Exposure
2.4. Body Composition
2.5. Blood Sampling
2.6. Skeletal Muscle Damage
2.7. Oxi-Inflammatory Mediators
2.8. Growth Factors
2.9. Lipoprotein-Lipid Profile
2.10. Haematological Variables
2.11. Statistical Analysis
3. Results
3.1. Skeletal Muscle Damage
3.2. Oxi-Inflammatory Mediators
3.3. Growth Factors
3.4. Haematological Variables
3.5. Lipoprotein–Lipid Profile
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Filippin, L.I.; Cuevas, M.J.; Lima, E.; Marroni, N.P.; Gonzalezgallego, J.; Xavier, R.M. Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide 2011, 24, 43–49. [Google Scholar] [CrossRef]
- Kuang, S.; Gillespie, M.A.; Rudnicki, M.A. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem. Cell 2008, 2, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tengan, C.H.; Rodrigues, G.S.; Godinho, R.O. Nitric oxide in skeletal muscle: Role on mitochondrial biogenesis and function. Int. J. Mol. Sci. 2012, 13, 17160–17184. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Davies, B.; Baker, J. Training in hypoxia: Modulation of metabolic and cardiovascular risk factors in men. Med. Sci. Sports Exerc. 2000, 32, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Bril, G.; Ploszczyca, K.; Piotrowicz, Z.; Chalimoniuk, M.; Roczniok, R.; Zembron-Lacny, A.; Gerasimuk, D.; Langfort, J. Intermittent hypoxic training at lactate threshold intensity improves aiming performance in well-trained biathletes with little change of cardiovascular variables. Biomed Res. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Wiesner, S.; Engeli, S.; Luft, F.C.; Jordan, J. Influence of normobaric hypoxia training on metabolic risk markers in human subjects. Med. Sci. Sports Exerc. 2008, 40, 1939–1944. [Google Scholar] [CrossRef]
- Chaillou, T.; Lanner, J.T. Regulation of myogenesis and skeletal muscle regeneration: Effects of oxygen levels on satellite cell activity. FASEB J. 2016, 30, 3929–3941. [Google Scholar] [CrossRef]
- Savla, J.J.; Levine, B.D.; Sadek, H.A. The effect of hypoxia on cardiovascular disease: Friend or foe? High. Alt. Med. Biol. 2018, 19, 124–130. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal. Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [PubMed]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, A.; Santos, C.X.; Shah, A.M.; Zhang, H.; Faber, J.E.; Kinter, M.T.; et al. Hypoxia induces heart regeneration in adult mice. Nature 2017, 541, 222–227. [Google Scholar] [CrossRef]
- Böger, R.H. The pharmacodynamics of L-arginine. J. Nutr. 2007, 137, 1650S–1655S. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Oh, S.K.; Lee, J.S.; Wu, C.; Lee, S.J. Effects of L-arginine on growth hormone and insulin-like growth factor 1. Food Sci. Biotechnol. 2017, 26, 1749–1754. [Google Scholar] [CrossRef]
- Curran, J.N.; Winter, D.C.; Bouchier-Hayes, D. Biological fate and clinical implications of arginine metabolism in tissue healing. Wound Rep. Reg. 2006, 14, 376–386. [Google Scholar] [CrossRef]
- Filippin, L.I.; Moreira, A.J.; Marroni, N.P.; Xavier, R.M. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide 2009, 21, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hinckson, E.A.; Hamlin, M.J.; Wood, M.R.; Hopkins, W.G. Game performance and intermittent hypoxic training. Br. J. Sports Med. 2007, 41, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Ziemann, E.; Zurek, P.; Hübner-Wozniak, E. Heat shock protein 27 response to wrestling training in relation to the muscle damage and inflammation. J. Strength Cond. Res. 2017, 3, 1221–1228. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Ambrose, K.R.; Golightly, Y.M. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Pract. Res. Clin. Rheumatol. 2015, 29, 120–130. [Google Scholar] [CrossRef]
- Chang, J.C.; Lien, C.F.; Lee, W.S.; Chang, H.R.; Hsu, Y.C.; Luo, Y.P.; Jeng, J.R.; Hsieh, J.C.; Yang, K.T. Intermittent Hypoxia Prevents Myocardial Mitochondrial Ca2+ Overload and Cell Death during Ischemia/Reperfusion: The Role of Reactive Oxygen Species. Cells 2019. [Google Scholar] [CrossRef]
- Dobson, J.L.; McMillan, J.; Li, L. Benefits of exercise intervention in reducing neuropathic pain. Front. Cell Neurosci. 2014, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.; Marsh, D. Ischaemia-reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. Int. J. Exp. Pathol. 2010, 91, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Kashimura, O.; Kano, Y.; Ohno, H.; Ji, L.L.; Izawa, T.; Best, T.M. Role of nitric oxide in muscle regeneration following eccentric muscle contractions in rat skeletal muscle. J. Physiol. Sci. 2013, 63, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Filippin, L.I.; Cuevas, M.J.; Lima, E.; Marroni, N.P.; Gonzalez-Gallego, J.; Xavier, R.M. The role of nitric oxide during healing of trauma to the skeletal muscle. Inflamm. Res. 2011, 60, 347–356. [Google Scholar] [CrossRef]
- Soneja, A.; Drews, M.; Malinski, T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005, 57, 108–119. [Google Scholar]
- Zembron-Lacny, A.; Tylutka, A.; Zeromska, A.; Kasperska, A.; Wolny-Rokicka, E. Does high volume of exercise training increase aseptic vascular inflammation in male athletes? Am. J. Men’s Health 2019, 13. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhu, H.F.; Dong, J.W.; Zhu, W.Z.; Yang, W.W.; Yang, H.T.; Zhou, Z.N. Inducible nitric oxide synthase contributes to intermittent hypoxia against ischemia/reperfusion injury. Acta Pharmacol. Sin. 2005, 26, 315–322. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Strijdom, H.; Muller, C.; Lochner, A. Direct intracellular nitric oxide detection in isolated adult cardiomyocytes: Flow cytometric analysis using the fluorescent probe, diaminofluorescein. J. Mol. Cell. Cardiol. 2004, 37, 897–902. [Google Scholar] [CrossRef]
- Strijdom, H.; Jacobs, S.; Hattingh, S.; Page, C.; Lochner, A. Nitric oxide production is higher in rat cardiac microvessel endothelial cells than ventricular cardiomyocytes in baseline and hypoxic conditions: A comparative study. FASEB J. 2006, 20, 314–316. [Google Scholar] [CrossRef]
- Vogt, M.; Hoppeler, H. Is hypoxia training good for muscles and exercise performance? Prog. Cardiovasc. Dis. 2010, 52, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kolár, F.; Szárszoi, O.; Neckárˇ, J.; Pecháňová, O.; Miková, D.; Hampl, V.; Oštádal, B. Role of nitric oxide and reactive oxygen species in reperfusion-induced arrhythmias and cardioprotection in chronically hypoxic rat. hearts. Physiol. Res. 2003, 52, 52. [Google Scholar]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.L.; Widstrom, L.; Goodrich, J.; Poddar, S.; Rueda, M.; Holliday, M.; San Millian, I.; Byrnes, W.C. A Retrospective Analysis of Collegiate Athlete Blood Biomarkers at Moderate Altitude. J. Strength Cond. Res. 2019, 33, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Vogt, M. Muscle tissue adaptations to hypoxia. J. Exp. Biol. 2001, 204, 3133–3139. [Google Scholar] [PubMed]
- Alvares, T.S.; Conte-Junior, C.A.; Silva, J.T.; Paschoalin, V.M. L-arginine does not improve biochemical and hormonal response in trained runners after 4 weeks of supplementation. Nutr. Res. 2014, 34, 31–39. [Google Scholar] [CrossRef]
- Forbes, S.C.; Bell, G.J. The acute effects of a low and high dose of oral L-arginine supplementation in young active males at rest. Appl. Physiol. Nutr. Metab. 2011, 36, 405–411. [Google Scholar] [CrossRef]
- Forbes, S.C.; Harber, V.; Bell, G.J. The acute effects of L-arginine on hormonal and metabolic responses during submaximal exercise in trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 369–377. [Google Scholar] [CrossRef]
- Meirelles, C.M.; Matsuura, C. Acute supplementation of L-arginine affects neither strength performance nor nitric oxide production. J. Sports Med. Phys. Fit. 2018, 58, 216–220. [Google Scholar]
- Meirelles, C.M.; Matsuura, C.; Silva, R.S., Jr.; Guimarães, F.F.; Gomes, P.S.C. Acute effects of l-arginine supplementation on oxygen consumption kinetics and muscle oxyhemoglobin and deoxyhemoglobin during treadmill running in male adults. Int. J. Exerc. Sci. 2019, 12, 444–455. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zembron-Lacny, A.; Krzywański, J.; Ostapiuk-Karolczuk, J.; Kasperska, A. Cell and molecular mechanisms of regeneration and reorganization of skeletal muscles. Ortop. Traumatol. Rehabil. 2012, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Breen, E.; Tang, K.; Olfert, M.; Knapp, A.; Wagner, P. Skeletal muscle capillarity during hypoxia: VEGF and its activation. High Alt. Med. Biol. 2008, 9, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Shashar, M.; Chernichovski, T.; Pasvolsky, O.; Levi, S.; Grupper, A.; Hershkovitz, R.; Weinstein, T.; Schwartz, I.F. Vascular Endothelial Growth Factor Augments Arginine Transport and Nitric Oxide Generation via a KDR Receptor Signaling Pathway. Kidney Blood Press. Res. 2017, 42, 201–208. [Google Scholar] [CrossRef]
- Gianni Barrera, R.; Di Maggio, N.; Melly, L.; Burger, M.G.; Mujagic, E.; Gürke, L.; Schaefer, D.J.; Banfi, A. Therapeutic vascularization in regenerative medicine. Stem Cells Transl. Med. 2020, 9, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.E. Hepatocyte growth factor and satellite cell activation. Adv. Exp. Med. Biol. 2016, 900, 1–25. [Google Scholar]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 2009, 94, 1153–1160. [Google Scholar] [CrossRef]
- Ogborn, D.I.; Gardiner, P.F. Effects of exercise and muscle type on BDNF, NT-4/5, and TrKB expression in skeletal muscle. Muscle Nerve 2010, 41, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Lieberman, P.; Protopapas, A.; Reed, E.; Youngs, J.W.; Kanki, B.G. Cognitive defects at altitude. Nature 1994, 372, 325. [Google Scholar] [CrossRef]
- Amann, M.; Romer, L.M.; Subudhi, A.W.; Pegelow, D.F.; Dempsey, J.A. Severity, of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J. Physiol. 2007, 581, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, Z.; Chalimoniuk, M.; Płoszczyca, K.K.; Czuba, M.; Langfort, J. Acute normobaric hypoxia does not affect the simultaneous exercise-induced increase in circulating BDNF and GDNF in young healthy men: A feasibility study. PLoS ONE 2019, 14, e0224207. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The many facets of erythropoietin physiologic and metabolic response. Front. Physiol. 2019, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Suzuki, N.; Yamamoto, M.; Gassmann, M.; Noguchi, C.T. Endogenous erythropoietin signaling facilitates skeletal muscle repair and recovery following pharmacologically induced damage. FASEB J. 2012, 26, 2847–2858. [Google Scholar] [CrossRef]
- Heeschen, C.; Aicher, A.; Lehmann, R.; Fichtlscherer, S.; Vasa, M.; Urbich, C.; Mildner-Rihm, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 2003, 102, 1340–1346. [Google Scholar] [CrossRef]
- Beleslin-Cokic, B.B.; Cokic, V.P.; Yu, X.; Weksler, B.B.; Schechter, A.N.; Noguchi, C.T. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 2004, 104, 2073–2080. [Google Scholar] [CrossRef]
- Mallet, R.T.; Manukhina, E.B.; Ruelas, S.S.; Caffrey, J.L.; Downey, H.F. Cardioprotection by intermittent hypoxia conditioning: Evidence, mechanisms, and therapeutic potential. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H216–H232. [Google Scholar] [CrossRef]
| Control n = 10 | Arg n = 7 | IHE n = 6 | Arg/IHE n = 9 | Control vs. Arg IHE Arg/IHE | |
|---|---|---|---|---|---|
| Age [yr.] | 24.6 ± 3.0 | 20.0 ± 1.6 | 22.8 ± 2.6 | 24.7 ± 4.4 | <0.05 0.622 0.999 |
| Height [cm] | 173.6 ± 8.8 | 179.0 ± 9.5 | 181.2 ± 7.3 | 175.6 ± 8.3 | 0.559 0.320 0.947 |
| Weight [kg] | 81.4 ± 21.8 | 79.9 ± 13.0 | 97.1 ± 22.7 | 87.9 ± 20.7 | 0.989 0.560 0.957 |
| BMI [kg/m2] | 26.6 ± 4.5 | 24.4 ± 1.4 | 29.3 ± 5.2 | 27.8 ± 4.5 | 0.552 0.773 0.990 |
| %FM | 18.1 ± 4.8 | 9.3 ± 3.0 | 14.5 ± 6.0 | 21.3 ± 6.5 | <0.05 0.844 0.252 |
| FM [kg] | 15.4 ± 7.4 | 7.6 ± 3.2 | 15.1 ± 9.5 | 19.3 ± 10.8 | 0.355 0.998 0.569 |
| FFM [kg] | 66.0 ± 15.0 | 72.3 ± 10.6 | 81.9 ± 14.0 | 67.2 ± 11.5 | 0.952 0.270 0.996 |
| 1st Day of Camp | 7th Day of Camp | 14th Day of Camp | 1st Day vs. 7th Day | 1st Day vs. 14th Day | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | |||
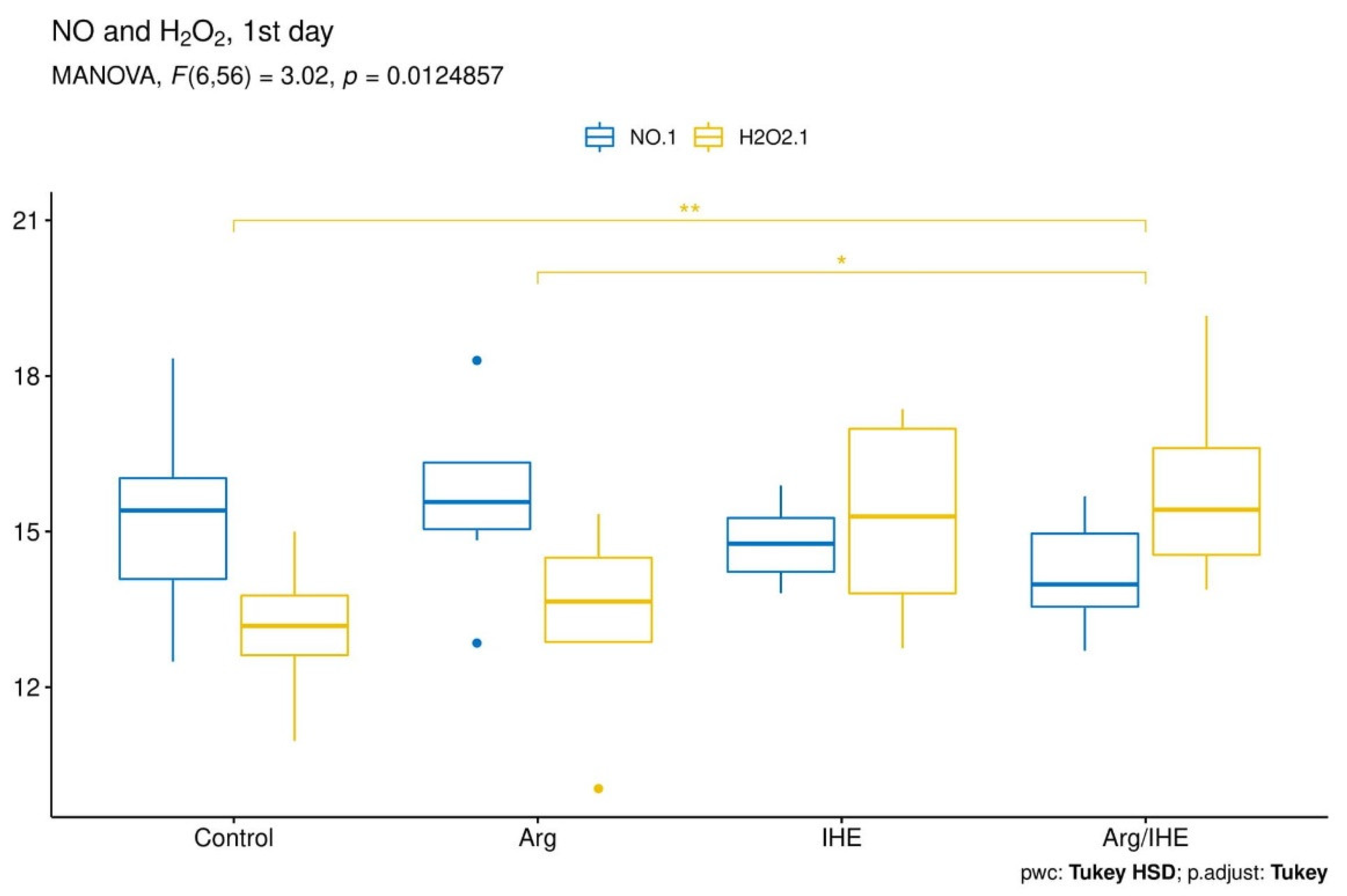
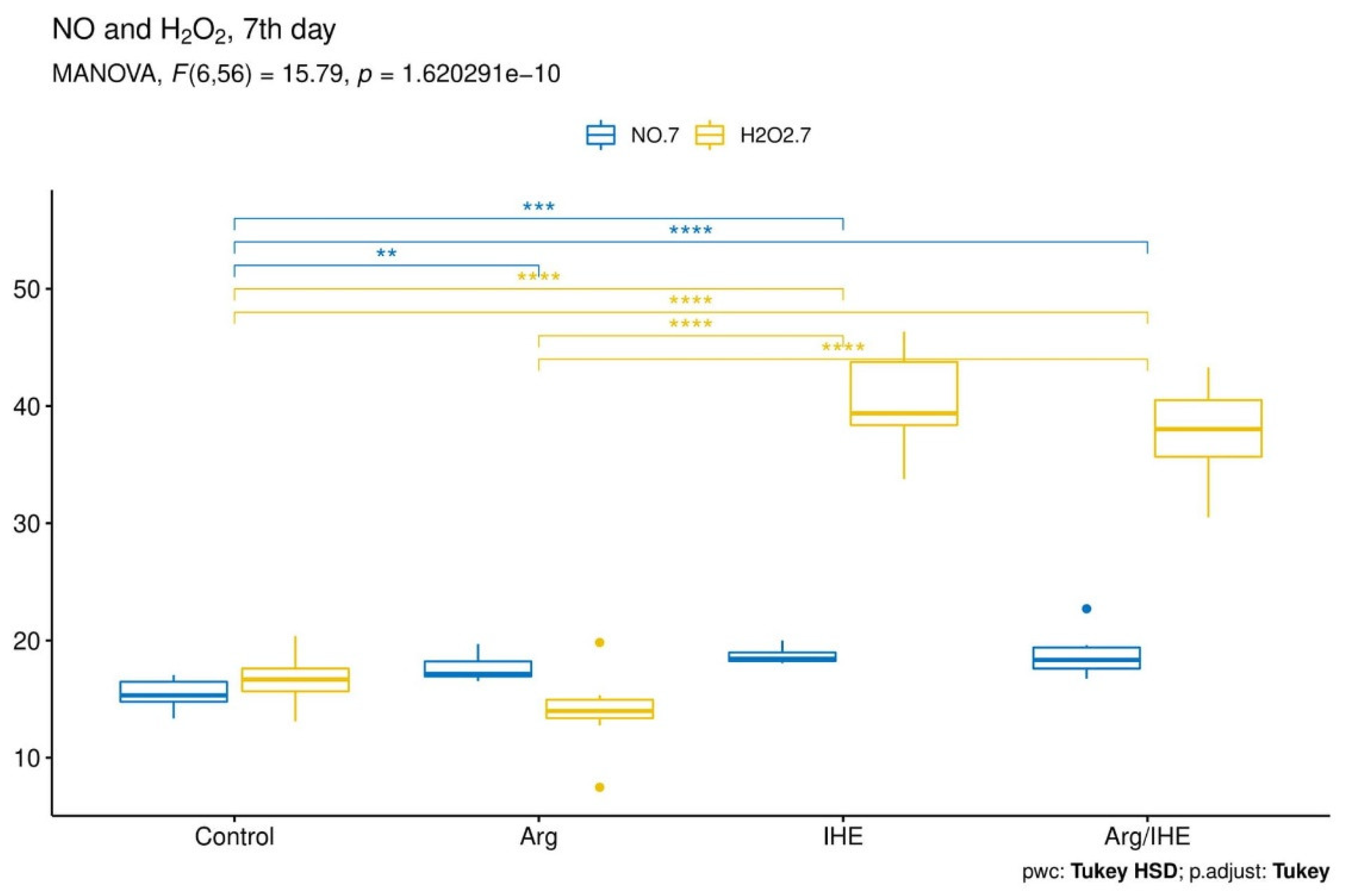
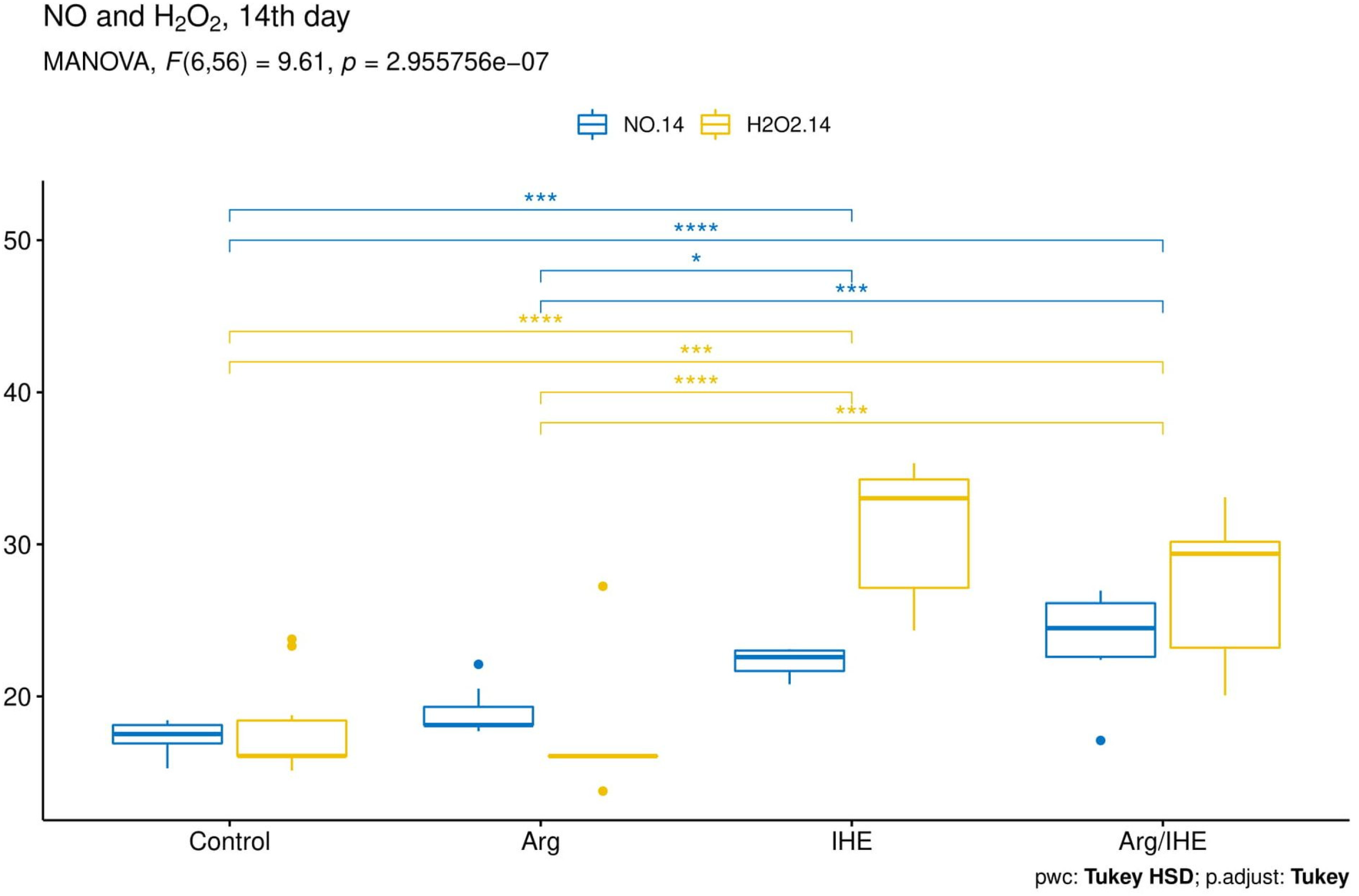
| NO [µmol/L] | |||||||||||
| Control | 15.37 ± 1.83 | - | 0.166 | 15.36 ± 1.26 | - | 0.569 | 17.33 ± 0.99 | - | 0.701 | 0.995 | <0.05 |
| Arg | 15.64 ± 1.66 | 0.98 | 17.67 ± 1.19 | <0.01 | 18.97 ± 1.67 | 0.326 | <0.01 | <0.01 | |||
| IHE | 14.78 ± 0.79 | 0.856 | 18.71 ± 0.73 | <0.001 | 22.26 ± 0.95 | <0.001 | <0.05 | <0.001 | |||
| Arg/IHE | 14.12 ± 0.95 | 0.251 | 18.67 ± 1.78 | <0.001 | 23.93 ± 3.02 | <0.001 | <0.001 | <0.01 | |||
| H2O2 [µmol/L] | |||||||||||
| Control | 13.16 ± 1.13 | - | 0.382 | 16.67 ± 2.00 | - | 0.92 | 17.87 ± 3.15 | - | 0.678 | <0.001 | <0.01 |
| Arg | 13.39 ± 1.76 | 0.992 | 13.99 ± 3.66 | 0.46 | 17.34 ± 4.45 | 0.994 | 0.748 | <0.05 | |||
| IHE | 15.26 ± 1.99 | 0.098 | 40.36 ± 4.64 | <0.001 | 30.93 ± 4.81 | <0.001 | <0.001 | <0.001 | |||
| Arg/IHE | 15.97 ± 1.91 | <0.01 | 39.47 ± 4.36 | <0.001 | 27.41 ± 4.63 | <0.001 | <0.001 | <0.001 | |||
| NO/H2O2 ratio [µmol/L] | |||||||||||
| Control | 1.17 ± 0.10 | - | 0.456 | 0.93 ± 0.13 | - | 0.99 ± 0.15 | - | 0.386 | <0.01 | <0.05 | |
| Arg | 1.19 ± 0.23 | 0.986 | 1.35 ± 0.42 | <0.01 | 1.13 ± 0.19 | 0.383 | 0.505 | 0.675 | |||
| IHE | 0.98 ± 0.13 | 0.095 | 0.47 ± 0.05 | <0.01 | 0.74 ± 0.15 | <0.05 | <0.001 | <0.05 | |||
| Arg/IHE | 0.90 ± 0.12 | <0.01 | 0.51 ± 0.10 | <0.01 | 0.90 ± 0.20 | 0.615 | <0.001 | 0.974 | |||
| CRP [mg/L] | |||||||||||
| Control | 1.57 ± 0.53 | - | 0.024 | 2.02 ± 0.35 | - | 0.552 | 2.29 ± 0.48 | - | 0.115 | <0.05 | <0.01 |
| Arg | 1.62 ± 0.49 | 0.997 | 1.99 ± 0.55 | 0.999 | 2.14 ± 0.41 | 0.917 | 0.182 | 0.097 | |||
| IHE | 1.45 ± 0.15 | 0.968 | 2.69 ± 0.41 | <0.05 | 2.46 ± 0.28 | 0.913 | <0.001 | <0.001 | |||
| Arg/IHE | 1.66 ± 0.57 | 0.977 | 3.09 ± 0.52 | <0.001 | 2.58 ± 0.63 | 0.589 | <0.001 | <0.01 | |||
| 1st Day of Camp | 7th Day of Camp | 14th Day of Camp | 1st Day vs. 7th Day | 1st Day vs. 14th Day | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | |||
| HGF [pg/mL] | |||||||||||
| Control | 587 ± 73 | - | 0.237 | 534 ± 77 | - | 0.467 | 602 ± 68 | - | 0.653 | <0.01 | 0.625 |
| Arg | 620 ± 60 | 0.687 | 568 ± 89 | 0.827 | 770 ± 69 | <0.001 | <0.05 | <0.01 | |||
| IHE | 623 ± 61 | 0.666 | 546 ± 95 | 0.992 | 792 ± 49 | <0.001 | 0.094 | <0.05 | |||
| Arg/IHE | 544 ± 42 | 0.425 | 708 ± 72 | <0.001 | 789 ± 71 | <0.001 | <0.001 | <0.001 | |||
| IGF-1 [ng/mL] | |||||||||||
| Control | 120 ± 41 | - | 0.19 | 116 ± 22 | - | 0.867 | 100 ± 15 | - | 0.911 | 0.16 | 0.084 |
| Arg | 126 ± 23 | 0.982 | 103 ± 19 | 0.834 | 98 ± 12 | 0.998 | 0.073 | <0.01 | |||
| IHE | 149 ± 11 | 0.231 | 248 ± 26 | <0.001 | 206 ± 29 | <0.001 | <0.001 | <0.01 | |||
| Arg/IHE | 148 ± 26 | 0.186 | 280 ± 51 | <0.001 | 293 ± 43 | <0.001 | <0.001 | <0.001 | |||
| PDGFBB [pg/mL] | |||||||||||
| Control | 2281 ± 513 | - | 0.084 | 2646 ± 289 | - | 0.381 | 2068 ± 368 | - | 0.706 | 0.074 | 0.063 |
| Arg | 2327 ± 418 | 0.995 | 2836 ± 293 | 0.806 | 1980 ± 161 | 0.927 | 0.062 | 0.088 | |||
| IHE | 2582 ± 231 | 0.456 | 2762 ± 160 | 0.953 | 3133 ± 263 | <0.001 | 0.15 | <0.01 | |||
| Arg/IHE | 2307 ± 266 | 0.999 | 3418 ± 686 | <0.01 | 2478 ± 274 | <0.05 | <0.01 | 0.226 | |||
| BDNF [pg/mL] | |||||||||||
| Control | 23,447 ± 3237 | - | 0.067 | 27,486 ± 1974 | - | 0.781 | 27,426 ± 2452 | - | 0.789 | <0.05 | <0.01 |
| Arg | 23,922 ± 3040 | 0.987 | 29,567 ± 2651 | 0.301 | 26,626 ± 1250 | 0.88 | <0.05 | 0.073 | |||
| IHE | 22,402 ± 3184 | 0.899 | 18,817 ± 1118 | <0.001 | 18,154 ± 1377 | <0.001 | 0.059 | 0.053 | |||
| Arg/IHE | 22,120 ± 2177 | 0.756 | 21,218 ± 3025 | <0.001 | 19,952 ± 2791 | <0.001 | 0.198 | <0.05 | |||
| VEGF [pg/mL] | |||||||||||
| Control | 341 ± 68 | - | 0.03 | 405 ± 54 | - | 0.112 | 234 ± 65 | - | 0.782 | 0.085 | <0.001 |
| Arg | 361 ± 64 | 0.861 | 408 ± 63 | 0.958 | 238 ± 77 | 0.999 | 0.154 | <0.05 | |||
| IHE | 330 ± 44 | 0.999 | 406 ± 46 | 0.974 | 389 ± 45 | <0.001 | <0.05 | 0.135 | |||
| Arg/IHE | 344 ± 78 | 0.991 | 452 ± 97 | 0.284 | 495 ± 64 | <0.001 | 0.054 | <0.01 | |||
| HGF [pg/mL] | IGF-1 [ng/mL] | PDGFBB [pg/mL] | BDNF [pg/mL] | VEGF [pg/mL] | |
|---|---|---|---|---|---|
| NO [µmol/L] | 0.662 | 0.554 | 0.160 | −0.286 | 0.274 |
| <0.001 | <0.001 | >0.05 | <0.01 | <0.01 | |
| H2O2 [µmol/L] | 0.321 | 0.780 | 0.479 | −0.525 | 0.368 |
| <0.01 | <0.001 | <0.001 | <0.001 | <0.001 |
| 1st Day of Camp | 7th Day of Camp | 14th Day of Camp | 1st Day vs. 7th Day | 1st day vs. 14th day | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | |||
| HB [g/dL] | |||||||||||
| Control | 15.3 ± 0.8 | - | 0.043 | 15.2 ± 0.7 | - | 0.165 | 15.5 ± 0.2 | - | 0.462 | 0.918 | 0.335 |
| Arg | 14.9 ± 0.8 | 0.757 | 14.8 ± 0.5 | 0.617 | 14.6 ± 0.3 | <0.01 | 0.271 | 0.306 | |||
| IHE | 15.1 ± 0.8 | 0.961 | 14.4 ± 1.0 | 0.137 | 14.3 ± 0.6 | <0.01 | 0.071 | 0.218 | |||
| Arg/IHE | 15.3 ± 0.7 | 1 | 15.1 ± 0.7 | 0.947 | 15.2 ± 0.8 | 0.517 | 0.16 | 0.851 | |||
| RBC [mln/mm3] | |||||||||||
| Control | 5.4 ± 0.3 | - | 0.189 | 5.4 ± 0.2 | - | 0.392 | 5.1 ± 0.2 | - | 0.736 | 0.411 | <0.05 |
| Arg | 5.3 ± 0.5 | 0.781 | 5.2 ± 0.3 | 0.286 | 4.6 ± 0.1 | <0.001 | 0.636 | <0.05 | |||
| IHE | 5.2 ± 0.4 | 0.852 | 5.0 ± 0.5 | <0.05 | 4.7 ± 0.3 | <0.01 | 0.055 | <0.05 | |||
| Arg/IHE | 5.0 ± 0.2 | 0.076 | 4.9 ± 0.2 | <0.01 | 5.5 ± 0.3 | <0.05 | 0.065 | <0.01 | |||
| RET [‰] | |||||||||||
| Control | 4.1 ± 1.1 | - | 0.222 | 5.2 ± 1.2 | - | 0.459 | 7.1 ± 2.2 | - | 0.505 | <0.05 | <0.01 |
| Arg | 4.1 ± 1.2 | 1 | 5.0 ± 1.4 | 0.994 | 10.1 ± 1.8 | <0.05 | 0.2 | <0.001 | |||
| IHE | 3.0 ± 0.6 | 0.139 | 8.7 ± 2.5 | <0.001 | 12.0 ± 1.4 | <0.001 | <0.01 | <0.001 | |||
| Arg/IHE | 3.3 ± 0.7 | 0.295 | 6.4 ± 1.1 | 0.319 | 9.3 ± 1.7 | 0.071 | <0.001 | <0.01 | |||
| HCT [%] | |||||||||||
| Control | 48.1 ± 3.0 | - | 0.4 | 48.2 ± 2.8 | - | 0.45 | 45.8 ± 1.5 | - | 0.403 | 0.825 | <0.05 |
| Arg | 47.9 ± 3.0 | 0.996 | 47.2 ± 1.8 | 0.878 | 43.0 ± 1.2 | <0.01 | 0.444 | <0.01 | |||
| IHE | 45.7 ± 1.1 | 0.221 | 45.1 ± 2.4 | 0.164 | 44.2 ± 2.4 | 0.176 | 0.598 | 0.096 | |||
| Arg/IHE | 43.8 ± 1.7 | <0.01 | 42.3 ± 3.7 | <0.001 | 45.8 ± 1.0 | 1 | 0.41 | <0.05 | |||
| MCV [fL] | |||||||||||
| Control | 89.3 ± 3.9 | - | 0.316 | 89.9 ± 3.1 | - | 0.427 | 89.8 ± 2.0 | - | 0.732 | 0.43 | 0.626 |
| Arg | 92.1 ± 3.0 | 0.35 | 91.8 ± 2.8 | 0.559 | 93.5 ± 0.3 | <0.05 | 0.647 | 0.278 | |||
| IHE | 85.5 ± 3.4 | 0.163 | 84.8 ± 2.4 | <0.05 | 83.8 ± 1.9 | <0.001 | 0.328 | 0.067 | |||
| Arg/IHE | 88.0 ± 3.2 | 0.842 | 87.8 ± 3.1 | 0.406 | 85.0 ± 3.5 | <0.001 | 0.681 | <0.05 | |||
| MCH [pg/RBC] | |||||||||||
| Control | 28.4 ± 1.0 | - | 0.393 | 28.5 ± 1.5 | - | 0.311 | 30.5 ± 0.9 | - | 0.643 | 1 | <0.001 |
| Arg | 28.7 ± 1.5 | 0.963 | 28.6 ± 0.9 | 1 | 32.0 ± 0.6 | <0.05 | 1 | <0.001 | |||
| IHE | 28.8 ± 1.4 | 0.934 | 29.7 ± 1.9 | 0.377 | 28.5 ± 1.0 | <0.01 | 0.079 | 0.451 | |||
| Arg/IHE | 30.7 ± 1.2 | <0.01 | 30.6 ± 1.1 | <0.05 | 28.6 ± 1.5 | <0.01 | 0.681 | <0.05 | |||
| MCHC [g/dL] | |||||||||||
| Control | 31.7 ± 0.9 | - | 0.826 | 31.7 ± 1.1 | - | 0.863 | 33.7 ± 0.4 | - | 0.158 | 0.968 | <0.01 |
| Arg | 31.0 ± 0.6 | 0.241 | 31.2 ± 0.3 | 0.522 | 34.2 ± 0.3 | 0.221 | 0.359 | <0.05 | |||
| IHE | 33.5 ± 0.2 | <0.001 | 35.1 ± 0.9 | <0.001 | 33.7 ± 0.6 | 1 | <0.001 | 0.336 | |||
| Arg/IHE | 34.8 ± 0.8 | <0.001 | 34.9 ± 0.3 | <0.001 | 33.8 ± 0.5 | 1 | 0.155 | <0.05 | |||
| RDW [%] | |||||||||||
| Control | 15,2 ± 1,7 | - | 0.525 | 15.0 ± 1.3 | - | 0.594 | 15,0 ± 0.9 | - | 0.85 | 0.797 | 0.743 |
| Arg | 14,7 ± 0.4 | 0.839 | 14.9 ± 0.6 | 0.973 | 14.5 ± 0.0 | 0.282 | 0.352 | 0.26 | |||
| IHE | 12.4 ± 0.4 | <0.001 | 12.4 ± 0.4 | <0.001 | 12.4 ± 0.3 | <0.001 | 0.741 | 0.618 | |||
| Arg/IHE | 15.3 ± 0.9 | 0.991 | 14.9 ± 0.6 | 0.975 | 12.3 ± 0.4 | <0.001 | 0.214 | <0.001 | |||
| EPO [mIU/mL] | |||||||||||
| Control | 3.25 ± 0.88 | - | 0.618 | 4.3 ± 1.37 | - | 0.466 | 4.84 ± 1.17 | - | 0.654 | <0.01 | <0.01 |
| Arg | 3.51 ± 0.42 | 0.922 | 6.08 ± 1.04 | <0.05 | 4.40 ± 1.68 | 0.994 | 0.618 | <0.05 | |||
| IHE | 3.12 ± 0.86 | 0.99 | 6.57 ± 0.71 | <0.01 | 9.70 ± 2.32 | <0.001 | <0.01 | <0.001 | |||
| Arg/IHE | 4.62 ± 0.8 | <0.01 | 6.49 ± 0.73 | <0.01 | 8.13 ± 1.62 | <0.001 | <0.001 | <0.01 | |||
| WBC [103/µL] | |||||||||||
| Control | 5.9 ± 1.0 | - | 0.149 | 6.7 ± 0.7 | - | 0.25 | 6.9 ± 0.4 | - | 0.173 | 0.066 | <0.05 |
| Arg | 5.6 ± 0.1 | 0.774 | 5.9 ± 0.8 | 0.3 | 6.5 ± 0.8 | 0.957 | 0.281 | <0.05 | |||
| IHE | 5.0 ± 0.3 | 0.148 | 5.3 ± 0.7 | <0.05 | 6.1 ± 0.3 | 0.619 | 0.114 | <0.01 | |||
| Arg/IHE | 5.5 ± 1.0 | 0.654 | 6.2 ± 1.2 | 0.699 | 7.6 ± 2.2 | 0.591 | 0.322 | <0.05 | |||
| 1st Day of Camp | 7th Day of Camp | 14th Day of Camp | 1st Day vs. 7th Day | 1st Day vs. 14th Day | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | mean ± SD | Control vs. Arg, IHE or Arg/IHE | η2 | |||
| TG [mg/dL] | |||||||||||
| Control | 97 ± 34 | - | 0.057 | 83 ± 24 | - | 0.231 | 118 ± 42 | - | 0.359 | <0.05 | 0.31 |
| Arg | 85 ± 24 | 0.925 | 76 ± 12 | 0.951 | 64 ± 4 | <0.01 | 0.331 | 0.057 | |||
| IHE | 111 ± 41 | 0.898 | 112 ± 18 | 0.138 | 122 ± 27 | 0.995 | 0.975 | 0.357 | |||
| Arg/IHE | 105 ± 50 | 0.969 | 78 ± 35 | 0.978 | 111 ± 32 | 0.964 | 0.06 | 0.764 | |||
| TC [mg/dL] | |||||||||||
| Control | 196 ± 25 | - | 0.143 | 189 ± 38 | - | 0.315 | 175 ± 19 | - | 0.306 | 0.323 | <0.05 |
| Arg | 160 ± 47 | 0.238 | 138 ± 25 | <0.05 | 148 ± 7 | 0.123 | 0.097 | 0.522 | |||
| IHE | 188 ± 38 | 0.976 | 161 ± 31 | 0.399 | 163 ± 27 | 0.789 | <0.05 | <0.05 | |||
| Arg/IHE | 196 ± 41 | 1 | 189 ± 34 | 1 | 188 ± 30 | 0.601 | 0.399 | 0.359 | |||
| LDL [mg/dL] | |||||||||||
| Control | 118 ± 27 | - | 0.149 | 121 ± 33 | - | 0.173 | 111 ± 18 | - | 0.059 | 0.707 | 0.232 |
| Arg | 93 ± 32 | 0.395 | 77 ± 17 | 0.555 | 97 ± 10 | 0.692 | 0.094 | 0.698 | |||
| IHE | 112 ± 38 | 0.985 | 103 ± 38 | 0.956 | 105 ± 37 | 0.963 | 0.406 | 0.386 | |||
| Arg/IHE | 128 ± 35 | 0.913 | 157 ± 117 | 0.664 | 112 ± 30 | 0.999 | 0.432 | 0.129 | |||
| HDL [mg/dL] | |||||||||||
| Control | 64 ± 43 | - | 0.094 | 51 ± 11 | - | 0.114 | 53 ± 10 | - | 0.188 | 0.234 | 0.76 |
| Arg | 50 ± 13 | 0.716 | 45 ± 9 | 0.675 | 53 ± 5 | 0.996 | 0.182 | 0.47 | |||
| IHE | 45 ± 10 | 0.498 | 56 ± 12 | 0.787 | 48 ± 7 | 0.667 | <0.01 | <0.05 | |||
| Arg/IHE | 47 ± 7 | 0.493 | 49 ± 7 | 0.998 | 45 ± 8 | 0.176 | <0.05 | 0.641 | |||
| Non-HDL [mg/dL] | |||||||||||
| Control | 132 ± 44 | - | 0.126 | 138 ± 36 | - | 0.312 | 122 ± 15 | - | 0.385 | 1 | 0.454 |
| Arg | 110 ± 35 | 1 | 92 ± 17 | <0.05 | 95 ± 2 | 0.105 | 0.084 | 0.298 | |||
| IHE | 143 ± 42 | 1 | 106 ± 39 | 0.234 | 116 ± 28 | 0.951 | <0.05 | <0.001 | |||
| Arg/IHE | 149 ± 40 | 1 | 139 ± 31 | 1 | 143 ± 33 | 0.215 | 0.205 | 0.313 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zembron-Lacny, A.; Gramacki, A.; Wawrzyniak-Gramacka, E.; Tylutka, A.; Hertmanowska, N.; Kasperska, A.; Czuba, M. Intermittent Hypoxic Exposure with High Dose of Arginine Impact on Circulating Mediators of Tissue Regeneration. Nutrients 2020, 12, 1933. https://doi.org/10.3390/nu12071933
Zembron-Lacny A, Gramacki A, Wawrzyniak-Gramacka E, Tylutka A, Hertmanowska N, Kasperska A, Czuba M. Intermittent Hypoxic Exposure with High Dose of Arginine Impact on Circulating Mediators of Tissue Regeneration. Nutrients. 2020; 12(7):1933. https://doi.org/10.3390/nu12071933
Chicago/Turabian StyleZembron-Lacny, Agnieszka, Artur Gramacki, Edyta Wawrzyniak-Gramacka, Anna Tylutka, Natalia Hertmanowska, Anna Kasperska, and Miłosz Czuba. 2020. "Intermittent Hypoxic Exposure with High Dose of Arginine Impact on Circulating Mediators of Tissue Regeneration" Nutrients 12, no. 7: 1933. https://doi.org/10.3390/nu12071933
APA StyleZembron-Lacny, A., Gramacki, A., Wawrzyniak-Gramacka, E., Tylutka, A., Hertmanowska, N., Kasperska, A., & Czuba, M. (2020). Intermittent Hypoxic Exposure with High Dose of Arginine Impact on Circulating Mediators of Tissue Regeneration. Nutrients, 12(7), 1933. https://doi.org/10.3390/nu12071933





