Correlation of Body Composition and Nutritional Status with Functional Recovery in Stroke Rehabilitation Patients
Abstract
1. Introduction
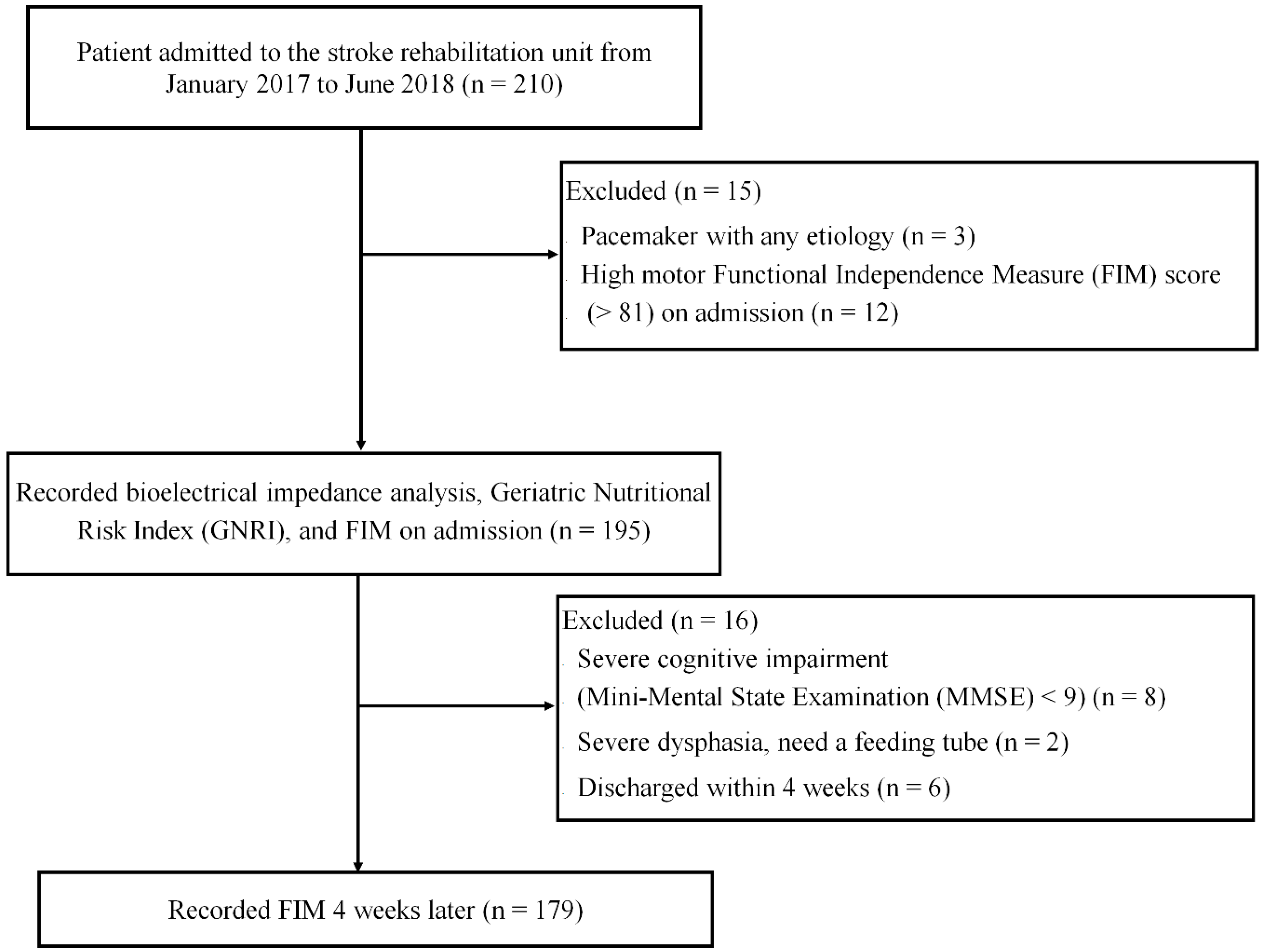
2. Materials and Methods
2.1. Bioelectrical Impedance Analysis
2.2. Nutritional Status
2.3. Functional Measurements
2.4. Stroke Rehabilitation Unit in Japan
2.5. Statistics
3. Results
3.1. Descriptive Characteristics
3.2. The Univariate Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Glabal and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Bohannon, R.W. Muscle strength and muscle training after stroke. J. Rehabil. Med. 2007, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakamura, R.; Yamada, Y.; Handa, T. Determinants of maximum walking speed in hemiparetic stroke patients. Tohoku J. Exp. Med. 1990, 162, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Krivickas, L.S.; Dover, D.J.; Ochaya, J.; Frontera, W.R. Relationship between force and size in human single muscle fibers. Exp. Physiol. 2011, 96, 539–547. [Google Scholar] [CrossRef]
- Frohlich-Zwahlen, A.K.; Casartelli, N.C.; Item-Glatthorn, J.F.; Maffiuletti, N.A. Validity of resting myotonometric assessment of lower extremity muscles in chronic stroke patients with limitedhypertonic: A preliminary study. J. Electromyogr. Kinesiol. 2014, 24, 762–769. [Google Scholar] [CrossRef]
- Pang, M.Y.; Eng, J.J.; McKay, H.; Dawson, A.S. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporous. Int. 2005, 16, 1769–1779. [Google Scholar] [CrossRef]
- Prada-Medeiros, C.L.; Silva, M.P.; Lessi, G.C.; Alves, M.Z.; Tannus, A.; Lindquist, A.R.; Salvini, T.F. Muscle atrophy and functional deficits of knee extensors and flexors in people with chronic stroke. Phys. Ther. 2012, 92, 429–439. [Google Scholar] [CrossRef]
- Ryan, A.S.; Buscemi, A.; Forrester, L.; Hafer-Macko, C.E.; Ivey, F.M. Atrophy and intramuscular fat in specific muscles of the thigh’ associated weakness and hyper insulin emit in stroke survivors. Neurorehabil. Neural Repair 2011, 25, 865–872. [Google Scholar] [CrossRef]
- Sunnerhagen, K.S.; Svantesson, U.; Lonn, L.; Krotkiewski, M.; Grimby, G. Upper motor neuron lessions’ their effect on muscle performance and appearance in stroke patients with minor motor impairment. Arch. Phys. Med. Rehabil. 1999, 80, 155–161. [Google Scholar] [CrossRef]
- Singer, K.P.O.P.; Breidahl, P. The use of computed tomography in assessing muscle cross-sectional area, and the relationship between cross-sectional area and strength. Aust. J. Physiother. 1987, 33, 75–82. [Google Scholar] [CrossRef]
- Freilich, R.J.; Kirsner, R.L.; Byrne, E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul. Disord. 1995, 5, 415–422. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Mori, N.; Kimura, M.; Ichihashi, N. Skeletal muscle quality asssessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur. J. Appl. Physiol. 2012, 112, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- The FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke. Stroke 2003, 34, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H. Rehabilitation and Clinical Nutrition. Jpn. J. Rehabil. Med. 2011, 48, 270–281. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Granger, C.V.; Hamilton, B.B.; Linacre, J.M.; Heinemann, A.W.; Wright, B.D. Performance profiles of the functional independence measure. Am. J. Phys. Med. Rehabil. 1993, 72, 84–89. [Google Scholar] [CrossRef]
- Haigh, R.; Tennant, A.; Biering-Sorensen, F.; Grimby, G.; Marincek, C.; Phillips, S.; Ring, H.; Tesio, L.; Thonnard, J.L. The use of outcome measures in physical medicine and rehabilitation within Europe. J. Rehabil. Med. 2001, 33, 273–278. [Google Scholar]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Kishimoto, M.; Shiude, K.; Tanaka, M. A Methodological evaluation of body composition analysis for patients with life-related disorders. J. Jpn. Soc. Nutr. Food Sci. 2009, 62, 253–258. (In Japanese) [Google Scholar] [CrossRef]
- English, C.; McLennan, H.; Thoirs, K.; Coates, A.; Bernhardt, J. Loss of skeletal muscle mass after stroke: A systematic review. Int. J. Stroke 2010, 5, 395–402. [Google Scholar] [CrossRef]
- Scherbakov, N.; von Haehling, S.; Anker, S.D.; Dirnagl, U.; Doehner, W. Stroke induced sarcopenia: Muscle wasting and disability after stroke. Int. J. Cardiol. 2013, 170, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Wagenaar, R.C.; Twisk, J.W.; Lankhorst, G.J.; Koetsier, J.C. Intensity of leg and arm training after primary middle cerebral artery stroke: A randomized trial. Lancet 1999, 354, 191–196. [Google Scholar] [CrossRef]
- Langhorne, P.; Wagenaar, R.C.; Partrige, C. Physiotherapy after stroke: More is better? Physiother. Res. Int. 1996, 1, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Wagenaar, R.C.; Koelman, T.W.; Lankhorst, G.J.; Koetsier, J.C. Effects of intensity of rehabilitation after stroke. A research synthesis. Stroke 1997, 28, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K. Bioelectrical impedance methods for noninvasive health monitoring: A review. J. Med. Eng. 2014. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobaus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.G.; Barros, A.J.D.; Wang, J.; Heymsfield, S.B.; Pierson, R.N., Jr. Bioelectrical impedance analysis: Poplation reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsh, J.F.; Lis, C.G. Bioelectrical impedance phase angle in clinical practice: Implications for the prognosis in stage IIIB and IV non-small cell lung cancer. BMC Cancer 2009, 9, 37. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Pichard, C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin. Nutr. 2013, 32, 294–299. [Google Scholar] [CrossRef]
- Foley, N.C.; Salter, K.L.; Robertson, J.; Teasell, R.W.; Woodbury, M.G. Which reported estimate of the prevalence of malnutrition after stroke is valid? Stroke 2009, 40, e66–e74. [Google Scholar] [CrossRef]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. International consensus guideline committee. Adult starvation and disease-related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J. Parenter. Enteral Nutr. 2010, 34, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; de Groot, L.C.; van Loon, L.J. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Anker, S.D. Treatment of cachexia: An overview of recent developments. J. Am. Med. Dir. Assoc. 2014, 15, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, E.D. Combination therapy in cachexia. Ann. Palliat. Med. 2019, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Mean | SD |
|---|---|---|
| Number of patients | 179 | |
| Age (years) | 79.7 | 11.5 |
| Sex (F/M) | 90/89 | |
| Mini-Mental State Examination | 20.2 | 8.0 |
| Days after stroke | 27.6 | 8.7 |
| Duration of rehabilitation program (min/day) | 159.8 | 21.6 |
| Motor Functional Independence Measure items on admission | 39.0 | 19.9 |
| Motor Functional Independence Measure items at 4 weeks | 53.6 | 26.8 |
| Men (n = 89) | Women (n = 90) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 78.6 | 13.3 | 80.7 | 8.3 |
| Height (cm) | 158.1 | 13.5 | 153.2 * | 7.1 |
| Weight (kg) | 52.4 | 15.8 | 45.7 * | 10.3 |
| BMI (kg/m2) | 20.0 | 3.76 | 19.4 | 3.9 |
| Albumin (g/dL) | 3.8 | 0.4 | 3.7 | 0.4 |
| GNRI | 97.3 | 10.4 | 93.5 | 11.3 |
| motor FIM items on admission | 39.5 | 18.5 | 38.6 | 21.5 |
| motor FIM items on 4 weeks | 55.7 | 27.6 | 52.8 | 25.4 |
| BIA data | ||||
| Body fat percentage (total body fat/body weight) (%) | 24.2 | 8.5 | 28.8 * | 10.9 |
| Body muscle percentage (skeletal muscle mass/body weight) (%) | 40.0 | 4.8 | 35.8 * | 5.7 |
| Body water percentage (total body water/body weight) (%) | 55.7 | 6.0 | 52.3 | 8.0 |
| Phase angle (%) | 4.2 | 1.1 | 3.3 * | 0.9 |
| Variables | Odds Ratios | 95% CI | p Value |
|---|---|---|---|
| Male gender | 2.11 | 0.86–5.21 | 0.10 |
| Advanced age (>80 years old) | 1.20 | 0.48–2.98 | 0.69 |
| No malnutrition (GNRI > 92) | 2.57 | 1.13–5.85 | 0.02 |
| High body fat percentage (male > 25%, female > 30%) | 1.81 | 0.70–4.70 | 0.69 |
| High body muscle percentage (male > 30%, female > 25%) | 2.42 | 1.05–5.59 | 0.03 |
| High body water composition percentage (>60%) | 2.84 | 0.50–16.23 | 0.24 |
| High phase angle (>3.0 degree) | 3.23 | 1.37–7.65 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irisawa, H.; Mizushima, T. Correlation of Body Composition and Nutritional Status with Functional Recovery in Stroke Rehabilitation Patients. Nutrients 2020, 12, 1923. https://doi.org/10.3390/nu12071923
Irisawa H, Mizushima T. Correlation of Body Composition and Nutritional Status with Functional Recovery in Stroke Rehabilitation Patients. Nutrients. 2020; 12(7):1923. https://doi.org/10.3390/nu12071923
Chicago/Turabian StyleIrisawa, Hiroshi, and Takashi Mizushima. 2020. "Correlation of Body Composition and Nutritional Status with Functional Recovery in Stroke Rehabilitation Patients" Nutrients 12, no. 7: 1923. https://doi.org/10.3390/nu12071923
APA StyleIrisawa, H., & Mizushima, T. (2020). Correlation of Body Composition and Nutritional Status with Functional Recovery in Stroke Rehabilitation Patients. Nutrients, 12(7), 1923. https://doi.org/10.3390/nu12071923





